Abstract
Background
The gut microbiota is associated with several of metabolic diseases, including obesity and type 2 diabetes and affects host physiology through distinct mechanisms. The microbiota produces a vast array of metabolites that signal to host cells in the intestine as well as in more distal organs.
Scope of review
Enteroendocrine cells acts as ‘chemo sensors’ of the intestinal milieu by expressing a large number of receptors, which respond to different metabolites and nutrients, and signal to host by a wide variety of hormones. However, enteroendocrine cells differ along the length of the gut in terms of hormones expressed and receptor repertoire. Also, the microbial ecology and dietary substrates differ along the length of the gut, providing further evidence for unique functions of specific subpopulations among enteroendocrine cells. Here we will review how the gut microbiota interacts with L-cells in the small and large intestine and the resulting effects on the host.
Major conclusions
Microbial metabolites can be sensed differently by specific subpopulations of enteroendocrine cells. Furthermore, hormones such as GLP-1 can have different functions when originating from the small intestine or colon. This article is part of a special issue on microbiota.
Keywords: Enteroendocrine, Microbiota, L cells, Metabolites, Gut
1. Introduction
The co-evolution of animals, from insects to humans, with microorganisms has created a symbiotic relationship with a delicate balance providing the host with functions it did not have to evolve itself. The microorganisms that colonize an animal, collectively known as the microbiota, reside on all mucosal surfaces in the body with the highest numbers in the gut. The gut microbiota is dominated by bacteria distributed over 500–1000 species. The microbiota have previously been estimated to outnumber human cells by 10 [1], [2], but a recent estimate reveals a ratio closer to 1 to 1 [3]. Regardless, the combined genomes of the microbiota, referred to as the microbiome, contains more than 10 million genes [4] including a vast array of genes controlling many metabolic pathways. The microbiome provides metabolic functions that the host did not have to develop itself, for example degradation of complex polysaccharides to short-chain fatty acids (SCFAs). Furthermore, the interaction between nutritional content of the diet and bacterial metabolism in the gut produces a metabolic footprint with an extensive number of bioactive metabolites that can influence the host in ways we are only starting to understand. Culture-independent methods to analyze the microbiota together with the use of gnotobiotic genetically modified animals have greatly increased the knowledge of host–microbial interactions and their impact on host physiology and identified that an altered microbiota is associated with metabolic diseases including obesity and type 2 diabetes [5].
The gut is an important sensor of energy balance and nutrient status and, as such, signals to the brain and periphery via neurons and hormones [6]. In contrast to many endocrine organs, enteroendocrine cells are sparsely distributed in the epithelial lining and account for ∼1% of the cells in the intestinal mucosa [7], [8]. Enteroendocrine cells secrete a vast number of peptides with profound effects on host physiology, which has been extensively reviewed elsewhere [9], [10]. Among the most studied peptides are the glucagon-like peptide 1 (GLP-1) and peptide YY (PYY). Both are secreted by L-cells that are most abundant in the distal small intestine and the colon and have several biological functions in host physiology ranging from controlling appetite and regulating stomach emptying and gut transit to acting as incretin hormones and promoting β-cell survival and proliferation. Here we will focus on how host–microbial interactions regulate enteroendocrine function in a spatio-temporal fashion and how they impact host physiology.
2. L-cell hormones
Enteroendocrine L-cells have mainly been studied for their function as regulators of postprandial response to nutrients. One prominent feature of L-cells is the expression of the proglucagon gene (Gcg) which is also expressed in pancreatic α-cells. The proglucagon peptide is cleaved by prohormone convertase to produce different proteins depending on the cell type and expression pattern of prohormone convertases. Prohormone convertase 2, which is abundant in pancreatic α-cells, generates glucagon, whereas prohormone convertase 1/3 in L-cells produces GLP-1, GLP-2, and oxyntomodulin [11].
GLP-2 has been shown to be important for gut epithelial function by stimulating epithelial cell regeneration and improving barrier function in the intestine [12]. Feeding ob/ob mice with prebiotics improved barrier function and reduced plasma LPS levels, which was associated with an increase in bifidobacteria and lactobacillus and dependent on GLP-2 [13]. Regeneration and growth of the intestine has also been shown to be promoted by GLP-1 and may, at least in part, be mediated through Fgf7 [14], [15].
Oxyntomodulin promotes satiety and serves as an agonist to both GLP-1 and glucagon receptors, albeit with a lower affinity than GLP-1 and glucagon [16], [17], [18], [19].
Perhaps the least studied gut hormone from L-cells is INSL5, which is expressed in colonic L-cells. INSL5 is upregulated by caloric restriction [20] as well as in germ-free mice [21], in which colonocytes are energy deprived due to the lack of SCFAs from fermenting bacteria [22]. Subsequently, INSL5 acts as an orexogenic hormone under conditions of energy deprivation where it stimulates food intake [20] and promotes hepatic glucose production [21]. These results suggest that INSL5 is an orexogenic hormone that may be physiologically important when energy is scarce, but studies in humans are required to determine the importance of this hormone in vivo.
In contrast to INSL5, the postprandially induced L-cell hormones PYY and GLP-1 have been shown to inhibit food intake [23], [24]. GLP-1 and PYY levels in the circulation increase within 15 min after a mixed meal and reach peak levels at 40 and 90 min [25], [26]. GLP-1 and PYY also have been shown reduce gastrointestinal transit and contribute to the ileal brake where gastric emptying is inhibited to balance food delivery to the intestine with digestion and absorption rate [27], [28], [29], [30], [31]. GLP-1 is well known for its role as an incretin hormone where it, together with the gastric inhibitory peptide (GIP), contributes to postprandial glucose clearance by stimulating insulin secretion from the pancreatic β-cells [11]. The mechanism by which GLP-1 stimulates insulin secretion was previously thought to be through an endocrine route where circulating GLP-1 acts directly on pancreatic β-cells since they express high levels of the GLP-1 receptor. However, recent studies have questioned this canonical signaling pathway, which will be discussed further below.
3. Receptor signaling pathways regulating GLP-1 in L-cells
L-cells are important sensors of nutrients present in the intestine, mediating signals to nearby cells as well as extra-intestinal organs of the body. These cells express a vast array of seven-transmembrane (7TM) G protein-coupled receptors (GPCRs) located in their cell membranes that can sense a broad spectrum of nutrients and metabolites, including microbially derived products [32].
4. Metabolic receptors
Medium and long chain fatty acids (MCFAs and LCFAs) are sensed by L-cells through the receptors GPR40 and 120, which stimulate the release of GLP-1 [33], [34]. Furthermore, GLP-1 is also induced by ethanolamides that signal through the receptor GPR119, which is expressed by L-cells, providing yet another signaling pathway in which fatty acids can affect enteroendocrine hormone secretion [35] that could serve as a potential therapeutic target to increase GLP-1 secretion.
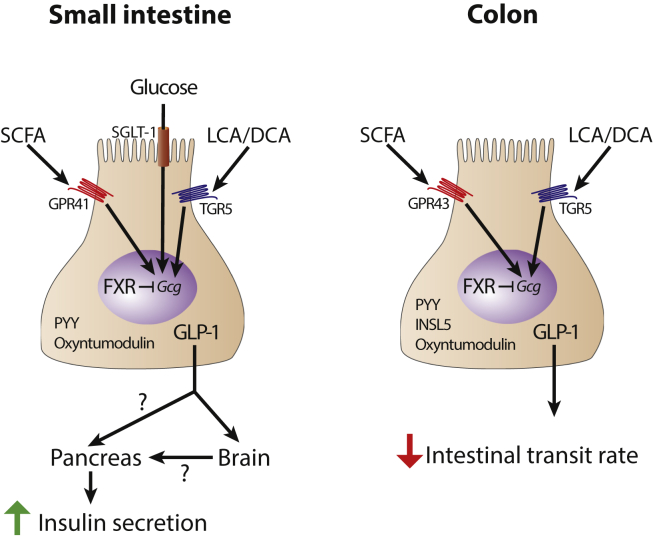
Glucose can directly promote GLP-1 release in a sodium glucose cotransporter 1 (SGLT-1) dependent pathway, and another study showed that the responsiveness to glucose in vivo is restricted to the small intestine whereas glucose injection in the colon did not affect GLP-1 levels [36], [37], lending evidence to distinct differences between small intestinal and colonic L-cells (Figure 1).
Figure 1.
Distinct functions of small intestinal and colonic L-cells. L-cells in the small intestine and colon are exposed to different microbes and metabolites derived from diet and diet-microbe metabolism. As such, they induce specific signaling pathways leading to different physiologic outputs. SCFA, short-chain fatty acids; LCA, lithocholic acid; DCA, deoxycholic acid; FXR, farnesoid X receptor; Gcg, proglucagon gene; PYY, peptide YY; GLP-1, glucagon-like peptide 1; SGLT-1, sodium glucose cotransporter 1.
5. Dietary fibers and SCFAs
SCFAs are the major products of microbial fermentation of fiber. The most abundant SCFAs produced by the gut microbiota are acetate, propionate, and butyrate [38], which can signal by several different pathways including GPCRs and histone deacetylase (HDAC) inhibitors but also act as substrates for intestinal gluconeogenesis and as an energy source [39] (Figure 1). SCFAs bind to the GPCRs GPR41 and GPR43, which show distinct expression patterns. GPR41 is predominantly expressed in small intestinal L-cells whereas GPR43 is predominantly expressed in colonic L-cells [40]. In humans, GPR41 and GPR43 are not expressed by the same cells [40], [41], [42], suggesting that distinct subpopulations of L-cells exist (see below). Binding of SCFAs to their receptors stimulates GLP-1 release [40], [43], providing a mechanistic explanation for the increased levels of GLP-1 upon dietary fiber supplementation. GPR41 knockout mice have resulted in conflicting results, showing either worsening of glucose tolerance [40] or no effect on glucose tolerance [44]. Knocking out GPR43 resulted in similar effects with reports on both having a worsened glucose tolerance [40] or no change [45]. The underlying reason for this discrepancy is unknown. However, diet may be an important factor, especially in combination with the microbiota in a given animal facility as different diets will yield different SCFA profiles and microbiota in different animal facilities produces specific metabolic profiles [46].
6. Bile acids
Another group of microbially modulated metabolites affecting host metabolic pathways are bile acids. They are produced in the liver from cholesterol and are secreted into the duodenum upon ingestion of a meal. Bile acids, originally considered to be detergents required for lipid absorption, are increasingly recognized as important signaling molecules affecting host metabolism. Bile acids are deconjugated by the microbiota in the lower small intestine and further metabolized in the colon to generate secondary bile acids [47], [48]. Bile acids signal through distinct receptors such as TGR5 and the nuclear receptor farnesoid X receptor (FXR), both of which are expressed by L-cells.
Activation of TGR5, which is more highly expressed in colonic L-cells compared with ileal L-cells, results in increased GLP-1 secretion [49], [50] (Figure 1). In contrast, activation of FXR leads to reduced GLP-1 expression both in ileal and colonic L-cells, and knockout of FXR increases GLP-1 gene expression and secretion in response to glucose (Figure 1). Interestingly, FXR activation in TGR5 knockout mice display differences between ileal and colonic L-cells. Whereas the reduction of proglucagon expression in the ileum is retained, the colonic reduction of proglucagon is blunted in the TGR5 knockout mice, suggesting a crosstalk between FXR and TGR5 in colonic but not ileal L-cells [51]. The differences between FXR and TGR5 signaling could be explained by their mode of action. Whereas TGR5 activation results in rapid responses in signaling pathways and release of preformed GLP-1, FXR activation is slower as it is a nuclear receptor that affects gene transcription.
7. Are all L-cells equal?
Whereas L-cells in the in the small intestine and colon secrete GLP-1, GLP-2, PYY, and oxyntomodulin [9], INSL5 expression is restricted to colonic L-cells [20], [21]. Similarly, the response to glucose is limited to small intestinal L-cells [36], [37] and expression of SCFA receptors GPR41 and GPR43 as well as the bile acid receptor TGR5 differs between small intestinal and colonic L-cells [36], [40] suggesting that there are several different subtypes of L-cells and that this nomenclature may need to be revised or at least the origin of the cell should be stated. This is supported by a transcriptional profiling study of L-cells in the small intestine and colon: L-cells in the small intestine had a transcription profile that were more similar to other small intestinal enteroendocrine cells than colonic L-cells, suggesting that the microenvironment is important for L-cell function [52]. The variety of expressed hormones in different enteroendocrine cell populations was further demonstrated by the fact that CCK expressing I-cells expressed different combinations of peptide hormones in addition to CCK, further emphasizing that there may be more populations of enteroendocrine cells than previously appreciated [53].
The microbial communities in the small and large intestine are different [54], and microbial cells are in direct contact with the epithelium and L-cells in the small intestine. In contrast, the microbiota is separated from the cells by a mucus layer in the colon [55]. Thus, it is clear that different cell populations may react to local environmental cues that can be regulated by the gut microbiota. For example, secondary bile acids, agonists for TGR5, are only produced in the colon [56] but may reach the ileum as conjugated after being recirculated from the colon. Furthermore, whereas SCFA is present in the 100 mM range in the colon exceeding the EC50 for GPR41 and 43 with 3–4 orders of magnitude [57], SCFA levels in the ileum are lower [38], suggesting that variation in SCFA concentrations may be more relevant for ileal L-cell biology. Finally, the interaction between the microbiota and GLP-1 is reciprocal as mice lacking the GLP-1 receptor have an altered microbiota, which may be attributed to altered regulation of intra epithelial lymphocyte (IEL) activity. IELs express the GLP-1 receptor, and activating the receptor increases the expression of immunomodulatory genes and antimicrobial peptides [58]. Thus, it would be interesting to examine how GLP-1 analogs modulate the gut microbiota in humans.
In addition to the differences between L-cells in different compartments there are also data suggesting that GLP-1 may have different actions depending on where it is released. As previously mentioned, GLP-1 is well known for its role as an incretin hormone, acting within 15 min after a meal, but the fact that the highest density of GLP-1 expressing cells reside in the colon, which will not sense nutrients from a meal until later, suggests that the postprandial rise in GLP-1 is not due to its release from colonic L-cells. Furthermore, GLP-1 is considered to be an anorectic hormone that reduces appetite but, at the same time, levels of GLP-1 are increased in situations of low energy as in anorexic patients and germ free mice that have increased food intake compared with conventionally raised counterparts [59], [60], [61]. Based on these facts, it is conceivable that GLP-1 may have different functions depending on where it is secreted and the fact that the peptide has a half-life of only 2 min [11], [62], which suggests that GLP-1 may mainly have paracrine rather than hormonal functions, or even signal directly to GLP-1R expressed on vagal afferent nerves that relay signals to the brain.
7.1. The role of GLP-1 as an incretin hormone in the small intestine
The role of GLP-1 as an incretin hormone is well established, and the classical view has been that it acts as an endocrine hormone. In this way, L-cells from the intestine release GLP-1 upon ingestion of a meal, and circulating GLP-1 reaches the pancreatic β-cells where it binds to the GLP-1 receptor, which stimulates insulin secretion. Indeed, GLP-1 is released from the intestinal L-cells, and stimulation of β-cells with GLP-1 induces insulin secretion. However, the very short half-life of the active peptide [62] and the relatively modest increase of postprandial GLP-1 in the circulation [63], [64], [65] suggests that the incretin effect of GLP-1 acts through alternate routes. Further support of an alternate route came from mouse knockout experiments in which the GLP-1 receptor was selectively knocked out in the pancreatic β-cells [66]. Interestingly, whereas intraperitoneal glucose tolerance was impaired in the knockout mice, oral glucose tolerance was not. Thus, it is likely that the incretin effect may involve nervous signaling, and recent data have demonstrated that GLP-1 receptors are expressed on nerves in the intestinal submucosa [67]. Thus, receptor activation could enable a neuronal signaling route mediating the incretin effect, which has experimental support [61], [68]. However, a recent study using tissue specific knockdown of GLP-1R showed that neither CNS nor vagal GLP1R signaling contributes to the incretin effect during a glucose tolerance test [69]. Furthermore, an experiment in which the GLP-1R was selectively expressed in β-cells in a Glp-1r −/− background showed that glucose tolerance was restored to normal. Intriguingly, this was not associated with improved postprandial insulin secretion [70]. Thus, GLP-1 released by intestinal L-cells in response to a meal increases glucose clearance through the incretin effect, possibly through a neuronal signaling pathway that does not require CNS or vagal GLP1R signaling. However, additional work is required to clarify how the GLP-1 mediates the incretin effect as well as how and if the microbiota directly or indirectly affects this process.
7.2. GLP-1 as an energy sensor in the colon
The highest density of GLP-1 expressing L-cells are in the colon [71], but nutrients do not reach the colon until long after (60 min) a meal. In contrast, the insulin peak occurs after 15 min. Most nutrients are absorbed in the small intestine, which makes it unlikely that colonic GLP-1 contributes to the incretin effect. This was further confirmed by the finding that the elevated levels of GLP-1 observed in germ-free mice did not contribute to improved glucose metabolism [31] and suggests that colonic produced GLP-1 has other biological function(s). We recently proposed that the physiological role of the increased basal GLP-1 expression from colonic L-cells function as an energy sensor to slow intestinal transit to allow more time for nutrient harvest in the small intestine. This hypothesis was supported by the fact that feeding germ-free mice an energy-rich Western diet was sufficient to normalize GLP-1 levels and gut transit [31]. This is likely an evolutionary advantage whereby in energy scarcity, the small intestinal transit is slow, providing more time for nutrient extraction, whereas in energy sufficient state, it is an advantage to have more rapid transit to expel potential pathogenic bacteria. Furthermore, this is more in line with the orexogenic effects of INSL5 that is secreted from colonic L-cells and contrasts the anorexic effects of GLP-1 from other cellular compartments. In support of an energy-sensing role for colonic GLP-1 are the observations that anorexic patients have increased GLP-1 levels and slower gastrointestinal transit [59], [60]. We recently observed that colonic INSL5 promotes hepatic glucose production, which is also in line with the notion that colonic L-cells function as energy sensors contributing to adaptation to famine [21]. Thus, the colonic L-cells may serve as energy sensors and respond to low energy levels by increasing appetite and hepatic glucose production through INSL5 and by slowing gastrointestinal transit through GLP-1.
8. Conclusion and perspectives
In conclusion, it has become clear that the gut microbiota affect host metabolism in multiple ways and changes in the gut microbiota undoubtedly correlate with metabolic disease. The mechanisms by which the gut microbiota interacts with diet and its host is becoming clearer, and some effects may involve signaling through L-cell hormones such as GLP-1, PYY, and INSL5. However, at present, there is little knowledge on how the microbiota modulates other L-cell hormones such as GLP-2 and oxyntomodulin. Considering the large pool of microbially derived metabolites with unknown receptors, there is reason to believe that microbiota-host signaling in L-cells goes way beyond SCFA and bile acid signaling. This notion emphasizes the need to identify novel signaling pathways to find possible new therapeutic targets in treatment of metabolic disease.
To understand the full complexity of L-cell hormone functions and the different roles of GLP-1 from different cellular sources, it would be necessary to specifically target Gcg expression in the different cell types. Separating small and large intestinal L-cells has been difficult, but by identifying genes that are specific to one of the compartments, as in the case of INSL5 in colonic L-cells, might open new ways of dissecting the different roles of GLP-1 and how the microbiota affects specific pools of L-cells, which would be of great interest considering the extensive use of GLP-1 mimetics in treatment today.
Acknowledgments
We would like to thank Anna Cederberg for help with the figure. Work in the authors laboratory is supported by the Swedish Research Council, the NovoNordisk foundation, Torsten Söderberg Foundation, Swedish Heart Lung Foundation, Göran Gustafsson Foundation, IngaBritt och Arne Lundberg Foundation, Knut and Alice Wallenberg Foundation, the FP7 sponsored program METACARDIS, the regional agreement on medical training and clinical research (ALF) between Region Västra Götaland and Sahlgrenska University Hospital. FB is a recipient of an ERC Consolidator Grant (European Research Council, Consolidator grant 615362 – METABASE).
Conflict of interest
FB is founder and shareholder of the company Metabogen AB.
References
- 1.Savage D.C. Microbial ecology of the gastrointestinal tract. The Annual Review of Microbiology. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Berg R.D. The indigenous gastrointestinal microflora. Trends in Microbiology. 1996;4(11):430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 3.Sender R., Fuchs S., Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164(3):337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Li J., Jia H., Cai X., Zhong H., Feng Q., Sunagawa S. An integrated catalog of reference genes in the human gut microbiome. Nature Biotechnology. 2014;32(8):834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 5.Tremaroli V., Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 6.Badman M.K., Flier J.S. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307(5717):1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 7.Hocker M., Wiedenmann B. Molecular mechanisms of enteroendocrine differentiation. Annals of the New York Academy of Sciences. 1998;859:160–174. doi: 10.1111/j.1749-6632.1998.tb11120.x. [DOI] [PubMed] [Google Scholar]
- 8.Rehfeld J.F. The new biology of gastrointestinal hormones. Physiological Reviews. 1998;78(4):1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- 9.Gribble F.M., Reimann F. Enteroendocrine cells: chemosensors in the intestinal epithelium. The Annual Review of Physiology. 2016;78:277–299. doi: 10.1146/annurev-physiol-021115-105439. [DOI] [PubMed] [Google Scholar]
- 10.Meek C.L., Lewis H.B., Reimann F., Gribble F.M., Park A.J. The effect of bariatric surgery on gastrointestinal and pancreatic peptide hormones. Peptides. 2016;77:28–37. doi: 10.1016/j.peptides.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Holst J.J. The physiology of glucagon-like peptide 1. Physiological Reviews. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 12.Drucker D.J., Erlich P., Asa S.L., Brubaker P.L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(15):7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58(8):1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simonsen L., Pilgaard S., Orskov C., Rosenkilde M.M., Hartmann B., Holst J.J. Exendin-4, but not dipeptidyl peptidase IV inhibition, increases small intestinal mass in GK rats. American Journal of Physiology, Gastrointestinal and Liver Physiology. 2007;293(1):G288–G295. doi: 10.1152/ajpgi.00453.2006. [DOI] [PubMed] [Google Scholar]
- 15.Koehler J.A., Baggio L.L., Yusta B., Longuet C., Rowland K.J., Cao X. GLP-1R agonists promote normal and neoplastic intestinal growth through mechanisms requiring Fgf7. Cell Metabolism. 2015;21(3):379–391. doi: 10.1016/j.cmet.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Dakin C.L., Gunn I., Small C.J., Edwards C.M., Hay D.L., Smith D.M. Oxyntomodulin inhibits food intake in the rat. Endocrinology. 2001;142(10):4244–4250. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 17.Wynne K., Park A.J., Small C.J., Patterson M., Ellis S.M., Murphy K.G. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–2395. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 18.Wynne K., Park A.J., Small C.J., Meeran K., Ghatei M.A., Frost G.S. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. International Journal of Obesity (London) 2006;30(12):1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 19.Pocai A., Carrington P.E., Adams J.R., Wright M., Eiermann G., Zhu L. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–2266. doi: 10.2337/db09-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosse J., Heffron H., Burling K., Akhter Hossain M., Habib A.M., Rogers G.J. Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(30):11133–11138. doi: 10.1073/pnas.1411413111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y.S., De Vadder F., Tremaroli V., Wichmann A., Mithieux G., Backhed F. Insulin-like peptide 5 is a microbially regulated peptide that promotes hepatic glucose production. Molecular Metabolism. 2016;5(4):263–270. doi: 10.1016/j.molmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donohoe D.R., Wali A., Brylawski B.P., Bultman S.J. Microbial regulation of glucose metabolism and cell-cycle progression in mammalian colonocytes. PLoS One. 2012;7(9):e46589. doi: 10.1371/journal.pone.0046589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon L.R., Wellman P.J. PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. American Journal of Physiology. 1998;274(1 Pt 2):R23–R29. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 24.Batterham R.L., Cowley M.A., Small C.J., Herzog H., Cohen M.A., Dakin C.L. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 25.Todd J.F., Edwards C.M., Ghatei M.A., Mather H.M., Bloom S.R. Subcutaneous glucagon-like peptide-1 improves postprandial glycaemic control over a 3-week period in patients with early type 2 diabetes. Clinical Science (London) 1998;95(3):325–329. [PubMed] [Google Scholar]
- 26.Batterham R.L., Bloom S.R. The gut hormone peptide YY regulates appetite. Annals of the New York Academy of Sciences. 2003;994:162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 27.Savage A.P., Adrian T.E., Carolan G., Chatterjee V.K., Bloom S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28(2):166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pironi L., Stanghellini V., Miglioli M., Corinaldesi R., De Giorgio R., Ruggeri E. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105(3):733–739. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- 29.Layer P., Holst J.J., Grandt D., Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Digestive Diseases and Sciences. 1995;40(5):1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 30.Nauck M.A., Niedereichholz U., Ettler R., Holst J.J., Orskov C., Ritzel R. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. American Journal of Physiology. 1997;273(5 Pt 1):E981–E988. doi: 10.1152/ajpendo.1997.273.5.E981. [DOI] [PubMed] [Google Scholar]
- 31.Wichmann A., Allahyar A., Greiner T.U., Plovier H., Lunden G.O., Larsson T. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host & Microbe. 2013;14(5):582–590. doi: 10.1016/j.chom.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Engelstoft M.S., Egerod K.L., Holst B., Schwartz T.W. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metabolism. 2008;8(6):447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Medicine. 2005;11(1):90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 34.Edfalk S., Steneberg P., Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57(9):2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauffer L.M., Iakoubov R., Brubaker P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58(5):1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reimann F., Habib A.M., Tolhurst G., Parker H.E., Rogers G.J., Gribble F.M. Glucose sensing in L cells: a primary cell study. Cell Metabolism. 2008;8(6):532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriya R., Shirakura T., Ito J., Mashiko S., Seo T. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. American Journal of Physiology, Endocrinology and Metabolism. 2009;297(6):E1358–E1365. doi: 10.1152/ajpendo.00412.2009. [DOI] [PubMed] [Google Scholar]
- 38.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016 doi: 10.1016/j.cell.2016.05.041. Accepted in principle. [DOI] [PubMed] [Google Scholar]
- 40.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karaki S., Tazoe H., Hayashi H., Kashiwabara H., Tooyama K., Suzuki Y. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. The Journal of Molecular Histology. 2008;39(2):135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- 42.Tazoe H., Otomo Y., Kaji I., Tanaka R., Karaki S.I., Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. Journal of Physiology and Pharmacology. 2008;59(Suppl. 2):251–262. [PubMed] [Google Scholar]
- 43.Nohr M.K., Pedersen M.H., Gille A., Egerod K.L., Engelstoft M.S., Husted A.S. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552–3564. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 44.Lin H.V., Frassetto A., Kowalik E.J., Jr., Nawrocki A.R., Lu M.M., Kosinski J.R. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjursell M., Admyre T., Goransson M., Marley A.E., Smith D.M., Oscarsson J. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. American Journal of Physiology, Endocrinology and Metabolism. 2011;300(1):E211–E220. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- 46.Ussar S., Griffin N.W., Bezy O., Fujisaka S., Vienberg S., Softic S. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metabolism. 2015;22(3):516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Midtvedt T. Microbial bile acid transformation. American Journal of Clinical Nutrition. 1974;27(11):1341–1347. doi: 10.1093/ajcn/27.11.1341. [DOI] [PubMed] [Google Scholar]
- 48.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. Journal of Lipid Research. 2006;47(2):241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Katsuma S., Hirasawa A., Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochemical and Biophysical Research Communications. 2005;329(1):386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 50.Brighton C.A., Rievaj J., Kuhre R.E., Glass L.L., Schoonjans K., Holst J.J. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156(11):3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trabelsi M.S., Daoudi M., Prawitt J., Ducastel S., Touche V., Sayin S.I. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nature Communication. 2015;6:7629. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Habib A.M., Richards P., Cairns L.S., Rogers G.J., Bannon C.A., Parker H.E. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153(7):3054–3065. doi: 10.1210/en.2011-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egerod K.L., Engelstoft M.S., Grunddal K.V., Nohr M.K., Secher A., Sakata I. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795. doi: 10.1210/en.2012-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoetendal E.G., Raes J., van den Bogert B., Arumugam M., Booijink C.C., Troost F.J. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME Journal. 2012;6(7):1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson M.E., Phillipson M., Petersson J., Velcich A., Holm L., Hansson G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metabolism. 2013;17(2):225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Le Poul E., Loison C., Struyf S., Springael J.Y., Lannoy V., Decobecq M.E. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. The Journal of Biological Chemistry. 2003;278(28):25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 58.Yusta B., Baggio L.L., Koehler J., Holland D., Cao X., Pinnell L.J. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes. 2015;64(7):2537–2549. doi: 10.2337/db14-1577. [DOI] [PubMed] [Google Scholar]
- 59.Kamal N., Chami T., Andersen A., Rosell F.A., Schuster M.M., Whitehead W.E. Delayed gastrointestinal transit times in anorexia nervosa and bulimia nervosa. Gastroenterology. 1991;101(5):1320–1324. doi: 10.1016/0016-5085(91)90083-w. [DOI] [PubMed] [Google Scholar]
- 60.Germain N., Galusca B., Le Roux C.W., Bossu C., Ghatei M.A., Lang F. Constitutional thinness and lean anorexia nervosa display opposite concentrations of peptide YY, glucagon-like peptide 1, ghrelin, and leptin. American Journal of Clinical Nutrition. 2007;85(4):967–971. doi: 10.1093/ajcn/85.4.967. [DOI] [PubMed] [Google Scholar]
- 61.Ruttimann E.B., Arnold M., Hillebrand J.J., Geary N., Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deacon C.F., Johnsen A.H., Holst J.J. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. Journal of Clinical Endocrinology & Metabolism. 1995;80(3):952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- 63.Vilsboll T., Krarup T., Sonne J., Madsbad S., Volund A., Juul A.G. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 2003;88(6):2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 64.Baggio L.L., Drucker D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 65.D'Alessio D.A. What if gut hormones aren't really hormones: DPP-4 inhibition and local action of GLP-1 in the gastrointestinal tract. Endocrinology. 2011;152(8):2925–2926. doi: 10.1210/en.2011-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith E.P., An Z., Wagner C., Lewis A.G., Cohen E.B., Li B. The role of beta cell glucagon-like peptide-1 signaling in glucose regulation and response to diabetes drugs. Cell Metabolism. 2014;19(6):1050–1057. doi: 10.1016/j.cmet.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards P., Parker H.E., Adriaenssens A.E., Hodgson J.M., Cork S.C., Trapp S. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vahl T.P., Tauchi M., Durler T.S., Elfers E.E., Fernandes T.M., Bitner R.D. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148(10):4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 69.Sisley S., Gutierrez-Aguilar R., Scott M., D'Alessio D.A., Sandoval D.A., Seeley R.J. Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. Journal of Clinical Investigation. 2014;124(6):2456–2463. doi: 10.1172/JCI72434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lamont B.J., Li Y., Kwan E., Brown T.J., Gaisano H., Drucker D.J. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. Journal of Clinical Investigation. 2012;122(1):388–402. doi: 10.1172/JCI42497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eissele R., Goke R., Willemer S., Harthus H.P., Vermeer H., Arnold R. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. European Journal of Clinical Investigation. 1992;22(4):283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]