Abstract
Summary: The current case describes a 78-year-old female with two previous episodes of major depression who presented with both symptoms of depression (amotivation and flattened affect) and typical symptoms of dementia (impaired memory and executive functioning). Even after a detailed clinical exam and neuropsychiatric testing, it remained difficult to definitively classify the diagnosis as either treatment-resistant depression or old-age dementia. After 8 weeks of inpatient treatment, including changing her reserpine-based antihypertensive medication, adjusting her antidepressants, and providing psychotherapy, her depressive and anxiety symptoms improved, but most of her cognitive symptoms persisted. Her symptoms did not change over 7 months of post-hospitalization follow-up. She subsequently developed advanced breast cancer and started chemotherapy; at this point her depressive and cognitive symptoms became more pronounced. We conclude that it will take two-to-three years of follow-up to determine whether the cognitive symptoms are residual to her depression or a newly emerging dementia (or both). This case shows that for elderly patients who have symptoms of both depression and dementia, detailed clinical examination and neuropsychiatric testing may need to be combined with longitudinal assessment of their responsiveness to treatment before a definitive diagnosis can be assigned.
Keywords: depression, dementia, pseudo-dementia, case report, China
Abstract
概述
抑郁伴痴呆在老年人中日益普遍。本报告描述了一个78岁的女性患者,先前有过两次抑郁发作,本次存在抑郁症状(动力缺乏和情感淡漠)和典型的痴呆症状(记忆力和执行功能受损)。即使经过详细的临床检查和神经心理测量,仍然难以明确诊断是难治性抑郁症还是老年痴呆。经过8周的住院治疗,更改了原先以利血平为主的降压药,调整抗抑郁药并予心理治疗,患者的抑郁和焦虑症状改善,但大多数认知症状仍然持续存在。在出院后7个月的随访中,这些症状也没有变化。随后,她出现了晚期乳腺癌并开始化疗,此时她的抑郁症状和认知症状更加明显。我们认为,需要2~3年的随访才可以确定认知症状是抑郁症的残留症状还是新出现的痴呆表现(或两者皆是)。该病例表明对于同时有抑郁症状和痴呆症状的老年患者,不仅需要详细的临床检查和神经心理测试,而且要结合对治疗疗效的长期评估才能明确诊断。
中文全文
本文全文中文版从2016年8月25日起在http://dx.doi.org/10.11919/j.issn.10020829.215085可供免费阅览下载
1. Case history
A 78-year-old female with vocational school education was admitted for a second hospitalization after a seven-year history of depressive symptoms, a two-year history of reduced physical activity, and a one-year history of memory loss. Initially, in 2008 after being frightened by the results of a urine test for a urinary tract infection, she became depressed and anorexic. She was diagnosed at a local general hospital with major depression (without psychotic symptoms) and treated with paroxetine and alprazolam for two months until the symptoms remitted. In 2012, while providing nursing care for her younger sister who had lung cancer, her depressive symptoms recurred and she made two suicide attempts by overdose with alprazolam. This led to a one-month psychiatric hospitalization that included treatment with sertraline, mirtazapine, and electroconvulsive therapy (ECT); after discharge she was able to do housework and grocery shopping. However, starting in June 2013, without any obvious trigger her activity level gradually decreased. She became uncommunicative and was unwilling to leave her home, neither watching TV nor doing any housework. She spent all of her time in bed, so her 82-year-old husband (who had hypertension and chronic bronchitis) had to assist with her eating and routine self-care. Starting in 2014 she reported experiencing short-term memory loss which made her forget what she had eaten for breakfast or what she had just read in the newspaper. She was initially treated as an outpatient, though most of the outpatient visits were with the husband alone because he was physically unable to regularly transport her to the outpatient department. She was prescribed a variety of medications including venlafaxine, citalopram, mianserin, Deanxit (a combined preparation of flupentixol and melitracen), mirtazapine, amisulpride, and quetiapine. Over 18 months of outpatient treatment there was no significant improvement, so she was readmitted to hospital.
On admission the patient reported a history of hypertension which had been controlled for the previous four years by taking 1-2 tablets per day of a combination anti-hypertensive (each tablet included reserpine, hydrochlorothiazide, dihydralazine, and promethazine), but she had no other significant personal or family history of illness. Physical examination revealed excoriated skin with infected lesions on her limbs and face due to poor hygiene and scratching. On mental status exam she was conscious, but she appeared apathetic, repeatedly asking to be allowed to go to sleep during the interview. She was inattentive, responding passively to questions with answers that were devoid of meaning. She was disoriented to time and space and had apparent memory lapses. She reported intermittent auditory hallucinations, saying that she could hear her brother speaking in the doorway.
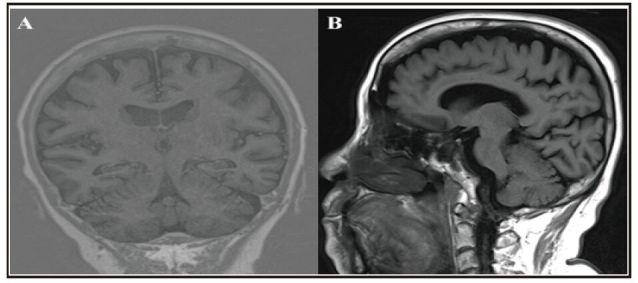
Further laboratory and neuropsychological examinations had the following results: (a) no abnormal blood chemistry results; (b) an abnormal electroencephalogram (EEG) (shown in Figure 1, Panel A), with the δ domain power increased and the α domain power decreased; (c) abnormal magnetic resonance imaging (MRI) test (shown in Figures 2 and 3), indicating ischemia in the right basal ganglia area and bilateral frontal lobe, hippocampus atrophy, and diffuse conical atrophy; (d) mild depression and anxiety as rated by the Hamilton Rating Scale for Depression (HAMD)[1] (score=15), and the Hamilton Anxiety Rating Scale (HAMA)[2] (score=8); and (c) decline in cognitive function as indicated by results of the Mini Mental State Examination (MMSE)[3] (score=21), the Montreal Cognitive Assessment Scale (MoCA)[4] (score=11), the Wechsler Adult Intelligence Scale-Revised in Chinese (WASI-RC)[5] (IQ score=64), the revised Chinese version of the Wechsler Memory (WMSRC)[6] (score=47), and supplemental tests that revealed difficulties in delayed memory, visual recognition, and verbal fluency.
Figure 1. Electroencephalogram (EEG). (A) Abnormal EEG on admission: prompting θ, δ domain power increased, and α domain power decreased. (B) EEG returns to normal after 6 weeks of treatment: θ domain power in occipitoparietal region increased and α domain power normalized.
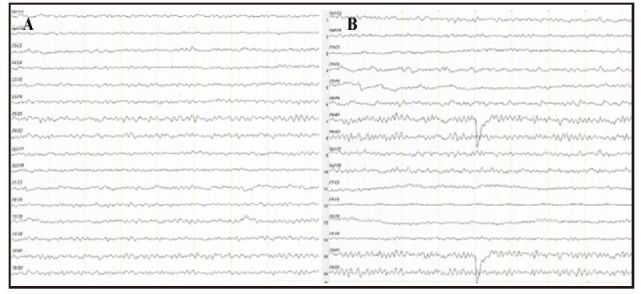
Figure 2. Cranial magnetic resonance imaging: right basal ganglia area and bilateral frontal area have punctiform ischemia (A is T1 weighted image; B is T2 weighted image).
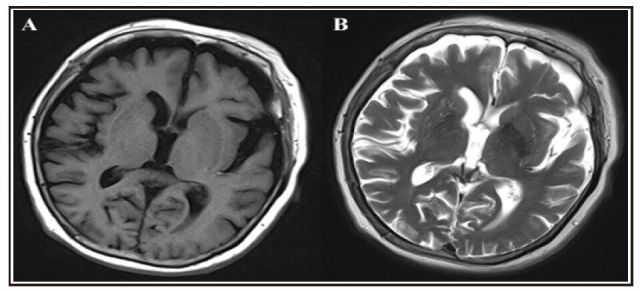
Figure 3. Hippocampus magnetic resonance imaging: hippocampus atrophy and brain atrophy (A is coronal view, B is sagittal view).
The admitting diagnosis was major depression and possible dementia (which had to be ruled out). On admission she continued on the medications she had been taking as an outpatient: citalopram 20 mg/d, mirtazapine 15 mg/d, quetiapine 25 mg/d, memantine 5 m g/d, oxiracetam 400 mg bid (to promote recognition), and lorazepam 0.5 mg qn (to improve sleep). Because reserpine, the anti-hypertensive medication she had been taking for four years, is a monoamine depletion agent which may exacerbate depression, we changed it to amlodipine 5 mg/d to control her blood pressure. While an inpatient she also participated in group cognitive behavioral therapy, family counseling, and art and music activities. There was no significant improvement after 2 weeks of treatment: her drowsiness, limited speech, lack of interest, and aversion to activity persisted. So we modified the treatment plan to the following: sertraline 50 mg/d, memantine 10 mg/d, rivastigmine 3 mg bid, and aripiprazole 2.5 mg/d. After 6 weeks of this new treatment regimen her test results indicated improvement in the depressive and anxiety symptoms: HAMD=9; HAMA=6. Her EEG had returned to normal, as shown in Panel B of Figure 1, the fragmentary auditory hallucinations had faded, and she had become somewhat more socially interactive. However, her cognitive problems with attention and executive functioning had only improved slightly: MMSE=22; and MoCA=16.
At the time of hospital discharge, 8 weeks after admission, her discharge diagnosis was treatment resistant depression and the discharge medications were amlodipine 5 mg/d, sertraline 100 mg/d, and rivastigmine 3 mg bid. After she returned home, we also arranged for the delivery of daily meals and for regular home visits from community workers. She attended monthly outpatient follow-up visits, but there was no further improvement in her depressive and cognitive symptoms. Seven months after discharge she became increasingly fatigued and experienced many falls; this resulted in admission to the surgery department of a general hospital where she was diagnosed with advanced breast cancer and started on chemotherapy. At that point her sertraline and rivastigmine were stopped and she was changed to trazadone 50 mg qn (to improve sleep). Her depressive and cognitive symptoms subsequently increased.
2. Discussion
Prior to the current admission the patient had been treated with several different antidepressants at an adequate dose for at least 6 weeks, but none of these courses of treatment were effective, so she met criteria for treatment resistant depression. Unlike her previous depressive episodes, the current episode included physical weakness and apathy, transient hallucinatory phenomena, and marked cognitive decline. Based on these findings and the diffuse conical atrophy and hippocampal atrophy seen on brain imaging, we concluded that the patient also met diagnostic criteria for Alzheimer's disease(AD).[8] The diagnostic difficulty was to determine whether she had two concurrent illnesses or a single illness that included both prominent affective and prominent cognitive features. And if it was a single disorder, which one?
Considering her prior episodes of depression, our initial diagnosis was depression with impaired cognitive functioning. Epidemiological studies[9] report that 30- 50% of patients with depression have concurrent cognitive dysfunction; in elderly depressed patients the cognitive dysfunction can include impairments in memory, attention, information processing, and executive functioning.[10] If the cognitive dysfunction in depressed elderly is improved with antidepressant treatment, this is considered 'pseudo dementia'[11] and, thus, is differentiated from AD. However, if residual cognitive symptoms remain after effective antidepressant treatment - as occurred in this case - these cognitive symptoms can either be considered residual symptoms of depression or, alternatively, the behavioral and cognitive manifestations of a separate disorder (dementia). In this patient, the presence of transient auditory hallucinations and other symptoms which were not present in her prior episodes of depression suggest an underlying organic brain disease; if this is the case, the current episode of depression could be an indicator of prodromal AD.[12]
Two additional factors further complicated the diagnosis. First, the patient had used reserpine (which depletes monoamines and is often a cause of depression) as a treatment for hypertension for several years prior to admission. As is true for the majority of such cases, [13] the depressive and anxiety symptoms improved after stopping the reserpine (and giving antidepressants). The failure to see substantial concurrent cognitive improvement after stopping reserpine could be to several reasons: there may be an independent dementing process; the long-term use of reserpine may have caused permanent brain damage; or cognitive improvement may take much longer than improvement in depression because it takes a long time to fully restore depleted monoamine reserves. Another complicating issue is the patient's lack of family support, something essential in the management of geriatric depression.[14, 15] Having no children, her elderly husband was her sole care-giver; her infrequent visits to the outpatient department made it impossible to manage her complicated condition appropriately and to observe the gradual onset of cognitive impairment.
Currently popular treatments for patients suffering from depression with cognitive dysfunction include medication combined with psychological and physical treatments such as repetitive transcranial magnetic stimulation (rTMS) and modified electroconvulsive therapy (MECT).[16] In this case, inpatient admission was required to re-adjust her medications and to provide psychotherapy and opportunities for increasing her social activities. After 8 weeks of treatment her depressive and anxiety symptoms had improved significantly and her indifference and social inactivity improved slightly, but there was relatively little improvement in her cognitive impairments. We conclude that long-term follow-up, probably for two-to- three years, will be needed to definitively determine the primary cause (or causes) of her cognitive symptoms.
Many elderly individuals who seek help from health services suffer from both depression and cognitive impairment. There are several important lessons that this case highlights. (a) Elderly patients with underlying cognitive impairment who experience a depressive episode are more likely to develop dementia[17] and have a poor response to medication.[18] This complicates the diagnosis and management of such patients and, thus, necessitates both a detailed physical exam and a thorough neuropsychological evaluation prior to the initiation of treatment. (b) Longitudinal observation of the course of illness may be needed to determine the correct diagnosis. A two-year longitudinal study of 201 non-demented elderly patients who experienced depression with cognitive dysfunction found that 50 (25%) recovered, 30 (15%) developed dementia, and 121 (60%) maintained some cognitive dysfunction.[19] (c) Clinicians who treat elderly patients must be aware of the cognitive effects of commonly used psychoactive drugs, and they must be up-to-date on the potential affective and cognitive side-effects of the full range of medications used to treat the physical illnesses experienced by elderly individuals. A research study in the United States estimated that there are 5.6 to 8.0 million people over the age of 65 who misuse medications that often cause cognitive impairment, including benzodiazepines, anticholinergics, opioids, analgesics, hypnotics, and antipsychotics.[20] (d) Family and social support is important to the quality of life and social functioning of all elderly individuals, but it is even more important for elderly persons with depression or dementia. These individuals need substantial help in managing their condition beyond what can be provided by the health care system. Part of the clinical assessment of such individuals should include an assessment of the types of social support available to the patient. Subsequent treatment planning should include provision of educational and psychological support to primary care-givers, and, when necessary, involvement of community-based social services to assist when the available family resources are insufficient.
Biography

Zhongyong Shi graduated with a bachelor's degree in clinical psychology from Henan University in 2013. She is currently a masters' degree student in the Tenth People's Hospital of Tongji University on a clinical rotation at the Shanghai Mental Health Center. Her main research interest is biomarkers of cognitive impairment.
Funding Statement
Preparation of this report was supported by grants from the Shanghai Clinical Center for Mental Disorders (2014), by the National Key Clinical Disciplines program at the Shanghai Mental Health Center (Office of Medical Affairs, Ministry of Health, 2011-873; OMAMH, 2011-873), and by the Science and Technology Commission of Shanghai's Medical Guide Project (No.15411961400).
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest related to this manuscript.
Informed consent: The patient and her guardian signed an informed consent form and agreed to the publication of this case report.
Authors' contribution: SZY participated in data collection and drafted the manuscript. XSF and LX carried out the clinical diagnosis and treatments. LX critically reviewed the manuscript. All authors read and approved the final manuscript.
References
- 1.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, et al. The prevalence of dementia and Alzheimer's disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27(4):428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 4.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 5.National Cooperative Group for Wechsler Adult Intelligence Scale-Revised. Wechsler Adult Intelligence Scale-Revised. Acta Psychologica Sinica. 1983;15(3):362–369. [Google Scholar]
- 6.Gong YX. [Wechsler Memory Scale-Revised in China]. Hunan: Hunan Medical University Publishing; 1989. Chinese. [Google Scholar]
- 7.Zhu ZQ, Ji JL, Xiao SF. [Key of Depression Treatment]. Jiangshu: Jiangsu Science and Technology Publishing House; 2003. pp. 142–143. Chinese. [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 9.Bhalla RK, Butters MA, Becker JT, Houck PR, Snitz BE, Lopez OL, et al. Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry. 2009;17(4):308–316. doi: 10.1097/JGP.0b013e318190b8d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto SS, Alexopoulos GS. Cognitive deficits in geriatric depression: clinical correlates and implications for current and future treatment. Psychiatr Clin North Am. 2013;36(4):517–531. doi: 10.1016/j.psc.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang H, Zhao F, You L, Giorgetta CDV, Sarkhel S, Prakash R. Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 2014;17(2):147–154. doi: 10.4103/0972-2327.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Blazer DG. Depression and cognition in the elderly. Annu Rev Clin Psychol. 2015;11:331–360. doi: 10.1146/annurev-clinpsy-032814-112828. [DOI] [PubMed] [Google Scholar]
- 13.Baumeister AA, Hawkins MF, Uzelac SM. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci. 2003;12(2):207–220. doi: 10.1076/jhin.12.2.207.15535. [DOI] [PubMed] [Google Scholar]
- 14.Michael Ebert PT, Looen BN. [Diagnosis and Treatment of Modern Mental Illness]. Sun XL, translator. Beijing: People's Health Publishing House; 2002. p. 316. Chinese. [Google Scholar]
- 15.Zheng Z, Mao J, Sun Y Y. [Impact of the family structure on elderly depression] Zhong Hua Xing Wei Yi Xue Yu Nao Ke Xue Za Zhi. 2013;22(11):1016. doi: 10.3760/cma.j.issn.1674-6554.2013.11.018. Chinese. [DOI] [Google Scholar]
- 16.Koenig AM, Butters MA. Cognition in late life depression: treatment considerations. Curr Treat Options Psychiatry. 2014;1:1–14. doi: 10.1007/s40501-013-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Potter GG, Wagner HR, Burke JR, Plassman BL, Welsh- Bohmer KA, Steffens DC. Neuropsychological predictors of dementia in late-life major depressive disorder. Am J Geriatr Psychiatry. 2013;21(3):297–306. doi: 10.1016/j.jagp.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sneed JR, Culang ME, Keilp JG, Rutherford BR, Devanand DP, Roose SP. Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry. 2010;18(2):128–135. doi: 10.1097/JGP.0b013e3181c796d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffens DC, McQuoid DR, Potter GG. Outcomes of older cognitively impaired individuals with current and past depression in the NCODE study. J Geriatr Psychiatry Neurol. 2009;22:52–61. doi: 10.1177/0891988708328213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blank K. Older adults & substance use: new data highlight concerns. Substance Abuse & Mental Health Service Administration (SAMHSA); 2009. [Google Scholar]


