Abstract
Collagen triple helix repeat containing-1 (CTHRC1), a secreted protein, has been demonstrated as an oncogene in many types of human cancers including pancreatic ductal adenocarcinoma (PDAC). However, little is known about the prognostic value of CTHRC1 in PDAC. In current study, we investigated the expression pattern and underlying clinical significance of CTHRC1 in PDAC. Data from public PDAC microarray datasets, real-time PCR and immunohistochemistry demonstrated that CTHRC1 expression was dramatically increased in PDAC compared with normal tissues at both mRNA and protein level, which was consistent with previous studies. Analysis of its correlation with clinicopathological parameters indicated that high protein expression level of CTHRC1 was significantly associated with lymph node metastasis, vascular invasion and perineural invasion. Kaplan-Meier survival analysis showed that patients with higher CTHRC1 expression exhibited a remarkably shorter overall survival in four different PDAC patient cohorts. Importantly, univariable and multivariable Cox regression analysis revealed that CTHRC1 protein expression level was a significant and independent prognostic factor for overall survival rate of PDAC patients. Together, these data suggested that CTHRC1 is an unfavorable biomarker of prognosis in PDAC and may serve as a potential therapeutic candidate for PDAC treatment.
Keywords: Pancreatic ductal adenocarcinoma, CTHRC1, prognosis, biomarker
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most aggressive and lethal malignancies worldwide [1]. It is the fourth and seventh leading cause of cancer-related deaths in USA and China, respectively [2,3], and is projected to rank the second by 2030 in USA [4]. Owing to lack of obvious specific symptoms, most PDAC patients are diagnosed at advanced stage with vascular dissemination or distant metastasis and ultimately the prognosis is extremely poor [5]. Therefore, new insights into the biology and genetics of PDAC are required and it is essential to identify novel biological markers for better diagnosis and prediction of prognosis, and thus develop new targeted therapy.
Collagen triple helix repeat containing-1 (CTHRC1), a 26-kDa secreted glycoprotein, was initially discovered in a rat model of balloon-injured vasculature where it is transiently expressed by fibroblasts of the remodelling adventitia and by smooth muscle cells of the neointima [6]. It is a highly conserved molecule sharing 92% of sequence identity in human compared with that of rat homolog [7]. Structurally, it contains a NH2-terminal signaling peptide for extracellular secretion, a short collagen triple helix repeat of 36 amino acids, and a COOH-terminal globular domain [6]. Recently, accumulating evidence indicated that high expression of CTHRC1 is present in a wide spectrum of human cancers and plays a significant role in oncogenic pathway in these malignancies, such as melanoma [8], hepatocellular carcinoma [9], colorectal cancer [10], non-small cell lung cancer [11], gastrointestinal stromal tumor [12], epithelial ovarian cancer [13], PDAC [14] and so on. It has also been reported that up-regulated CTHRC1 in PDAC carcinogenesis contributed to tumor cell invasion and metastasis via activating several key signaling molecules, including Src, focal adhesion kinase, paxillin, mitogen-activated protein kinase kinase (MEK), extracellular signal-regulated kinase and Rac1 [14]. However, little is known about the relationship of CTHRC1 expression with clinicopathological parameters and prognosis in PDAC patients.
In this retrospective study, we further validated the expression pattern of CTHRC1 at both mRNA and protein level in a relative large scale, and investigated the relationship of CTHRC1 expression with corresponding clinicopathological parameters and prognosis. Importantly, the results suggested that high expression of CTHRC1 is a reliable indicator for the poor prognosis of PDAC patients.
Materials and methods
Patients and samples
A total of 40 freshly-frozen primary pancreatic cancer and matched adjacent non-tumor tissues (Ren Ji cohort 1) were collected from patients who underwent pancreatic surgical resection at Ren Ji Hospital between January 2012 and December 2013. Tissue microarrays containing 205 pairs of PDAC specimens and corresponding noncancerous tissues (Ren Ji cohort 2) were obtained from Ren Ji Hospital from January 2002 to June 2013. The pathological information was retrieved from the Pathology Department. None of the patients had received radiotherapy, chemotherapy, hormone therapy or other related anti-tumor therapies before surgery. For all samples from PDAC patients, clinical information was available. The follow-up time was calculated from the date of surgery to pancreatic cancer-related death, or November 17, 2015, the ultimate deadline. All the patients were provided with written informed consent before enrolment, and the study was approved by the Research Ethics Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University.
PDAC datasets acquisition and process
PDAC microarray datasets GSE71729 (Agilent-014850 Whole Human Genome Microarray 4x44K), E-MEXP-2780 (Affymetrix Human Genome U133 Plus 2.0 Array) and corresponding clinical data in this study were directly downloaded from Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) and ArrayExpress (http://www.ebi.ac.uk/arrayexpress/), respectively. Arrays were analyzed using log2-transformed background-corrected expression signal and annotated using GEO platform GPL4133 (GSE71729) and GPL570 (E-MEXP-2780) through R 3.2.2 software. Multiple probes mapping to the same gene symbol were collapsed by mean probe expression. Samples were normalized to each other via quantile normalization. After that, a set of gene ID-centric gene expression values were retrieved for downstream analysis.
Total RNA extraction and quantitative real-time PCR
Total RNA was extracted from tissues (Ren Ji Cohort 1) by using Trizol reagent (Takara, Japan) according to the manufactuer’s instruction. cDNA was synthesized using a PrimeScript RT Reagent Kit (Takara, Japan) in accordance with the protocol of manufacturer. StepOne Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA) was applied to detect the expression level of target gene using the SYBR Premix Ex Taq II (Takara, Japan), and GAPDH acted as an internal control. The data was calculated by the 2-ΔΔCt method. Primer sequences are listed as follows: CTHRC1 forward, 5’-CAATGGCATTCCGGGTACAC-3’, reverse, 5’-GTACACTCCGCAATTTTCCCAA-3’; GAPDH forward, 5’-GCATTGCCCTCAACGACCAC-3’, reverse: 5’-CCACCACCCTGTTGCTGTAG-3’.
Immunohistochemistry
The tissue microarray sections were deparaffinized in xylene and rehydrated with graded ethanol, and then treated with 0.3% hydrogen peroxide, followed by antigen retrieval. After being blocked with 10% normal goat serum for 30 min, the sections were incubated with primary antibodies at 4°C overnight, followed by incubation with a HRP-labeled secondary antibody for 30 min at room temperature. Finally, Diaminobenzidine tetrahydrochloride (DAB; Maixin Biotech, China) was used for the color-reaction followed by nucleus counterstaining with hematoxylin. The following antibodies were used: rabbit anti-CTHRC1 (1:200, Abcam, UK); Elivision plus Polyer HRP (Mouse/Rabbit) IHC Kit (Maixin Biotech, China).
The slides were inspected independently by two investigators in a blinded manner. Protein expression was evaluated according to the extent and intensity of staining (percentage of positive cells was measured on a scale of 0-3: < 5% scored 0; 6-25% scored 1; 25-50% scored 2; more than 50% scored 3, the intensity of staining was measured on a scale of 0-3: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining). And a final score was created to determine the cut-off value for low and high expression group by using grades of the extent × grades of intensity staining. Then the protein expression was sorted into four categories: “-” for a score of 0-1, “+” for a score of 2-3, “++” for a score of 4-6 and “+++” for a score of > 6; low expression was defined as a final score < 4 and high expression with a final score ≥ 4.
Statistical analysis
Data were presented as the means ± SD. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Graphical representations were carried out with GraphPad Prism 6 (San Diego, CA, USA) software. For clinicopathological analysis, the chi-square test or Fisher’s exact test was performed. The survival calculations were illustrated with Kaplan-Meier curves and differences between survival curves were tested by the log-rank test. Cox proportional hazards model was used to identify the prognostic factors by univariable and multivariable analysis. The student’s t-test or Mann-Whitney U test was used for comparison between two groups depending on distribution. P values (two-sided) less than 0.05 were considered statistically significant.
Results
Expression of CTHRC1 in PDAC tissues at mRNA and protein level
To further verify the expression pattern of CTHRC1 in PDAC, we first analyzed one independent microarray dataset in PDAC research from GEO. The results demonstrated that CTHRC1 mRNA expression was significantly enhanced in PDAC tissues compared with normal pancreatic tissues (P < 0.0001, Figure 1A). Then we analyzed the expression level of CTHRC1 in two independent patient cohorts from Ren Ji Hospital. We first performed real-time PCR to measure the mRNA expression of CTHRC1 in PDAC tissues and paired adjacent normal tissues (n=40, cohort 1, fresh tissues). Consistent with the data from GEO database, CTHRC1 mRNA expression was also obviously elevated in PDAC tissues (P < 0.0001, Figure 1B). We next detected CTHRC1 protein expression in tissue microarray containing 205 pairs of PDAC samples by immunohistochemistry (n=205, cohort 2, paraffin-embedded tissues). The results also confirmed that CTHRC1 protein displayed higher expression in tumor tissues than normal pancreatic tissues (69.3% vs. 27.3%, P < 0.0001), and the immunoreactivity of CTHRC1 was mostly distributed in both cytoplasm and cell membrane of PDAC cells (Figure 2). Collectively, these results further validated that both the mRNA and protein level of CTHRC1 were significantly upregulated in PDAC tissues, which was in agreement with previous studies.
Figure 1.
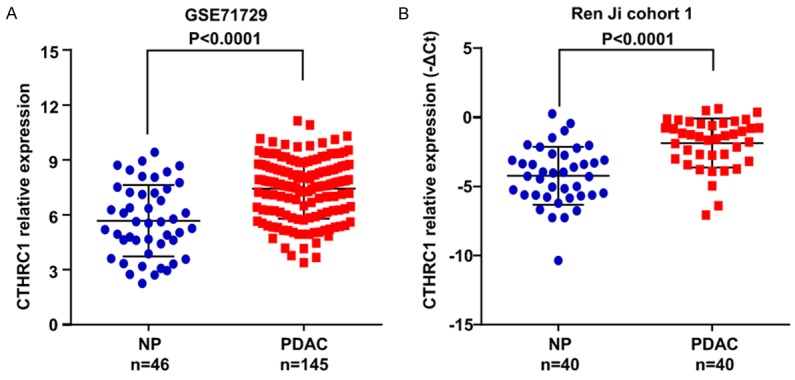
CTHRC1 expression in PDAC at mRNA level. A. CTHRC1 expression in GSE71729 derived from GEO grouped by normal pancreas (NP, n=46) and PDAC (n=145). P-values were calculated using the Mann-Whitney test. B. Increased CTHRC1 mRNA expression in 40 pairs of PDAC/normal pancreas (NP) was detected by real-time PCR (paired t-test). Error bars in the scatter plots represent SD.
Figure 2.

CTHRC1 expression in PDAC at protein level. CTHRC1 protein level was measured by immunohistochemical analysis in normal pancreas (NP) and PDAC tissue samples from Ren Ji cohort 2. A. Representative images are shown at 40×, 200×, 400× magnification, respectively. B. Chi-square test was used for comparison of CTHRC1 expression between two groups.
Correlation between CTHRC1 expression and clinicopathological parameters
To explore the clinical significance of CTHRC1 expression in PDAC, we analyzed the relationship between CTHRC1 expression and corresponding patients’ clinicopathological characteristics in Ren Ji cohort 2. As shown in Table 1, CTHRC1 expression was significantly associated with adverse clinicopathological features of PDAC, including lymph node metastasis (P=0.047), vascular invasion (P=0.018) and perineural invasion (P=0.025). No correlations between CTHRC1 expression and age, gender, tumor location, TNM stage, tumor size, T classification, distant metastasis, histological differentiation and serum CA199 level were found.
Table 1.
Correlations between CTHRC1 expression and clinicopathologic features in Ren Ji cohort 2 patients with pancreatic ductal adenocarcinoma (PDAC)
| Clinicopathological feature | Total 205 | Expression of CTHRC1 | P value (χ2 test) | |
|---|---|---|---|---|
|
| ||||
| Low (n=63, 30.7%) | High (n=142, 69.3%) | |||
| Age (years) | ||||
| < 65 | 97 | 36 (37.1) | 61 (62.9) | 0.060 |
| ≥ 65 | 108 | 27 (25.0) | 81 (75.0) | |
| Gender | ||||
| Male | 117 | 34 (29.1) | 83 (70.9) | 0.550 |
| Female | 88 | 29 (33.0) | 59 (67.0) | |
| Tumor location | ||||
| Head | 139 | 44 (31.7) | 95 (68.3) | 0.678 |
| Body/tail | 66 | 19 (28.8) | 47 (71.2) | |
| TNM stage (AJCC) | ||||
| Stage I | 38 | 11 (28.9) | 27 (71.1) | 0.830 |
| Stage II | 132 | 43 (32.6) | 89 (67.4) | |
| Stage III | 21 | 6 (28.6) | 15 (71.4) | |
| Stage IV | 14 | 3 (21.4) | 11 (78.6) | |
| Tumor size | ||||
| ≤ 3 cm | 69 | 19 (27.5) | 50 (72.5) | 0.480 |
| > 3 cm | 136 | 44 (32.4) | 92 (67.6) | |
| T classificattion | ||||
| T1 | 12 | 4 (33.3) | 8 (66.7) | 0.950 |
| T2 | 30 | 8 (26.7) | 22 (73.3) | |
| T3 | 139 | 43 (30.9) | 96 (69.1) | |
| T4 | 24 | 8 (33.3) | 16 (66.7) | |
| Lymph node metastasis | ||||
| Absent | 136 | 48 (35.3) | 88 (64.7) | 0.047 |
| Present | 69 | 15 (21.7) | 54 (78.3) | |
| Distant metastasis | ||||
| Absent | 191 | 60 (31.4) | 131 (68.6) | 0.434 |
| Present | 14 | 3 (21.4) | 11 (78.6) | |
| Vascular invasion | ||||
| Absent | 178 | 60 (33.7) | 118 (66.3) | 0.018 |
| Present | 27 | 3 (11.1) | 24 (88.9) | |
| Perineural invasion | ||||
| Absent | 106 | 40 (37.7) | 66 (62.3) | 0.025 |
| Present | 99 | 23 (23.2) | 76 (76.8) | |
| Histological differentiation | ||||
| Well/moderate | 132 | 43 (32.6) | 89 (67.4) | 0.442 |
| Poor | 73 | 20 (27.4) | 53 (72.6) | |
| CA199 level (U/ml) | ||||
| ≤ 35 | 26 | 8 (30.8) | 18 (69.2) | 0.734 |
| > 35 | 105 | 36 (34.3) | 69 (65.7) | |
| Missing | 74 | |||
Values in parentheses indicate percentage values. The bold number represents the P-values with significant differences.
Relationship between CTHRC1 expression and prognosis in PDAC patients
To further evaluate the prognostic value of CTHRC1, we examined the correlation of CTHRC1 expression with overall survival using Kaplan-Meier analysis and log-rank test in PDAC patients from GSE71729, E-MEXP-2780, Ren Ji cohort 1 and 2, respectively. Among the four different cohorts, in order to exclude the effect of complications, PDAC patients who died within 2 months after surgery were removed, leaving 109 cases in GSE71729, 30 in E-MEXP-2780, 40 in Ren Ji cohort 1 and 193 in Ren Ji cohort 2. The result revealed that CTHRC1 expression was dramatically associated with PDAC patients’ overall survival in GSE71729 (P=0.0343, Figure 3A), E-MEXP-2780 (P=0182, Figure 3B), Ren Ji cohort 1 (P=0.0215, Figure 3C) and cohort 2 (P=0.0064, Figure 3D), which indicated that patients with higher CTHRC1 expression exhibited significantly shorter survival time than those with lower CTHRC1 expression.
Figure 3.
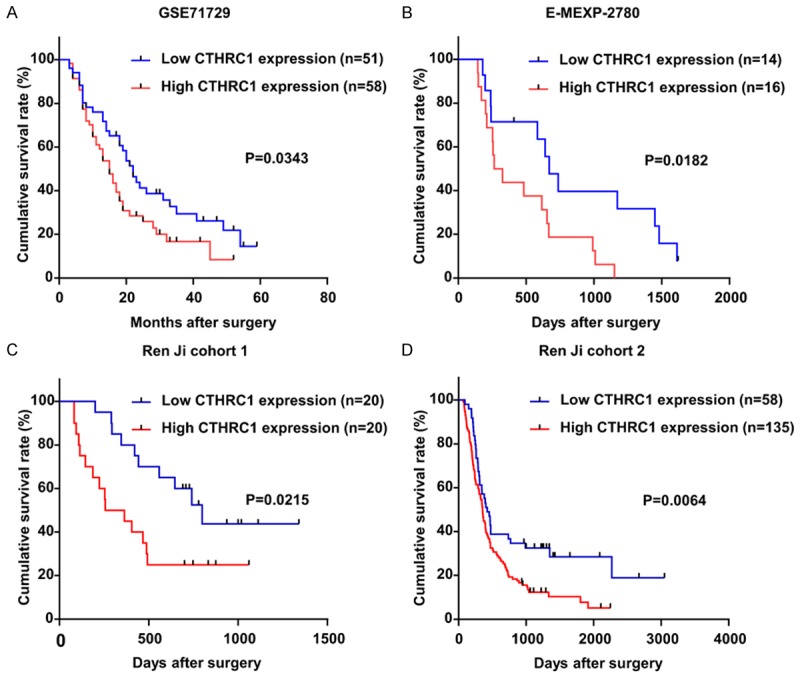
The potential value of CTHRC1 expression in predicting PDAC patients prognosis. A-D. The association of CTHRC1 expression and patients survival was conducted in GSE71729, E-MEXP-2780, Ren Ji cohort 1 and cohort 2. Kaplan-Meier survival curves show that patients with higher CTHRC1 expression have a poorer overall survival after surgery than their corresponding counterparts in PDAC. P-values were calculated by log-rank test.
Furthermore, univariable and multivariable Cox regression analyses were performed to identify the risk factors correlated with patients’ prognosis in Ren Ji cohort 2. Univariable analyses displayed that CTHRC1 expression, age, TNM stage, tumor size, lymph node metastasis, distant metastasis and histology were significant prognostic factors for overall survival prediction (Table 2). Meanwhile, multivariable analysis suggested that CTHRC1 expression, age, tumor size, lymph node metastasis and histology were independent predictors of the overall survival in patients with PDAC after pancreatectomy (Table 2). Taken together, these data demonstrated that high CTHRC1 expression status may be a predictor for prognosis in PDAC patients.
Table 2.
Univariable and multivariable analyses of prognostic parameters for survival in Ren Ji cohort 2 patients with pancreatic ductal adenocarcinoma (PDAC)
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Prognostic parameter | HR | 95% CI | P value | HR | 95% CI | P value |
| Expression of CTHRC1 (high vs. low) | 1.712 | 1.158-2.531 | 0.007 | 1.588 | 1.059-2.381 | 0.025 |
| Age (≥ 65 vs. < 65) | 1.601 | 1.140-2.251 | 0.007 | 1.842 | 1.294-2.622 | 0.001 |
| Gender (male vs. female) | 0.823 | 0.584-1.160 | 0.266 | - | - | - |
| TNM stage (III, IV vs. I, II) | 1.725 | 1.114-2.672 | 0.015 | 1.242 | 0.696-2.216 | 0.463 |
| Tumor Size (> 3 cm vs. ≤ 3 cm) | 1.590 | 1.111-2.276 | 0.011 | 1.619 | 1.123-2.334 | 0.010 |
| T classification (T3, 4 vs. T1, 2) | 1.540 | 0.988-2.402 | 0.057 | - | - | - |
| Lymph node metastasis (present vs. absent) | 1.869 | 1.313-2.660 | 0.001 | 1.776 | 1.207-2.613 | 0.004 |
| Distant metastasis (present vs. absent) | 2.029 | 1.091-3.775 | 0.025 | 1.308 | 0.584-2.927 | 0.514 |
| Vascular invasion (present vs. absent) | 1.309 | 0.796-2.151 | 0.289 | - | - | - |
| Perineural invasion (present vs. absent) | 1.090 | 0.779-1.525 | 0.614 | - | - | - |
| Tumor location (head vs. body/tail) | 1.065 | 0.746-1.522 | 0.728 | - | - | - |
| Histology (poor vs. well/moderate) | 1.794 | 1.269-2.536 | 0.001 | 1.729 | 1.216-2.459 | 0.012 |
HR: Hazard ratio; CI: Confidence interval. The bold number represents the P-values with significant differences.
Discussion
Despite tremendous endeavors on biological markers for better diagnosis and prognosis of PDAC, the clinical outcome of this fatal malignancy remains almost stagnant [2,15]. Since CTHRC1 was originally found in balloon-injured rat arteries where it participates in vascular remodelling by limiting collagen matrix deposition and promoting cell migration [6], the biological oncogenic roles of CTHRC1 have been implicated in various types of human cancers including PDAC [8-14]. However, a comprehensive investigation remains to elucidate the clinical significance and prognostic value of CTHRC1 in PDAC specifically. Here, we attempted to illustrate them in PDAC, especially the prognostic significance.
High expression of CTHRC1 was found in a variety of cancers [8-14]. And our study further validated CTHRC1 expression at both mRNA and protein level in PDAC. The mRNA expression of CTHRC1 was obviously elevated in 40 PDAC tissues compared with paired adjacent normal tissues. Furthermore, CTHRC1 protein expression in tissue microarray containing 205 pairs of samples by immunohistochemistry displayed higher in tumor tissues than normal pancreatic tissues. Collectively, the data from GEO database and our current study confirmed that CTHRC1 expression was elevated in PDAC tissues compared with adjacent normal tissues, which was consistent with previous studies [14,16]. Therefore, it is reasonable to suggest that upregulated CTHRC1 exhibits oncogenic function. And now, it has been reported that overexpression of CTHRC1 increased the phosphorylation of Src and Erk, and vice versa, and then enhanced the tumor cells’ migration and metastatic properties in PDAC [14].
For further confirming the oncogenic role of CTHRC1 in PDAC, the relationship between CTHRC1 expression and corresponding clinicopathological parameters was determined and our results revealed that elevated CTHRC1 expression in PDAC was significantly correlated with lymph node metastasis, vascular invasion and perineural invasion. Since the clinicopathological parameters above represent partially the deterioration and development of the tumor, it offers more evidence that CTHRC1 was a factor related to PDAC progression from the clinical perspective. And similar results were also reported in non-small cell lung cancer [11], epithelial ovarian cancer [13], gastric cancer [17], breast cancer [18] and so on.
To address the prognostic value of CTHRC1 in PDAC, we performed Kaplan-Meier survival analyses in four independent PDAC patient cohorts and found that elevated expression of CTHRC1 was inversely associated with clinical overall outcomes of PDAC patients. Meanwhile, univariable and multivariable Cox regression analysis indicated that upregulated CTHRC1 expression might be an independent predictor of unfavorable overall outcome for PDAC patients. This observation was in agreement with previous findings in many other types of cancers concluding that elevated CTHRC1 promotes tumor malignancy and confers an indicator for the poor prognosis [9,11,12,17-20]. For example, in gastrointestinal stromal tumors, CTHRC1 was demonstrated to act as a prognostic factor and promote invasiveness by activating Wnt/PCP-Rho signaling [12]; In colorectal cancer, high levels of CTHRC1 could promote cancer cell invasion ability through ERK-dependent induction of MMP9 expression and predict poor clinical outcomes [10]. And to our best knowledge, this is the first report showing that CTHRC1 may be a predictor of survival in a sizable group of PDAC patients.
In summary, our study further demonstrated that upregulation of CTHRC1 is associated with poor survival in PDAC patients, indicating that CTHRC1 may serve as a valuable prognostic marker in PDAC and may represent a potential molecular target for the treatment of PDAC.
Acknowledgements
We thank all the subjects of this study for their participation. This work was supported by grants from the science and technology funds of School of Medicine, Shanghai Jiao Tong University (No. 14XJ10022) and National Basic Research Program of the National Natural Science Foundation (No. 81401931) to ZJF.
Disclosure of conflict of interest
None.
References
- 1.Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol. 2012;9:435–444. doi: 10.1038/nrgastro.2012.119. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X. Report of cancer incidence and mortality in China, 2010. Ann Transl Med. 2014;2:61. doi: 10.3978/j.issn.2305-5839.2014.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 5.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 6.Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. [DOI] [PubMed] [Google Scholar]
- 7.Bian Z, Miao Q, Zhong W, Zhang H, Wang Q, Peng Y, Chen X, Guo C, Shen L, Yang F, Xu J, Qiu D, Fang J, Friedman S, Tang R, Gershwin ME, Ma X. Treatment of cholestatic fibrosis by altering gene expression of Cthrc1: Implications for autoimmune and non-autoimmune liver disease. J Autoimmun. 2015;63:76–87. doi: 10.1016/j.jaut.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ip W, Wellman-Labadie O, Tang L, Su M, Yu R, Dutz J, Wang Y, Huang S, Zhang X, Huang C, Zhou Y. Collagen triple helix repeat containing 1 promotes melanoma cell adhesion and survival. J Cutan Med Surg. 2011;15:103–110. doi: 10.2310/7750.2011.10014. [DOI] [PubMed] [Google Scholar]
- 9.Chen YL, Wang TH, Hsu HC, Yuan RH, Jeng YM. Overexpression of CTHRC1 in hepatocellular carcinoma promotes tumor invasion and predicts poor prognosis. PLoS One. 2013;8:e70324. doi: 10.1371/journal.pone.0070324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim JT, Kim BY, Lee SJ, Choe YK, Kim DH, Kim SH, Chae SW, Kim KD, Lee HG. Collagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cells. Oncotarget. 2014;5:519–529. doi: 10.18632/oncotarget.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke Z, He W, Lai Y, Guo X, Chen S, Li S, Wang Y, Wang L. Overexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. Oncotarget. 2014;5:9410–9424. doi: 10.18632/oncotarget.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H, Zhang WM, You H, Qin W, Gu J, Yang S, Cao H, Zhang ZG. CTHRC1 acts as a prognostic factor and promotes invasiveness of gastrointestinal stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia. 2014;16:265–278. 278.e261–213. doi: 10.1016/j.neo.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y, Feng C, Chen X, Zhang Y, Lin M, Wang L, Ke Z. High expression of CTHRC1 promotes EMT of epithelial ovarian cancer (EOC) and is associated with poor prognosis. Oncotarget. 2015;6:35813–35829. doi: 10.18632/oncotarget.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park EH, Kim S, Jo JY, Kim SJ, Hwang Y, Kim JM, Song SY, Lee DK, Koh SS. Collagen triple helix repeat containing-1 promotes pancreatic cancer progression by regulating migration and adhesion of tumor cells. Carcinogenesis. 2013;34:694–702. doi: 10.1093/carcin/bgs378. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson C, Earl J, Ghaneh P, Halloran C, Carrato A, Greenhalf W, Neoptolemos J, Costello E. Biomarkers for early diagnosis of pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2015;9:305–315. doi: 10.1586/17474124.2015.965145. [DOI] [PubMed] [Google Scholar]
- 16.Tang L, Dai DL, Su M, Martinka M, Li G, Zhou Y. Aberrant expression of collagen triple helix repeat containing 1 in human solid cancers. Clin Cancer Res. 2006;12:3716–3722. doi: 10.1158/1078-0432.CCR-06-0030. [DOI] [PubMed] [Google Scholar]
- 17.Gu L, Liu L, Zhong L, Bai Y, Sui H, Wei X, Zhang W, Huang P, Gao D, Kong Y, Lou G. Cthrc1 overexpression is an independent prognostic marker in gastric cancer. Hum Pathol. 2014;45:1031–1038. doi: 10.1016/j.humpath.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Baek TH, Yim HS, Kim KH, Jeong SH, Kang HB, Oh SS, Lee HG, Kim JW, Kim KD. Collagen triple helix repeat containing-1 (CTHRC1) expression in invasive ductal carcinoma of the breast: the impact on prognosis and correlation to clinicopathologic features. Pathol Oncol Res. 2013;19:731–737. doi: 10.1007/s12253-013-9636-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee CE, Vincent-Chong VK, Ramanathan A, Kallarakkal TG, Karen-Ng LP, Ghani WM, Rahman ZA, Ismail SM, Abraham MT, Tay KK, Mustafa WM, Cheong SC, Zain RB. Collagen Triple Helix Repeat Containing-1 (CTHRC1) Expression in Oral Squamous Cell Carcinoma (OSCC): Prognostic Value and Clinico-Pathological Implications. Int J Med Sci. 2015;12:937–945. doi: 10.7150/ijms.11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan F, Liu F, Liu H, Hu Y, Liu D, Li G. CTHRC1 is associated with peritoneal carcinomatosis in colorectal cancer: a new predictor for prognosis. Med Oncol. 2013;30:473. doi: 10.1007/s12032-013-0473-3. [DOI] [PubMed] [Google Scholar]


