Abstract
Amyloidosis is caused by extracellular deposition of abnormal protein fibrils, resulting in destruction of tissue architecture and impairment of organ function. The most common forms of systemic amyloidosis are light-chain (AL) and transthyretin-related (ATTR). ATTR can result from an autosomal dominant hereditary transmission of mutated genes in the TTR (ATTRm) or from a wild-type form of disease (ATTRwt), previously known as senile cardiac amyloidosis (SCA). With the aging of the worldwide population, ATTRwt will emerge as the most common type of cardiac amyloidosis that clinicians encounter. Diagnosis of systemic amyloidosis is often delayed, either due to the false assumption that it is a rare disease, or due to misdiagnosis as a result of mistaking it with other conditions. Clinicians must integrate clinical clues from history, physical exam, and common diagnostic tests to raise suspicion for ATTRwt. The historical gold standard for diagnosis of cardiac amyloid is endomyocardial biopsy (EMB) with pathological distinction of precursor protein type, but this method often results in delayed diagnosis given the limited availability of expertise to perform and interpret the EMB. Emerging noninvasive imaging modalities provide easier, accurate screening for ATTRwt. These modalities include: advanced echocardiography, using strain imaging and the myocardial contraction fraction; nuclear scintigraphy, which can differentiate between ATTR and AL cardiac amyloid; and cardiac magnetic resonance, using extracellular volume measurement, late gadolinium enhancement, and distinct T1 mapping. These novel approaches reveal insights into the prevalence, clinical course, morphological effects, and prognosis of ATTRwt.
Introduction
Amyloidosis is caused by extracellular deposition of abnormal protein fibrils, resulting in destruction of tissue architecture and impairment of organ function1. Amyloidosis may be localized or systemic, and is categorized by the type of precursor protein2. Clinical manifestations of systemic amyloidosis depend on the site of amyloid deposition. The most common forms are light-chain (AL) and transthyretin-related (ATTR). AL amyloidosis is caused by deposition of immunoglobulin light chains resulting from an underlying plasma cell dyscrasia3,4. ATTR can result from an autosomal dominant hereditary transmission of mutated genes in the TTR protein (there are more than 100 known mutations in the gene) or as a wild-type form of disease (with a normal TTR sequence)4,5. TTR is produced by the liver and in the mutated state deposits in the peripheral nervous system, causing familial amyloid polyneuropathy (FAP), or the myocardium, causing familial amyloid cardiomyopathy (FAC).
Epidemiology
The most common TTR mutation in the United States is Val122Ile (Valine to Isoleucine substitution at position 122). TTR amyloid predominantly affects individuals of African American descent; Val122lle mutation prevalence has been reported in 3.43% of African Americans under age 656. Wild-type TTR (ATTRwt), previously known as Senile Cardiac Amyloidosis (SCA), is caused by deposition of amyloid fibrils formed from misfolded TTR proteins, and has been described almost exclusively in older adult males1-3. While AL Amyloid is a relatively rare disorder, with approximately 3,000 new cases in North America annually4, ATTRwt is rapidly increasing in recognition. Wild-type TTR deposits are already found in >25% of people over age 80 at autopsy, and its percentage of clinical representation at amyloid centers is rapidly accelerating7. Additionally, with the aging of the worldwide population, ATTRwt will likely become the most common type of cardiac amyloidosis (CA) encountered by clinicians.
Impediments to Diagnosis
Despite its clinical importance, the diagnosis of cardiac amyloidosis can be delayed or missed altogether. Several factors may be responsible. First, there is the assumption that amyloid is a rare condition. Indeed, AL amyloid is rare; only 30-50% of patients with AL amyloid have cardiac involvement4 and there are an estimated 10,000 patients with AL in United States. ATTRwt, however, is not rare, and universally involves the heart. Second, there still exists an erroneous belief that cardiac amyloid is untreatable and that current management has no impact on outcomes. However, accurate diagnosis is critical for guiding amyloid-directed therapies as well as management of amyloid sequelae, especially heart failure and arrhythmias. Third, cardiac amyloidosis can be clinically subtle. Extra-cardiac manifestations such as peripheral neuropathy may predominate and become the clinical focus1. Diagnosis of ATTRwt-CA can also be confounded by the high prevalence of monoclonal proteins that occur with advancing age; up to 20% of elderly patients with TTR cardiac amyloidosis have concomitant monoclonal gammopathy of undetermined significance1. Symptoms of ATTRwt-CA are relatively non-specific, and are often attributed to the syndrome of heart failure. Indeed, recent investigations have shown that a significant percentage of patients with HFPEF have ATTRwt cardiac amyloidosis8,9. In fact, many of the clinical disorders with which ATTRwt-CA is associated – including HFPEF, aortic stenosis, stroke, atrial fibrillation, syncope, and angina – are especially common in the elderly, and the underlying cause is often not defined. Fourth, the phenotypic heterogeneity of amyloidosis often causes protean, non-specific symptoms (e.g. fatigue) or extra-cardiac manifestations that prompt care with a non-cardiac specialist. Finally, the necessity of target organ tissue histological diagnosis with confirmation of amyloid type is often associated with delays in diagnosis, because of the expertise required for both EMB and precursor protein confirmation by mass spectroscopy.
Clinical Clues to Diagnosis of ATTRwt
Cardiac amyloidosis is frequently misdiagnosed as hypertensive heart disease and hypertrophic cardiomyopathy (HCM), since their clinical and imaging feature can overlap10. A clinician whose differential diagnosis includes CA may discern findings that increase its likelihood. These include HFPEF in the absence of an elevated blood pressure, recent-onset hypotension (e.g. in a formerly hypertensive patient), intolerance of commonly-used cardiovascular medications such as ACE inhibitors or beta blockers, or a history of bilateral carpal tunnel syndrome. While dyspnea and lower extremity edema are common findings in heart failure, CA is often characterized by a predominance of findings related to right heart failure such as of hepatomegaly, ascites, abdominal boating/satiety and edema. Pulmonary edema, however, is quite rare10. CA patients may also have positive troponins, frequently without typical angina, prompting diagnostic evaluation for acute coronary syndrome, yet without obstructive lesions on coronary angiography. Angina, if present, may also be due to amyloid infiltration of small intramyocardial vessels or myocardiotoxicity from the amyloid deposits11.
Prognosis, Treatment, and the Need for Better Diagnostics
ATTRwt is not a benign diagnosis. A prospective cohort study found the median survival of ATTRwt after diagnosis to be 43 months, with a decline in 6-minute walk test and ejection fraction (EF) over the first 18 months12. A recent prospective study found a similar median survival from time of biopsy of 46.7 months with a 5-year survival was 35.7%. Notably, 78% of the deaths were attributed to cardiac causes13. Fortunately, potential treatments for ATTRwt are in late phase clinical trials. Pharmacologic therapies include TTR stabilizers such as tafamidis and diflunisal, which have been shown to be effective for FAP14,15; RNA silencers and monoclonal antibodies are also being tested16. Regarding heart failure therapy, it is important to recognize that non-dihydropyridine calcium channel blockers are contra-indicated in patients with cardiac amyloidosis, as they cause significant negative inotropy and high degree heart block. Additionally, there is potential increased risk of digoxin toxicity, due to digitalis’ binding of amyloid fibrils17. Diuresis is the mainstay of clinical management, while ACE inhibition and nitrates carry a greater risk of symptomatic hypotension. High dose beta blockade is also often poorly tolerated, due to fixed stroke volume and reliance on an increased heart rate to maintain cardiac output18.
Yet, there is much still to learn about the actual prevalence and clinical course of the disease. As therapies emerge, imaging modalities may have a role not just in diagnostics, but also in monitoring disease progression or response. Newly developed non-invasive imaging methods have already provided novel insights into amyloid and associated conditions, such as heart failure with preserved ejection fraction (HFPEF), atrial fibrillation, and aortic stenosis with paradoxical low flow. This review will focus on the evaluation of ATTRwt cardiac amyloid and the novel insights that have recently been garnered from a growing array of imaging modalities.
Classical Diagnostic Tests
Electrocardiography
Common diagnostic tools can assist in the detection of cardiac amyloidosis. Typical ECG changes reflect the displacement of myocardium with amyloid deposits, affecting the conduction system3. The classically reported ECG finding ascribed to CA is low QRS voltage (amplitude of ≤ 0.5mV in limb leads or ≤ 1.0 mV in precordial leads), especially in disproportion to a hypertrophic left ventricle (LV) seen on echocardiogram3,5. However, in ATTRwt, the sensitivity of low voltage in isolation is poor (< 30%), as the fibrils are less toxic to the myocardium than in AL, and up to 15% of subjects may even have electrocardiographic evidence of LVH5,17,19,20. The lack of sensitivity of the electrocardiogram is especially true among older adults with the Val122Ile mutation, which almost exclusively affects African-Americans, in whom the presence of increased QRS amplitude (independent of hypertension) can potentially confound detection of amyloid-related voltage decrements20. Irrespective of race, hypertension-related LVH can decrease sensitivity of low voltage as a diagnostic criterion for CA5, as can left bundle branch block conduction abnormalities17. Diagnostic approaches that incorporate both ECG voltage with echocardiographically-determined wall thickness in order to calculate voltage-to-mass ratios have been shown to be sensitive and specific for diagnosing CA than voltage criteria alone21. Another classic sign on ECG is a pseudo-infarct pattern, either poor R-wave progression or QS waves in the precordial leads, yet these are reported more frequently in AL than ATTR12,19.
Endomyocardial Biopsy
The current reference standard for diagnosis of cardiac amyloid involves EMB. This approach can be challenging, especially in context of large scale population-based screening. EMB is a procedure often restricted to centers with significant expertise in its performance, typically large referral centers with transplant programs1. While rare, procedural complications can include arrhythmia, perforation with pericardial tamponade, and pneumothorax22. In addition to technical expertise required for cardiac biopsy, pathologic expertise is required for accurate identification of amyloid and appropriate subtyping23. Histological staining using amyloid-specific stains (Congo red) reveals apple-green birefringence under polarized light. Pathologic verification of the amyloid precursor protein by immunohistochemistry and/or sequence analysis by mass spectroscopy should be performed for further diagnostic confirmation1,3,5. In ATTR, extra-cardiac biopsies can have low sensitivity, thereby emphasizing the need for accurate non-invasive screening tool1. The aforementioned limitations of ECG, EMB, or extra-cardiac biopsies to diagnose ATTRwt yields a need for accurate and practical diagnostic imaging techniques.
Insights from Novel Imaging for ATTRwt-CA
Non-invasive imaging is widely used for diagnosis and serial evaluation of patients with known or suspected cardiac amyloid. Traditional imaging methods have predicated diagnosis on cardiac morphology. Newly developed techniques have enabled more sensitive assessment of myocardial function, as well as quantitative evaluation of myocardial tissue characteristics that can be altered in the context of amyloid deposition. The following sections will provide an overview of advances in echocardiography, nuclear scintigraphy, and cardiac magnetic resonance as relevant to diagnosis and management of ATTRwt-CA.
Echocardiography
Morphology and Function
Many previously described characteristics of ATTRwt-CA can be demonstrated by conventional 2D echocardiography. These findings include left and right ventricular (LV, RV) wall thickening, normal to small LV cavity – initially with preserved ejection fraction, bi-atrial enlargement, and a small pericardial effusion. ATTR-CA is characterized by progressively worsening diastolic dysfunction, ultimately to a restrictive pattern, with shorter deceleration time (<150ms) and high early (E-wave) and relatively low atrial (A-wave) velocities (E/A waves >2). Severity of diastolic dysfunctional has been reported to be proportional to magnitude of cardiac amyloid deposition18. A non-specific but frequently mentioned finding of ATTRwt-CA is a granular, speckled appearance of the myocardium. Speckling, however, has been shown to be dependent on data acquisition parameters (e.g. gain settings) and may not be apparent using relatively recent echocardiography machines3,12,17,24.
Wall Thickness
Another basic echocardiography hallmark of ATTRwt-CA is increased wall thickness. Diagnostic suspicion should be increased when wall thickness is increased in the absence of a stimulus such as hypertension, predisposing hemodynamic factors (e.g. aortic stenosis), or genetic conditions (e.g. hypertrophic cardiomyopathy, Fabry's disease)1,3,5,6,12. While morphologic features of AL and ATTRwt can overlap, prior studies have reported patients with ATTRwt to manifest greater LV wall thickness than those with AL2,25,26,27 – mean wall thickness of 17mm±2 versus 15mm±2 in a recent study27. Irrespective of diagnostic subtype, increased wall thickness in patients with amyloid is due to protein (AL or TTR) deposition rather than actual myocyte hypertrophy, thereby explaining concomitant “low voltage” on ECG1,5.
Strain and Strain Rate
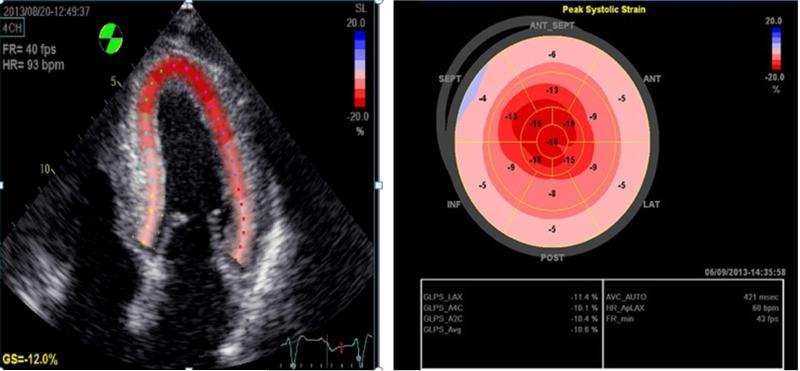
Advanced echocardiographic techniques reveal more sophisticated characteristics of ATTRwt-CA. Echocardiographic strain imaging, for example, provides a means of quantifying regional myocardial deformation. Longitudinal strain (LS) can be analyzed using tissue-Doppler imaging (TDI) and 2D speckle-tracking. Furthermore, 3D speckle-tracking can be used to differentiate between ATTRwt-CA and other hypertrophic diseases by potentially yielding improved assessment of global radial, circumferential, and longitudinal strain1,3,5,6,15,25,28. Radial and longitudinal strain have each been shown to be reduced in ATTRwt-CA as well as other conditions, although the pattern of strain decrement can be used to identify CA. A novel insight from strain imaging is that patients with ATTRwt-CA typically demonstrate marked decrease in LS in basal and mid-wall areas with relative apical sparing, referred to as “cherry on top” or apical preservation17 (Figure 1). Conversely, patients with HCM or aortic stenosis related LVH typically demonstrate reduced LS in regions of maximal hypertrophy5,6,17,25,29. Generally, abnormal LS portends a worse survival. Curiously, survival in ATTRwt is better than in AL despite similarly abnormal LS, likely due to the greater toxicity of AL amyloid17,27.
Figure 1. “Apical Sparing” Strain Pattern of ATTR Cardiac Amyloid on Echocardiogram.
Patients with CA typically demonstrate marked decrease in longitudinal strain in basal and mid-wall areas with relative apical sparing, referred to as “cherry on top” or apical preservation.
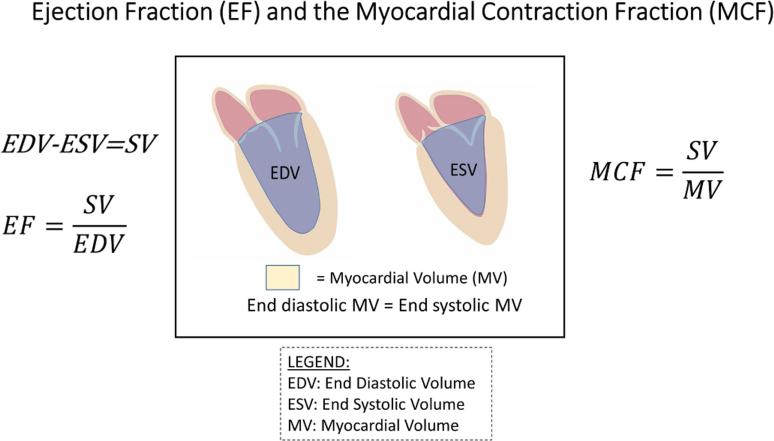
Myocardial Contraction Fraction
Conventional measures of left ventricular function, notably the ejection fraction, are often initially normal/near-normal in CA. However, actual physiologic performance of the LV is not intact, due to amyloid-associated changes in ventricular remodeling (e.g. chamber size and wall thickness), reduced chamber capacitance and decreased chamber contractility. A novel metric of myocardial contraction, the myocardial contraction fraction (MCF), which is the ratio of stroke volume to myocardial volume, is frequently abnormal in ATTR-CA patients (Figure 2). Normal or “preserved” EF in ATTR-CA is due to a similar percentage decrease in both stroke volume and LV end-diastolic volume (LVEDV), which occurs as the ventricular wall thickens with concomitant decline in ventricular capacitance and upward shifts in the end diastolic pressure volume relation.29 The MCF, while analogous to the ejection fraction in terms of being unitless and free of the need for indexation for body size, offers several distinct theoretical advantages. First, capitalizing on the fundamental principle that the myocardium is incompressible from end diastole to end systole, and by indexing the stroke volume to the myocardial volume, the MCF is an index of the volumetric shortening of the myocardium that is independent of chamber size and geometry. By eliminating chamber volume from the assessment of shortening, the MCF expresses the strain relationship only in terms of the heart structure which shortens, namely the myocardium. As a measure of myocardial shortening, the stroke volume is therefore most appropriately assessed relative to the myocardium (specifically to myocardial volume). The MCF, though operationalized prior to the advent and widespread measurement of strain using echocardiography, is highly correlated with global longitudinal strain30. The novel insight from MCF is that it is a better marker of pathology in ATTRwt cardiac amyloid than EF. In a recent study, MCF <30 in CA patients was associated with increased risk of death, while EF did not correlate31. Emerging data also suggest that MCF has superior diagnostic ability for ATTR-CA, i.e. correlates better with 99mTc-PYP positivity than ECG voltage-to-mass ratio1,32.
Figure 2. Myocardial Contraction Fraction.
The myocardial contraction fraction (MCF) is based on the principle that the myocardium is nearly incompressible and does not change volume significantly from end-diastole to end-systole. By indexing the stroke volume to the myocardial volume, the MCF is an index of the volumetric shortening of the myocardium that is independent of chamber size and geometry. The MCF, while analogous to EF in terms of being unitless and free of the need for indexation for body size, offers several theoretical advantages including expressing the strain relationship only in terms of that which shortens, namely the myocardium, thereby providing an ability to distinguish pathologic from physiologic hypertrophy.
Nuclear Scintigraphy
Several nuclear scintigraphy tracers are currently being evaluated for detection of cardiac amyloid. These include bone imaging agents such as (99m) Tc-3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD) and (99m)Tc-pyrophosphate (99mTc-PYP).
The history of nuclear scintigraphy to detect CA originates when Kula et al. (1977) visualized calcifications in amyloid deposits with 99mTc –diphosphanate23. In 1982, there was a report of two patients without acute myocardial infarction who demonstrated 99mTc-PYP myocardial uptake and confirmed cardiac amyloidosis, one diagnosed by EMB and the other at autopsy32. Yet, in the 1980s, sensitivity for detecting CA was determined to be poor, likely due to inability to differentiate AL and TTR cardiac amyloid, lack of a quantitative scoring system for measuring myocardial tracer retention, and uncommon usage of advanced imaging at that time17,23.
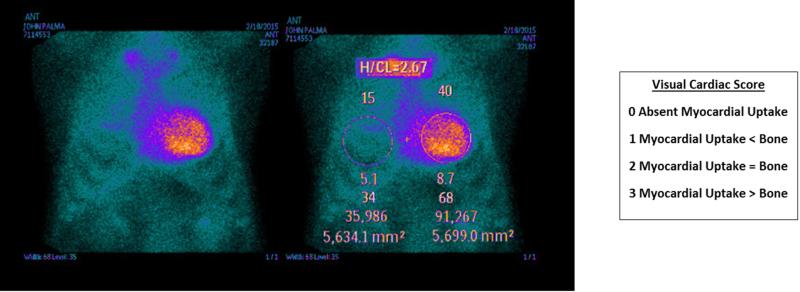
The two commonly used nuclear isotopes, 99mTc-DPD and 99mTc-PYP, both localize to cardiac TTR deposits. While 99mTc-DPD has been more widely studied, it is not available in the United States and in Canada. 99mTc-PYP, however, is FDA-approved for use in both planar and Single Photon Emission Computed Tomography (SPECT) imaging. It is speculated that both agents are taken up by TTR-infiltrated myocardium via a calcium-mediated mechanism1,3,5. While several studies show that 99mTc-DPD and 99mTc-PYP show only ATTR uptake and thus can differentiate from AL33, there is some evidence that both can be taken up as well in patients with AL, albeit with less intense myocardial uptake. The reason for increased uptake in ATTR versus AL is unknown, but has been hypothesized to be due to higher amount of calcium, or longer duration of amyloid deposition, as ATTR-CA is more indolent and less myocardiotoxic than AL-CA. Bokhari et al. (2013) demonstrated that a 99mTc-PYP visual score of 2 or greater and a heart-to-contralateral ratio (HCL), a quantitative visual score measured one hour after injection, of greater than 1.5 can differentiate between ATTR and AL with sensitivity and specificity near 100%34 (Figure 3). Nuclear scintigraphy results can also have prognostic value. Rapezzi et al. (2011) showed that an increased 99mTc-DPD heart-to-whole body ratio, another index of CA uptake, predicts poorer outcomes35. Similarly, increased uptake of 99mTc-PYP has been shown to correlate with disease severity such as wall thickness and LV mass35. High 99mTc-PYP uptake, though, as measured by the H/CL ratio, was recently shown to remain constant over time despite clinical progression of CA, which may reflect a threshold of amyloid burden beyond which the clinical course has been triggered, despite slowing of amyloid deposition36.
Figure 3. Semiquantitative and Quantitative analysis of 99mTc-PYP Myocardial Uptake.
Semiquantitative visual cardiac score was assigned 0-3 according to the scale detailed (A). The representative image demonstrates a visual cardiac score of 3. Quantitative heart-to-contralateral (H/CL) ratio was calculated by drawing a region of interest (ROI) over the heart, copying and mirroring it to the contralateral chest, and calculating the ratio of heart ROI mean counts to contralateral ROI mean counts (B). The representative image demonstrates H/CL ratio 40/15=2.67.
Nuclear scintigraphy may ultimately obviate the need for biopsy to diagnose ATTR-CA and as has already yielded several new insights. First, prevalence of undiagnosed or misdiagnosed ATTR-CA is much higher than previously recognized. For example, among a cohort of 120 HPEF patients (≥ 60 years old) with LV hypertrophy (wall thickness ≥ 12mm), 13.3% showed a moderate-to-severe 99mTc-DPD uptake15. Additionally, preliminary data reveal that 16% of 75 patients with severe aortic stenosis, mostly men, had strong 99mTc-PYP uptake37. Discovering cardiac amyloid as the underlying etiology directs clinical focus on preventing or treating amyloid deposition rather than just the apparent cardiac disorders. Finally, the composition of precursor protein in CA (ATTR versus AL) has been shown to be more prognostic than the change in morphologic strain or myocardial function27.
Cardiac Magnetic Resonance (CMR)
Cardiac magnetic resonance (CMR) is widely accepted as a non-invasive reference standard for cardiac function and structure. CMR entails no radiation exposure and provides excellent image quality independent of patient body habitus, making this modality well suited for cross-sectional study of patients with known or suspected amyloid. CMR has been shown to be more reproducible than echocardiography38, enabling detection of subtle impairments in cardiac contractility. Prior validation studies have shown CMR to yield near exact agreement with ex-vivo chamber volumes as well as necropsy-quantified myocardial mass39,40. Importantly, the above indices can be quantified using non-contrast cine-CMR, which can be used to provide accurate quantification of amyloid-associated morphological changes (such as LVH, decreased chamber size, and atrial dilation) even in patients with contraindications to contrast use such as advanced renal insufficiency. Beyond cardiac function and remodeling, CMR can directly assess myocardial tissue characteristics. Post-contrast tissue characterization methods – including inversion recovery imaging for regional late gadolinium enhancement (LGE) and T1 mapping for extracellular volumes fractionation – have been used to diagnose amyloid and correspond to adverse clinical outcomes41,42,43. Thus, within a single exam, CMR can evaluate distinct facets of amyloid-related cardiomyopathy: myocardial structure, function, and tissue characteristics.
Late Gadolinium Enhancement
Post-contrast tissue characterization via LGE is predicated on increased gadolinium deposition (with consequent T1 shortening) in regions of increased myocardial extracellular volume. Multidisciplinary studies have shown patients with CA to demonstrate increased interstitial expansion on biopsy due to amyloid protein deposition, corresponding to LGE on CMR44. Novel insights from LGE on CMR include that amyloid-associated patterns of LGE can vary: subendocardial, mid-myocardial, or transmural in location, but typically occurs in a non-coronary arterial distribution. Diffuse subendocardial LGE, however, has been reported to be more common in the context of AL amyloid, although overlap can occur (Figure 4A). For example, among a cohort of 250 amyloid patients, subendocardial LGE was more prevalent in AL (39% in AL versus 24% in TTR, p<0.05) whereas transmural LGE was more common in TTR (63% versus 27% in AL, p<0.001)45. Similar results have been reported in retrospective datasets, which have reported LGE to be more extensive in patients with TTR (versus AL) amyloid46. Importantly, pattern of LGE can sometimes be challenging to differentiate from other infiltrative processes or from myocarditis. In this context, ancillary morphologic findings such as LVH can be of utility. Moreover, as amyloid-directed treatment varies based on subtype, CMR may be of greatest utility as an initial screening test, on which presence of LGE may prompt further testing (via biopsy or adjuvant testing) to establish amyloid sub-type (AL versus TTR) and guide tailored therapies.
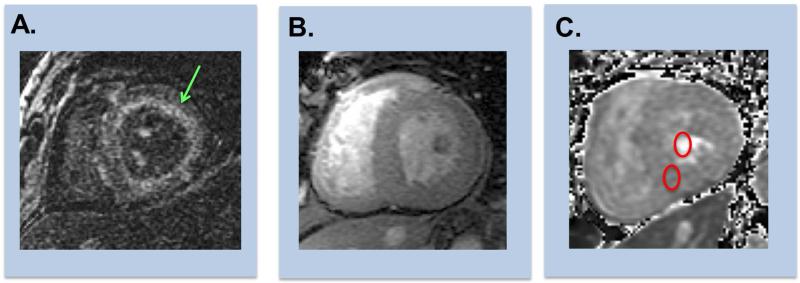
Figure 4. Representative Examples of Cardiac Amyloid on CMR.
Representative examples of CMR evidenced enhancement patterns among patients with cardiac amyloid.
(A) Diffuse subendocardial enhancement (green arrow) on DE-CMR (inversion time [TI] 300msec).
(B) Diffuse transmural enhancement on DE-CMR (left [equivalent TI]).
(C) Corresponding T1 map enables quantification of extracellular volume fraction, which can be can be calculated via measurement of T1 in myocardium and blood pool (red circles) on matched pre- and post-contrast images.
Beyond diagnosis, another novel insight from CMR is that LGE has been shown to stratify clinical prognosis among patients with known or suspected amyloid. Several LGE pulse sequences have been used for this purpose. Among a cohort of 154 patients (64 hypertensive, 90 suspected amyloid), White et al. (2014) demonstrated that diffuse LGE, assessed via an inversion time [TI] scout method for which TIs were sequentially increased to assess myocardial T1 shortening versus blood pool (Figure 4B,C), independently predicted mortality (hazard ratio: 6.0, 95% CI 3.0-12.1; p < 0.0001)42. Among a mixed cohort of 250 patients with AL and TTR amyloid, Fontana et al. (2015) reported that transmural LGE (on phase sensitive inversion recovery imaging) predicted subsequent mortality (hazard ratio, 5.4; 95% CI 2.1-13.7; p<0.0001) and remained an independent predictor of death after adjustment for N-terminal pro-brain natriuretic peptide, EF, stroke volume index, E/E′, and LV mass index (hazard ratio, 4.1; 95% CI, 1.3-13.1; P<0.05). Of note, 15% of patients in this amyloid cohort had negative LGE; patients with negative LGE had lower LV mass, lesser diastolic dysfunction, and less advanced ECG and biomarker (BNP) abnormalities.46 In this context, it is possible that patients with biopsy-proven cardiac amyloid but negative LGE have less advanced disease – corresponding to improved outcomes – irrespective of biopsy results. Further studies are warranted to investigate.
T1 mapping
T1 mapping represents a new CMR approach that enables quantification of diffuse alterations in myocardial longitudinal relaxation (T1). Whereas amyloid itself produces increased T1 on pre-contrast imaging, post-contrast T1 is shortened due to amyloid-associated increments in extracellular volume of distribution (within which gadolinium can accumulate). A novel insight derived from T1 on CMR is that pre- and post-contrast T1 mapping data (obtained from regions of interest in the LV blood pool and myocardium) can be used to calculate myocardial extracellular volume (ECV) fraction, which holds the potential to provide a quantitative index of amyloid disease burden. Additionally, non-contrast T1 mapping has been shown to differentiate between patients with and without amyloid, as well as between patients with LVH of differing etiologies. Among a mixed cohort of patients with AL-CA, ATTRCA, hypertrophic cardiomyopathy (HCM), and normal controls, Fontana et al. (2014) reported that native T1 was higher in ATTR-CA than HCM and controls (1,097 ± 43 ms versus 1,026 ± 64 ms versus 967 ± 34 ms, respectively; both p < 0.0001), but lower than that in AL-CA (AL 1,130 ± 68 ms; p = 0.01). 47
Another insight into the diagnosis and understanding of ATTR-CA arises from the revelation that ECV may even be elevated in patients with biopsy proven amyloid but negative LGE. For example, among a cohort of 36 patients with biopsy proven systemic amyloid (30 AL, 6 TTR) and 30 normative controls, Barison et al. (2015) reported ECV to be higher in amyloid patients versus controls (0.43 ± 0.12 versus 0.26 ± 0.04, P < 0.05), and notably even higher among amyloid patients without LGE (0.35 ± 0.10) as compared to controls (P < 0.01)48. Other studies have shown ECV to be useful for stratifying between amyloid subtypes, as evidenced by slightly higher ECV among patients with TTR versus AL amyloid (p=0.008)49. Ongoing research is aimed at testing prognostic utility of T1 mapping (inclusive of native T1 and calculated ECV), as well as relative utility of different CMR approaches for predicting therapeutic response among patients with AL and TTR amyloid50.
Diagnosis Using Advanced Imaging
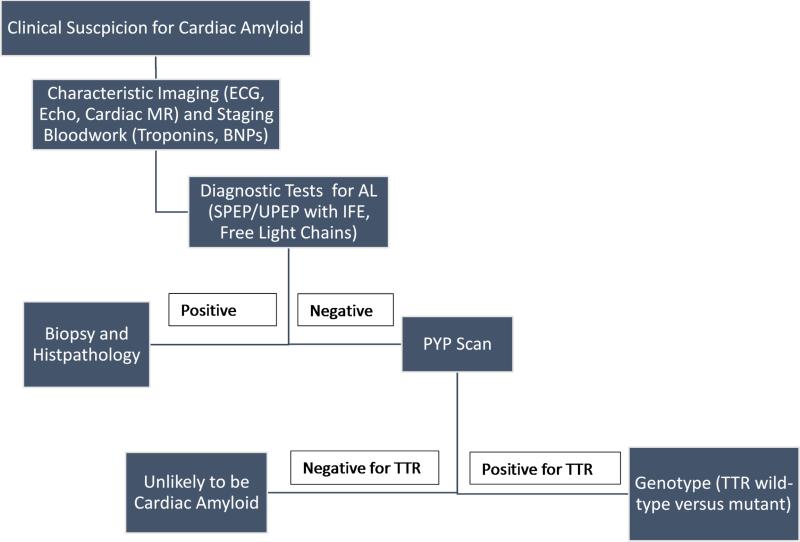
These novel imaging approaches can be incorporated into the diagnostic approach to a patient with cardiac amyloid. Once patient history and exam – including the clues described earlier – elicit enough clinical suspicion for CA, data from ECG and echocardiogram may support this diagnosis. CMR can also be used to confirm morphologic or functional changes typical of cardiac amyloid. For these patients, ruling out AL amyloid is imperative to avoid delaying vital therapies, and laboratory testing should include serum and urine protein electrophoresis with immunofixation as well as kappa and lambda free light chains. Natriuretic peptides and troponins could be used for staging purposes as well. If the immunologic bloodwork shows the presence of a monoclonal protein, these patients warrant histopathology confirmation of the diagnosis to exclude AL amyloid, although even in these patients a highly-positive scintigraphy scan may indicate ATTR-CA with concomitant monoclonal gammopathy, which is common with advanced age. If there is no evidence of a monoclonal protein by serum or urine protein electrophoresis with immunofixation and kappa/lambda free light chains, high myocardial retention of the tracer on nuclear scintigraphy51 is indicative of ATTR (and should be clarified into wild type versus mutant by genotyping), while low uptake is unlikely to represent cardiac amyloid, although with enough clinical suspicion an endomyocardial biopsy could still be justified52. (Figure 5)
Figure 5.
Diagnosis Algorithm for ATTRwt Cardiac Amyloid using PYP scanning as an alternative to EMB
Conclusion
Improved diagnostic screening for TTR cardiac amyloidosis is necessary, as the prevalence of ATTR-CA from wild type disease is significantly greater than previously known, and will continue to increase as the population ages. Classical methods of diagnosis that rely upon symptomatology, physical exam findings, ECG, and basic echocardiography, are either insensitive or nonspecific to confirm the diagnosis of ATTRwt-CA. The diagnostic gold standard, endomyocardial biopsy, is frequently considered too invasive or resource-intensive to adequately keep pace with the diagnostic need. Novel imaging modalities, though, have been shown to aid in early, non-invasive, and specific detection of ATTR-CA once the diagnosis is suspected. For example, advanced echocardiography strain patterns, which show characteristic apical sparing and a reduction in the ratio of stroke volume to myocardial volume (i.e. the MCF), are strongly suggestive of ATTRwt-CA. Additionally, nuclear scintigraphy is excellent at detecting ATTRwt-CA and differentiating it from AL via qualitative and quantitative scoring systems. Finally, CMR tissue characterization methods such as LGE and T1 mapping data can help to differentiate amyloid from other hypertrophic cardiac disease and stratify prognosis. These novel imaging modalities offer improved mechanisms for accurate diagnosis of ATTRwt-CA, enable a greater understanding of the disease and its progression, and can even aid in prognosis.
Table 1.
Comparison of non-invasive imaging modalities for Cardiac Amyloidosis
| Modality | Advantages | Limitations | Novel Insights |
|---|---|---|---|
| Echocardiography | - Widely available and relatively inexpensive - Can provide insight into morphological and functional cardiac changes - Emerging role for strain imaging for diagnosis and prognosis |
- Best for assessing advanced LV remodeling (although diastolic dysfunction may suggest early changes) - Limited by patient-specific factors (e.g. windows, image artifacts) - Morphologic and functional changes non-specific (amyloid abnormalities may overlap with other cardiomyopathies, cannot differentiate ATTR from AL) - Strain imaging not widely clinically available |
- LV Wall thickness of ATTR > AL, usually - Apical sparing very suggestive of cardiac amyloid - Strain imaging may be prognostic - Myocardial contraction fraction (MCF) may be useful for identification and is prognostic for ATTR |
| Nuclear (99mTc-PYP, 99mTc-DPD) | - Highly sensitive for TTR amyloid due to either mutation or wild type - Can differentiate ATTR from AL - 99mTc-PYP available for use in US and Canada |
- Negative result does not exclude AL cardiac amyloid - Minimal radiation exposure (equivalent to mammogram) - 99mTc-DPD not FDA approved |
- Prevalence of TTR cardiac amyloid is higher than previously recognized in conditions such as HFPEF and aortic stenosis - Extent of myocardial retention correlates with poorer prognosis - TTR amyloid deposits accumulate slowly - Clinical disease progression occurs despite lack of changes with imaging |
| CMR | - High spatial and temporal resolution - No radiation exposure, reproducible (excellent for serial assessment of function, morphology) - Cine-CMR and native T1 mapping do not require contrast - Tissue characterization may enable disease detection prior to advanced changes in LV morphology |
- Contra-indicated in patients with ferromagnetic implants (e.g. pacemaker; frequently among CA patients with conduction disease) - Gadolinium contraindicated in patients with advanced renal insufficiency - Patients with claustrophobia or advanced HF may not tolerate CMR - LGE patterns non-specific; may require biopsy to establish diagnosis and distinguish amyloid subtypes (TTR, AL) |
- LGE and ECV based tissue characterization provide incremental diagnostic utility beyond standard morphological indices (e.g. LVH, EF) - LGE pattern and ECV elevation magnitude may help to distinguish between amyloid subtypes (TTR and AL) - LGE and ECV stratifies prognosis (mortality) among amyloid patients independent of LV morphology, functional, and biomarker indices |
Brief summary.
Wild-type transthyretin cardiac amyloid (ATTRwt), previously known as senile cardiac amyloidosis (SCA), is an under-recognized disease. Diagnosis of systemic amyloidosis is often delayed due to misdiagnosis or logistical difficulties related to the need for invasive methods. Emerging non-invasive imaging techniques can facilitate early and specific identification of ATTRwt. Advanced echocardiography, nuclear scintigraphy, and cardiac magnetic resonance have yielded important insights concerning prevalence, clinical course, and prognosis of ATTRwt.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rapezzi C, Lorenzini M, Longhi S, et al. Cardiac amyloidosis: the great pretender. Heart Fail Rev. 2015;20:117–124. doi: 10.1007/s10741-015-9480-0. [DOI] [PubMed] [Google Scholar]
- 2.Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 3.Bejar D, Colombo PC, Latif F, Yuzefpolskaya M. Infiltrative Cardiomyopathies. Clinical Medicine Insights: Cardiology. 2015;9(S2):29–38. doi: 10.4137/CMC.S19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falk RH, Quarta CC, Dorbala S. How to image cardiac amyloidosis. Circ Cardiovasc Imaging. 2014;7(3):552–62. doi: 10.1161/CIRCIMAGING.113.001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohty D, Damy T, Cosnay P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106:528–540. doi: 10.1016/j.acvd.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22(3):171–4. doi: 10.3109/13506129.2015.1051219. [DOI] [PubMed] [Google Scholar]
- 7.Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in cardiac amyloidosis: a review. J Am Heart Assoc. 2012;1:e000364. doi: 10.1161/JAHA.111.000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–94. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 9.Mohammed SF, Mirzoyev SA, Edwards WD, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aljaroudi WA, Desai MY, Tang WH, et al. Role of imaging in the diagnosis and management of patients with cardiac amyloidosis: state of the art review and focus on emerging nuclear techniques. J Nucl Cardiol. 2014;21:271–283. doi: 10.1007/s12350-013-9800-5. [DOI] [PubMed] [Google Scholar]
- 11.Di Bella G, Pizzino F, Minutoli F, et al. The mosaic of the cardiac amyloidosis diagnosis: role of imaging in subtypes and stages of the disease. Eur Heart J Cardiovasc Imaging. 2014;15:1307–1315. doi: 10.1093/ehjci/jeu158. [DOI] [PubMed] [Google Scholar]
- 12.Ruberg FL, Maurer M, Judge D, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;64:222–228. doi: 10.1016/j.ahj.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Connors LH, Sam F, Skinner M, et al. Heart Failure Resulting From Age-Related Cardiac Amyloid Disease Associated With Wild-Type Transthyretin: A Prospective, Observational Cohort Study. Circulation. 2016;133(3):282–90. doi: 10.1161/CIRCULATIONAHA.115.018852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelho T, Maia LF, Martins da Silva A, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79:785–792. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berk JL, Suhr OB, Obici L, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310:2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castano A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20:163–178. doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–1288. doi: 10.1161/01.cir.63.6.1285. [DOI] [PubMed] [Google Scholar]
- 18.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 19.Cyrille NB, Goldsmith J, Alvarez J, Maurer MS. Prevalence and prognostic significance of low QRS voltage among the three main types of cardiac amyloidosis. Am J Cardiol. 2014;114:1089–1093. doi: 10.1016/j.amjcard.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Okin PM, Wright JT, Nieminen MS, et al. Ethnic differences in electrocardiographic criteria for left ventricular hypertrophy: The LIFE Study. Am J Hypertens. 2002;15:663–671. doi: 10.1016/s0895-7061(02)02945-x. [DOI] [PubMed] [Google Scholar]
- 21.Quarta CC, Perlini S, Longhi S, et al. A simple voltage/mass index improves diagnosis of cardiac amyloidosis: an electrocardiographic and echocardiographic study of 570 patients with left ventricular hypertrophy. J Am Coll Cardiol. 2012;59(13s1):E1586–E1586. Abst. [Google Scholar]
- 22.Bokhari S, Shahzad R, Castaño A, Maurer MS. Nuclear imaging modalities for cardiac amyloidosis. J Nucl Cardiol. 2014;21(1):175–84. doi: 10.1007/s12350-013-9803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linke RP. On typing amyloidosis using immunohistochemistry: detailed illustrations, review and a note on mass spectrometry. Prog Histochem Cytochem. 2012;47:61–132. doi: 10.1016/j.proghi.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Baccouche H, Maunz M, Beck T, et al. Differentiating cardiac amyloidosis and hypertrophic cardiomyopathy by use of three-dimensional speckle tracking echocardiography. Echocardiography. 2012;29:668–677. doi: 10.1111/j.1540-8175.2012.01680.x. [DOI] [PubMed] [Google Scholar]
- 25.Cappelli F, Baldasseroni S, Bergesio F, et al. Echocardiographic and biohumoral characteristics in patients with AL and TTR amyloidosis at diagnosis. Clin Cardiol. 2015;38(2):69–75. doi: 10.1002/clc.22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quarta CC, Solomon SD, Uraizee I, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129(18):1840–1849. doi: 10.1161/CIRCULATIONAHA.113.006242. [DOI] [PubMed] [Google Scholar]
- 27.Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain–associated (AL) amyloidosis. Arch Intern Med. 2005;165:1425–1429. doi: 10.1001/archinte.165.12.1425. [DOI] [PubMed] [Google Scholar]
- 28.Engvall C, Henein M, Holmgren A, Suhr OB, Mörner S, Lindqvist P. Can myocardial strain differentiate hypertrophic from infiltrative etiology of a thickened septum? Echocardiography. 2011;28(4):408–15. doi: 10.1111/j.1540-8175.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 29.Bhuiyan T, Helmke S, Patel AR, et al. Pressure-volume relationships in patients with transthyretin (ATTR) cardiac amyloidosis secondary to V122I mutations and wild-type transthyretin: Transthyretin Cardiac Amyloid Study (TRACS). Circ Heart Fail. 2011;4:121–128. doi: 10.1161/CIRCHEARTFAILURE.109.910455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milani P, Dispenzieri A, Gertz MA, et al. 1774 In Patients with Light-Chain (AL) Amyloidosis Myocardial Contraction Fraction (MCF) Is a Simple, but Powerful Prognostic Measure That Can be Calculated from a Standard Echocardiogram (ECHO). American Society of Hematology. 2015 Abst. [Google Scholar]
- 31.Tendler A, Helmke S, Teruya S, Alvarez J, Maurer MS. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. 2014;16:1–6. doi: 10.3109/13506129.2014.994202. [DOI] [PubMed] [Google Scholar]
- 32.Sobol SM, Brown JM, Bunker SR, Patel J, Luli RJ. Noninvasive diagnosis of cardiac amyloidosis by technetium-99m-pyrophosphate myocardial scintigraphy. Am Heart J. 1982;103:563–566. doi: 10.1016/0002-8703(82)90344-1. [DOI] [PubMed] [Google Scholar]
- 33.Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 34.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rapezzi C, Quarta CC, Guidalotti PL, et al. Role of 99mTc-PYP scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4:659–670. doi: 10.1016/j.jcmg.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Castaño A, DeLuca A, Weinberg R, et al. Serial scanning with technetium pyrophosphate (99mTc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol. 2015 doi: 10.1007/s12350-015-0261-x. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narotsky DL, Kodali SK, Maurer MS, et al. Screening for Transthyretin Cardiac Amyloidosis Using 99mTc-Pyrophosphate Scintigraphy in Patients Undergoing Transcatheter Aortic Valve Replacement. Circulation. 2015 Abst. [Google Scholar]
- 38.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 39.Codella NC, Weinsaft JW, Cham MD, Janik M, Prince MR, Wang Y. Left ventricle: automated segmentation by using myocardial effusion threshold reduction and intravoxel computation at MR imaging. Radiology. 2008;248(3):1004–1012. doi: 10.1148/radiol.2482072016. [DOI] [PubMed] [Google Scholar]
- 40.Codella NC, Lee HY, Fieno DS, et al. Improved left ventricular mass quantification with partial voxel interpolation: in vivo and necropsy validation of a novel cardiac MRI segmentation algorithm. Circ Cardiovasc Imaging. 2012;5(1):137–146. doi: 10.1161/CIRCIMAGING.111.966754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White JA, Kim HW, Shah D, et al. CMR imaging with rapid visual T1 assessment predicts mortality in patients suspected of cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(2):143–56. doi: 10.1016/j.jcmg.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banypersad SM, Sado DM, Flett AS, et al. Quantification of myocardial extracellular volume fraction in systemic AL amyloidosis: an equilibrium contrast cardiovascular magnetic resonance study. Circ Cardiovasc Imaging. 2013;6(1):34–9. doi: 10.1161/CIRCIMAGING.112.978627. [DOI] [PubMed] [Google Scholar]
- 43.Austin BA, Tang WH, Rodriguez ER, et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2(12):1369–77. doi: 10.1016/j.jcmg.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 44.Maceira AM1, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111(2):186–93. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 45.Fontana M, Pica S, Reant P, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–9. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dungu JN, Valencia O, Pinney JH, et al. CMR-based differentiation of AL and ATTR cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7:133–142. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Fontana M, Banypersad SM, Treibel TA, et al. Native t1 mapping in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2014;7:157–165. doi: 10.1016/j.jcmg.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Barison A, Aquaro GD, Pugliese NR, et al. Measurement of myocardial amyloid deposition in systemic amyloidosis: insights from cardiovascular magnetic resonance imaging. J Intern Med. 2015;277(5):605–14. doi: 10.1111/joim.12324. [DOI] [PubMed] [Google Scholar]
- 49.Fontana M, Banypersad S, Treibel TA, et al. AL and ATTR cardiac amyloid are different: native T1 mapping and ECV detect different biology. J Cardiovasc Magn Reson. 2014;16(Suppl 1):P341. [Google Scholar]
- 50.Fontana M, Banypersad SM, Treibel TA, et al. Differential Myocyte Responses in Patients with Cardiac Transthyretin Amyloidosis and Light-Chain Amyloidosis: A Cardiac MR Imaging Study. Radiology. 2015;277(2):388–97. doi: 10.1148/radiol.2015141744. [DOI] [PubMed] [Google Scholar]
- 51.ASNC Practice Points 99mTechnetium-Pyrophosphate Imaging for Transthyretin Cardiac Amyloidosis. http://www.asnc.org/Files/Practice%20Resources/Practice%20Points/ASNC%20Practice%20Point-99mTechnetiumPyrophosphateImaging2016.pdf. March 2016.
- 52.Gillmore JD, Maurer MS, Falk RH, et al. Non-Biopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016 doi: 10.1161/CIRCULATIONAHA.116.021612. Epub ahead of print. [DOI] [PubMed] [Google Scholar]