Abstract
Diffuse low grade gliomas (DLGG) are continuously progressive primary brain neoplasms that lead to neurological deficits and death. Treatment strategies are controversial. Randomized trials establishing the prognostic value of surgery do not exist. Here, we report the results of a nine-year near-randomized patient distribution between resection and biopsy. Until 2012, the Department of Neurosurgery and the Department of Stereotactic Neurosurgery at the University Medical Center Freiburg were organized as separate administrative units both coordinating DLGG patient treatment independently. All consecutive adult patients with a new diagnosis of DLGG by either stereotactic biopsy or resection were included. Pre- and post-operative tumor volumetry was performed. 126 patients, 87 men (69%), 39 women (31%), median age 41 years, were included. 77 (61%) were initially managed by biopsy, 49 (39%) by resection. A significant survival benefit was found for patients with an initial management by resection (5-year OS 82% vs. 54%). The survival benefit of patients with initial resection was reserved to patients with a residual tumor volume of less than 15 cm3. Maximum safe resection is the first therapy of choice in DLGG patients if a near-complete tumor removal can be achieved. Accurate prediction of the extent-of-resection is required for selection of surgical candidates.
Diffuse supratentorial low grade glioma in adults (DLGG) is a heterogenous neuroectodermal tumor entity accounting for about 15% of all glial brain neoplasms1. It is a chronic progressive disease of the central nervous system that, by infiltrative growth and malignant transformation, leads to neurological deficits and death. Since class I evidence for the benefit of any medical treatment does not exist, optimal management of DLGG is still controversial2,3,4,5,6,7,8,9. An increasing body of evidence suggests, that an early surgical intervention is superior compared to a “watch and wait” strategy8,10.
Different surgical strategies have been tried including stereotactic biopsy followed by brachytherapy, chemotherapy or external radiation or primary resection.
To date, no randomized controlled trial comparing surgical resection with biopsy has been conducted in patients with DLGG11. Given the growing evidence of the benefits of an early surgical intervention, it is unlikely that such a trial will ever be performed12,13. The closest approach to a patient randomization between biopsy and surgery originates from a retrospective population-based study comparing two Norwegian hospitals with different management strategies14. Herein, a significant survival benefit (74% vs. 60% 5-year OS, 68% vs. 44% 7-year OS) was found if the principal initial management strategy of patients with DLGG was surgery. Consequently and in line with present international guidelines8, surgical resection to the maximum safe extent at present is the suggested first-line therapy for DLGG.
A starting position comparable to that described by Jakola et al. historically existed at our center. Until 2012, patients with a first diagnosis of DLGG were treated and followed either by the department of Stereotactic Neurosurgery or the Department of Neurosurgery – both acting independently - at our university medical center. This situation led to a near-randomized distribution of patients with DLGG to initial management either by biopsy or surgical resection.
In the present study, the results of an early resective intervention are compared to a management with biopsy alone with special emphasis on a pre- and post-operative tumor volumetry.
Materials and Methods
The study design and methods were approved by the Ethics Committee of the University Medical Center Freiburg. The methods were carried out in accordance with the approved guidelines. The trial was registered with the German Clinical Trial Registry (DRKS, Unique identifier: DRKS00009264). All persons or their relatives gave their informed consent within written treatment contract on admission and therefore prior to their inclusion in the study.
Clinical Setting
Until 2012, the Department of Neurosurgery and the Department of Stereotactic Neurosurgery at the University Medical Center Freiburg were organized as two separate administrative units. Both Departments acted independently in terms of patient care. Patients with DLGG were allocated to either department by a near-randomized process resulting from random referral. Both departments followed their patients in separate outpatient clinics and independently decided on choices of adjuvant treatment strategies. The mean distance between the patients’ place of residence at diagnosis and our medical center was calculated using Google Maps (http://maps.google.com/maps, access: 01/2016) to test for comparability of the catchment area. Stereotactic and surgical samples were equally analyzed by the Department of Neuropathology, Medical Center – University of Freiburg.
Eligibility
All patients 18 years or older with a first histological diagnosis of a supratentorial DLGG (WHO°II) from either a stereotactic biopsy or a neurosurgical resection between 2004 and 2012 in the Department of Neurosurgery or the Department of Stereotactic and Functional Neurosurgery of the Medical Center – University of Freiburg, were included in the study. Patients with evidence of a gemistocytic histology were excluded due to the known aggressive clinical behavior of gemistocytic gliomas15,16. Patients with gliomatosis cerebri (defined as evidence of initial tumor infiltration of 3 or more lobes) were excluded.
Variables
Patient and treatment characteristics were retrieved from medical records (primary source). If patients received a surgical resection within 3 months after biopsy the initial management was classified as “resection”. Pre-operative MRI were available in 118 of 126 patients (94%) [FLAIR/T2w in 100 (79%), T1w in 18 (14%)]. Pre-operative tumor volumetry was based on computed tomography (CT) in the remaining 8 patients (6%). Contrast-enhanced pre-operative T1w MRI were available in 114 patients (90%). At least one follow-up MRI obtained within 6 months after resection was available in 45 patients (92% of patients with an initial resection). Post-operative tumor volumetry was based on CT in 4 patients (8%). Pre- and post-operative tumor volumetry was manually performed using the open source software OSIRIX (http://www.osirix-viewer.com).
Presence and severity of comorbidities were evaluated by the Charlson Comorbidity Index17. The Karnofsky Performance Score (KPS) was used to determine the functional status of the patients upon study inclusion. The items of the Pignatti-Score18 (i.e. age ≥ 40 years, tumor diameter ≥ 6 cm, tumor crossing midline, presence of neurological deficit, astrocytic histology) were included in the statistical analysis. Tumor location, initial contrast enhancement, maximum tumor diameter and eloquence were independently reviewed by two neurosurgeons (P.R., R.R.). Eloquence was graded according to the Sawaya-Score19. Post-interventional neurological deficits were dichotomized in transient (<6 months) or permanent (>6 months) deficits.
Follow-up and Statistical Analysis
Overall survival (OS) was the primary end point of the study. Survival was calculated from the date of the first histological diagnosis. Patients were followed until death or September 30, 2015. Six patients (5%) were lost to follow-up. They were censored at the date of the recorded last contact.
Univariate statistics were performed by log-rank test, Fisher exact test, Pearson χ2 test, Mann-Whitney-U test or one-way ANOVA (Kruskal-Wallis test with Dunns multiple comparison test), as appropriate. To determine independent predictors of patient survival significant variables from the initial univariate analyses were included in two multivariable cox-regression models. All patients were included in the first model. The second model assessed only patients with initial resection and included the new variable residual tumor volume (RTV). Insignificant variables were excluded by backward elimination in both models. The Pignatti-Score was excluded from the multivariable analysis despite being a significant variable for OS in univariate statistics as it represents a compound variable. All reported p-values were two sided, and p < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 5 (GraphPad Software, San Diego, USA) or Stata 13 statistical software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX, USA).
Results
Biopsy vs. Resection, Near-Randomisation Between Two Departments
Between 2004 and 2012, 126 patients with a first histological diagnosis of DLGG (excluding gemistocytic gliomas and gliomatosis cerebri) were surgically treated at the University Medical Center Freiburg. 77 patients were managed by the Department of Stereotactic Neurosurgery (hereafter referred to as “Biopsy” group) and initial management was stereotactic biopsy. 49 patients underwent an early surgical resection and were treated at the Department of Neurosurgery (hereafter referred to as “Resection” group). 3 patients received an initial biopsy and were resected within 3 months. These patients were included in the “Resection” group. The postal codes of patients at the date of diagnosis were available for 47 of 49 patients (96%) with initial resection and 75 of 77 (97%) with initial biopsy. The catchment area (i.e. the mean distance between the patients’ place of residence at diagnosis and our medical center) was comparable for both departments: Patients in the “Resection” group lived a mean 84.6 (±123.6) and patients in the “Biopsy” group a mean 90.9 (±104.4) kilometres from our centre (p = 0.36).
A dichotomized overview comparing patient, tumor and treatment characteristics of both patient cohorts is summarized in Table 1. Both groups were comparable for most variables. However, significant differences were found for the year of diagnosis (“Biopsy”: 2006 vs. “Resection”: 2008). The “Biopsy” cohort contained significantly more patients with an astrocytoma histology (57 vs 37%). The mean tumor volume was significantly larger (54 vs 75 cm3) in the “Resection” group. Biopsy patients were more likely to receive early radiotherapy (33 vs. 4%) or chemotherapy (29 vs. 10%). The median follow-up of “Biopsy” patients was 4.3 (IQR: 2.1–8.2) years and 5.6 (IQR: 4.0–8.9) years for “Resection” patients. By the end of the observation period, significantly more “Biopsy” patients had died (56% vs. 27%). Median OS was 6.7 years in the “Biopsy” group and not reached in the “Resection” group (Fig. 1). OS after 5 years was 82% for “Resection” patients compared to 54% with initial biopsy and 67% vs. 38% after 10 years. There was no surgical mortality. No post-operative deficits occurred in the “Biopsy” cohort. 7 patients (14%) with an initial management by resection developed a new post-operative deficit which was permanent (i.e. persisting >6 months) in 4 (8%).
Table 1. Comparison of Patient, Tumor and Treatment Characteristics in Both Departments.
| Initial Management |
Univariate Statistics | ||
|---|---|---|---|
| Biopsy | Resection | ||
| Patient characteristics | |||
| Number of patients | 77 | 49 | |
| Year of first diagnosis, (IQR), y | 2006 (2005–2008) | 2008 (2006–2011) | p = 0.0025 |
| Sex | p = 0.24 | ||
| Male | 50 (65%) | 37 (76%) | |
| Female | 27 (35% | 12 (25%) | |
| Age at diagnosis, median (IQR), y | 44 (35–60) | 39 (33–46) | p = 0.096 |
| Follow-up, median, (IQR), y | 4.3 (2.1–8.2) | 5.6 (4.0–8.9) | P = 0.066 |
| Status | χ2 (2, N = 126) = 10.4 p = 0.005 | ||
| Dead | 43 (56%) | 13 (27%) | |
| Alive | 31 (40%) | 33 (67%) | |
| Unknown | 3 (4%) | 3 (6%) | |
| Overall Survival, median, y | 6.7 | Not reached | p = 0.003 |
| 5-year survival rate (%) | 54% | 82% | |
| 10-year survival rate (%) | 38% | 69% | |
| Preoperative KPS, mean (SD), % | 90 (8.1) | 92 (8.6) | p = 0.08 |
| Charlson comorbidity index, mean (SD) | 0.32 (1.1) | 0.37 (0.8) | p = 0.19 |
| Initial symptoms | χ2 (3, N = 126) = 0.45 p = 0.93 | ||
| None (incidental) | 5 (6%) | 2 (4%) | |
| Seizure | 49 (64%) | 34 (69%) | |
| Headache | 10 (13%) | 6 (12%) | |
| Neurological deficit | 23 (30%) | 15 (31%) | |
| Pignatti score, mean (SD) | 1.8 (1.0) | 1.7 (1.3) | p = 0.39 |
| Tumor Characteristics | |||
| Histopathology | χ2 (2, N = 126) = 8.5 p = 0.014 | ||
| Astrocytoma WHO°II | 44 (57%) | 18 (37%) | |
| Oligoastrocytoma WHO°II | 27 (35%) | 19 (39%) | |
| Oligodendroglioma WHO°II | 6 (8%) | 12 (24%) | |
| Tumor size | |||
| Maximum diameter, mean, (SD), mm | 50 (19) | 58 (23) | p = 0.051 |
| Tumor volume, mean, (SD), mm3 | 54 (53) | 75 (61) | p = 0.045 |
| Eloquent location (Sawaya-Score) | χ2 (2, N = 126) = 3.2 p = 0.20 | ||
| 1 | 5 (6%) | 8 (16%) | |
| 2 | 46 (60%) | 25 (51%) | |
| 3 | 26 (34%) | 16 (33%) | |
| Bilateral tumor extension | 4 (5%) | 3 (6%) | p = 1.00 |
| Left Hemisphere | 45 (58%) | 25 (51%) | p = 0.45 |
| Preoperative contrast enhancement | 14/73§ (19%) | 13/41§ (32%) | p = 0.17 |
| Location of tumor | χ2 (4, N = 126) = 5.0 p = 0.29 | ||
| Frontal | 29 (38%) | 22 (45%) | |
| Temporal | 10 (13%) | 11 (22%) | |
| Parietal | 7 (9%) | 2 (4%) | |
| Insula | 20 (26%) | 11 (22%) | |
| Other | 11 (14%) | 3 (6%) | |
| Surgical Characteristics | |||
| Number of tumor resections | |||
| 0 (Biopsy only) | 55 (71%) | — | |
| 1 | 13 (17%) | 25 (51%) | χ2 (2, N = ) = 2.2 p = 0.34 |
| 2 | 6 (8%) | 21 (43%) | χ2 (2, N = ) = 2.2 p = 0.34 |
| >2 | 3 (4%) | 3 (6%) | |
| Post operative deficits† | 0 (0%) | 7 (14%) | p = 0.001 |
| Post-operative deficits >6 months | 0 (0%) | 4 (8%) | p = 0.02 |
| χ2 (4, N = ) = 13.2 p = 0.011 | |||
| Adjuvant Therapy | Available: 66 (86%) | Available: 49 (100%) | χ2 (4, N = ) = 14.9 p = 0.005 |
| None | 22 (33%) | 23 (47%) | |
| Early radiotherapy | 22 (33%) | 2 (4%) | |
| Ever radiotherapy | 37 (56%) | 21 (43%) | |
| Early chemotherapy | 19 (29%) | 5 (10%) | |
| Ever chemotherapy | 41 (62%) | 23 (47%) | |
§Number of available pre-operative contrast-enhanced MRI.
†Deficits after first intervention (biopsy or 1st surgery), permanent deficit was defined as a new deficit persisting >6 months.
Figure 1. Overall survival of patients with a first diagnosis of low grade glioma and initial management by either resection or biopsy.
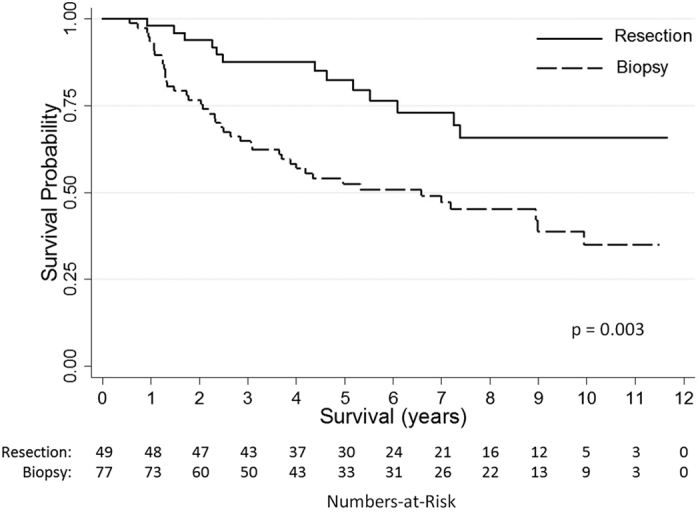
Both departments pursued different policies regarding the timing of adjuvant therapy. Patients initially managed by biopsy were more frequently referred to an early (within 6 months after diagnosis) radiotherapy (33% vs. 8%) or chemotherapy (30 vs. 11%) if the initial histological diagnosis was Astrocytoma (Supplementary Figure 1 and Table 1, see Adjuvant Therapy).
A new or worsened post-operative deficit was observed in 7 “Resection” patients (14%). Recovery was observed in 3 of these and 4 patients (8%) remained with a permanent deficit. No permanent deficits were observed among patients initially treated by biopsy.
Prognostic Factors for Overall Survival
In order to establish prognostic factors for a longer OS, patient data from both departments were pooled (Table 2). Patient, tumor and treatment characteristics that had a significant negative impact on OS in univariate analyses were higher age at diagnosis, higher Pignatti-Score, astrocytoma histology, a tumor location in the parietal lobe or otherwise not specified location (other, i.e.: 7 thalamic, 4 bilobar, 2 occipital, 1 basal ganglia), and initial management with biopsy. Early radiotherapy was also associated with a shorter OS. However, this treatment strategy was predominantly followed in patients with initial management by biopsy and astrocytoma histology, both being established risk factors for shorter OS (Supplementary Figure 1). “Early radiotherapy” was therefore excluded as a variable from further statistical analyses.
Table 2. Multivariate Analysis of Prognostic Factors.
| Overall Survival Analysis Failure Event: Death | LGG Patients | Univariate Statistics | Multivariate Cox-Regression |
|---|---|---|---|
| Patient characteristics | |||
| Number of patients | 126 | ||
| Year of first diagnosis, (IQR), y | 2007 (2005–2010) | ||
| Sex | p = 0.71 | ||
| Male | 87 (69%) | ||
| Female | 39 (31%) | ||
| Age at diagnosis, median (IQR), y | 41 (35–51) | p = 0.001 | p = 0.001 (95% CI: 1.01–1.05) HR: 1.03 (per year) |
| Follow-up, median (IQR), y | 4.9 (2.5–8.4) | ||
| Status | |||
| Dead | 56 (44%) | ||
| Alive | 64 (51%) | ||
| Lost to follow-up | 6 (5%) | ||
| Overall Survival, median, y | 9.1 | ||
| 5-year survival rate (%) | 65% | ||
| 10-year survival rate (%) | 49% | ||
| Preoperative KPS, mean (SD), % | 90.6 (8.3) | p = 0.12 | |
| Charlson comorbidity index, mean (SD) | 0.34 (1.0) | p = 0.76 | |
| Initial symptoms | |||
| None (incidental) | 7 (6%) | p = 0.61 | |
| Seizure | 83 (66%) | p = 0.61 | |
| Headache | 16 (13%) | p = 0.73 | |
| Neurological deficit | 38 (30%) | p = 0.22 | |
| Pignatti score, mean (SD) | 1.7 (1.1) | p < 0.001 | |
| Tumor Characteristics | |||
| Histopathology | |||
| Oligodendroglioma WHO°II | 18 (14%) | Reference | Reference |
| Oligoastrocytoma WHO°II | 46 (37%) | p = 0.098 | |
| Astrocytoma WHO°II | 62 (49%) | p = 0.002 | p = 0.010 (95% CI: 1.3–4.2) HR: 2.25 |
| Tumor size | |||
| Maximum diameter, mean, (SD), mm | 53 (21) | p = 0.85 | |
| Tumor volume, mean, (SD), mm3 | 62 (57) | p = 0.69 | |
| Eloquent location (Sawaya-Score) | p = 0.066 | ||
| 1 | 13 (10%) | ||
| 2 | 81 (64%) | ||
| 3 | 42 (33%) | ||
| Bilateral tumor extension | 7 (6%) | p = 0.29 | |
| Left Hemisphere | 70 (56%) | p = 0.40 | |
| Preoperative contrast enhancement | 27/114§ (24%) | p = 0.71 | |
| Location of tumor | |||
| Frontal | 51 (40%) | Reference | Reference |
| Temporal | 21 (17%) | p = 0.78 | p = 0.69 |
| Parietal | 9 (7%) | p = 0.006 | p = 0.05 |
| Insula | 31 (25%) | p = 0.21 | p = 0.64 |
| Other | 14 (11%) | p < 0.001 | p = 0.007 (95% CI: 1.4–7.9) HR: 3.3 |
| Initial management by biopsy | 77 (61%) | p = 0.0018 | p = 0.083 |
| Resection after initial management by biopsy | 22 (29%) | p = 0.67 | |
| Adjuvant Therapy | Available: 115 (91%) | ||
| None | 45 (39%) | p = 0.18 | |
| Early radiotherapy | 25 (22%) | p < 0.001 | |
| Ever radiotherapy | 58 (50%) | p = 0.11 | |
| Early chemotherapy | 24 (21%) | p = 0.91 | |
| Ever chemotherapy | 64 (56%) | p = 0.51 | |
§Number of available pre-operative contrast-enhanced MRI.
22 patients (29%) with an initial management with biopsy underwent a surgical resection after a mean time of 2.8 (±2.4) years. This, however, had no effect on OS. The median OS of patients who crossed over to a resection after an initial management by biopsy was 7.8 years vs. 5.0 years in patients who never underwent a resection (p = 0.67).
In a multivariable Cox-regression model statistical significance for OS was maintained for age at diagnosis (HR 1.03 per year), astrocytoma histology (HR 2.25) and otherwise not specified tumor location (HR 3.3).
Remarkably, the important impact of the initial management strategy (biopsy vs. resection) on OS (Fig. 1) was not maintained in the multivariable model. A comparison of both groups (“Resection” vs. “Biopsy”) regarding each histological subtype did not show significant differences of OS (Supplementary Figure 2). Consequently, factors other than the initial management strategy were essential determinants for survival differences between patients initially managed by biopsy or resection.
We therefore evaluated the residual tumor volume (RTV) in patients with an initial management by resection. A significant correlation between better OS and smaller RTV was found (Fig. 2). A dichotomization of these patients was performed according to the RTV (RTV < 15 cm3, RTV > 15 cm3).
Figure 2. Correlation between overall survival and residual tumor volume.
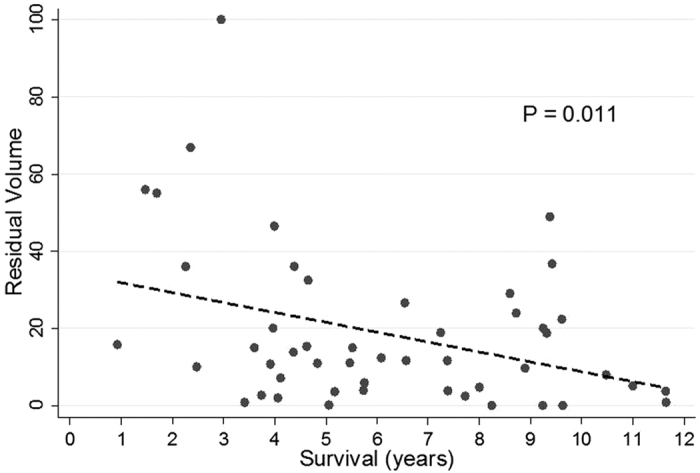
A multivariable Cox-regression model to assess significant factors for OS was applied to the patients with initial resection including the RTV (Table 3). Herein, RTV > 15 cm3 (HR 3.8) and astrocytoma histology (HR 5.4) were predictors of shorter survival. Age at diagnosis was not associated with inferior OS.
Table 3. Multivariate Analysis of Prognostic Factors in Patients with Initial Resection.
| Overall Survival Analysis Failure Event: Death | LGG Patients Resection | Multivariate Cox Regression |
|---|---|---|
| Number of patients | 49 | |
| Age at diagnosis, median (IQR), y | 39 (33–46) | p = 0.71 |
| Histopathology | ||
| Oligodendroglioma WHO°II | 12 (24%) | Reference |
| Oligoastrocytoma WHO°II | 19 (39%) | |
| Astrocytoma WHO°II | 18 (37%) | p = 0.005 (95% CI: 1.7–17.8) HR: 5.4 |
| Residual Tumor Volume | ||
| Residual tumor volume <15 cm3 | 27 (55%) | Reference |
| Residual tumor volume >15 cm3 | 22 (45%) | p = 0.034 (95% CI: 1.1–13.0) HR: 3.8 |
Based on this analysis the patients were re-grouped into three prognostically relevant Residual Tumor Volume groups (RTV < 15 cm3, >15 cm3 and Biopsy), (Supplementary Table 1). 21 of 27 patients (78%) with a RTV < 15 cm3 were alive at the end of follow up. The 5 and 10-year OS rate in this group was 82 and 69%, respectively. In comparison, OS did not differ significantly between patients managed by either biopsy or resection with a RTV > 15 cm3 (6.7 vs. 10.6 years median OS). 43 patients (56%) initially managed by biopsy and 12 patients (55%) with a RTV > 15 cm3 were dead by the end of follow-up (Fig. 3).
Figure 3. Overall survival of low grade glioma patients according to the residual tumor volume (RTV).
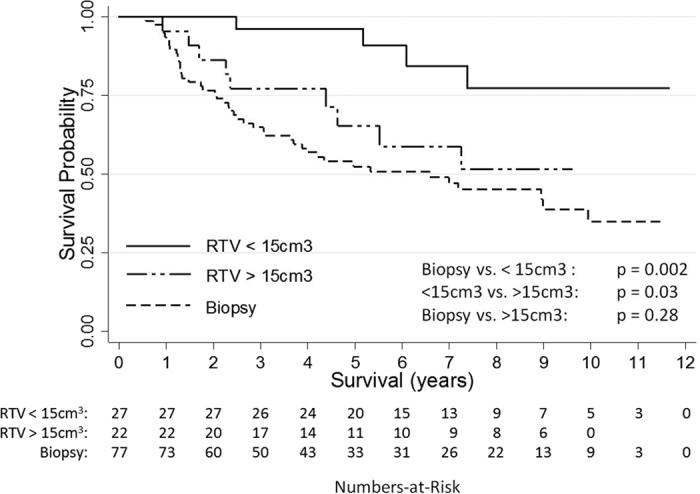
Overall survival of patients with a RTV of more than 15 cm3 did not have an improved prognosis compared to patients with an initial management by biopsy.
There was no surgical mortality. New post-operative deficits occurred in 5 patients from the RTV < 15 cm3 group (19%) and 2 patients from the RTV > 15 cm3 (9%). Permanent deficits were observed in 2 patients from both surgical groups (7% and 9%, respectively).
Notably, a large heterogeneity was found for patient, tumor and treatment characteristics between these three groups rendering a comparability questionable. Most impressively, the tumor volume of patients with a RTV > 15 cm3 was significantly bigger than of the “Biopsy” and RTV < 15 cm3 group. In order to answer the question whether the comparably poor OS of the RTV > 15 cm3 group was a result of selection bias, a group matching the characteristics of the RTV > 15 cm3 group was extracted from the “Biopsy” patients (Supplementary Table 2). No relevant difference in OS (9.1 vs 10.6 years) was observed between these groups (Supplementary Figure 3).
Discussion
In agreement with the closest approach to a randomization of patients with DLGG between surgery and biopsy reported by Jakola et al. survival analysis of patients initially managed by either biopsy or resection revealed a significantly better OS for patients managed by an early surgical intervention. The 5-year OS rate was 82% for “Resection” patients compared to 54% for “Biopsy” patients (67% vs. 38% after 10 years). We hypothesize that the survival benefit of the surgical group is even underestimated as patients initially managed by biopsy were more likely to receive an early adjuvant treatment (53% vs. 14%). A later surgical intervention after initial management by biopsy (as performed in 22 patients initially managed by biopsy after a mean 2.8 years) did not have an impact on OS corroborating the importance of the initial treatment choice.
A more differentiated analysis of our patient data, especially with respect to the pre- and post-operative tumor volume revealed however, that the OS benefit was exclusively reserved to patients in whom a resection with a RTV of less than 15 cm3 was achieved. While these patients had a good prognosis (77% 10-year OS) patients with a RTV of more than 15 cm3 did not fare better than patients managed by biopsy alone. This effect was maintained when the RTV > 15 cm3 cohort was compared to a subset of “Biopsy” patients that was matched for known prognostic factors.
In line with the pertinent literature but in contrast to the series of Jakola et al. initial management by stereotactic biopsy in our study was not associated with surgical morbidity20.
Interestingly, tumor size per se, previously validated as a strong prognostic factor in large patient series18,21, is not prognostic in a near-randomized setting between surgery and biopsy. We suggest that this finding further supports the prognostic value of an early surgical intervention, since a poor starting position provided by tumor characteristics (evaluated here: tumor size) can possibly be reversed by an aggressive surgical therapy.
In large patient cohorts, astrocytoma histology was associated with an inferior prognosis compared to mixed gliomas and oligodendrogliomas8,22,23. In the present study, a pure astrocytoma histology was significantly more frequently diagnosed in specimen obtained by biopsy compared to surgery (57% vs. 37%) potentially indicating a selection bias of prognostically poor patients to biopsy. We suggest, however, that this represents a sampling error due to the known limited representation of histological tumor features in biopsy specimen24,25,26,27. Per se, there is no unequivocal association between histological and molecular features and resectability of DLGG22,23. Further, the frequency of astrocytoma histology was balanced between patients with a RTV < 15 cm3 and RTV > 15 cm3 supporting this notion.
The precise delineation of a beneficial extent of resection or residual tumor volume remains a matter of debate. While the largest investigation addressing this aspect by Capelle et al. suggested a residual tumor volume of less than 10 cm3 to be the crucial factor for a good prognosis, in particular due to a delay of anaplastic transformation21, others have suggested different percentages of tumor removal/residual tumor that proved valuable28,29,30,31,32,33. In light of the present study, we clearly challenge the notion, that any surgical intervention improves patient survival in DLGG. Instead, a differentiated and personalized view is necessary for appropriate treatment decisions.
Form our data we would recommend performing surgery in patients if a resection with optimally none tumor residual or at least a residual volume in the range of up to a maximum of 15 cm3 can be achieved at an acceptable risk. In cases, where this maximal residual tumor volume is likely to be exceeded, a preferred strategy could be (stereotactic) biopsy followed by adjuvant therapy bearing a neoadjuvant approach with a resection after tumor volume reduction by chemotherapy in mind34,35.
Taken together, the data summarized here underscore the fact that most DLGG cannot be considered benign lesions unless a significant tumor volume reduction can be achieved. Adjuvant therapy is indicated in patients with poorly resectable DLGG. Furthermore, neurosurgeons are encouraged to continue the quest for an increased resectability of DLGG by improving surgical techniques while preserving function and quality of life at the same time29. Prediction of the attainable RTV and risk stratification at the individual level (surgeon and patient) needs to be refined to ameliorate treatment decisions36,37,38.
Limitations of the study
Our study is subject to the common constraints of a retrospective investigation. We limited the study to patients with a first diagnosis of DLGG. A histological sampling error cannot be ruled out as the diagnostic accuracy of biopsy specimen may be variable24,25,26,27. The greatest possible conformity of histological analyses is provided by the fact that all specimen were analyzed by the same neuropathology unit. Due to the retrospective nature of this study that included patients with a first diagnosis as early as 2004 molecular tumor characteristics were only available in part and therefore not included into the present analysis. A certain treatment selection bias has to be acknowledged as patients harboring larger tumors were more likely to receive a resection (mean tumor volume, “Resection”: 75 mm3 “Biopsy”: 54 mm3). In turn, patients with tumors within the basal ganglia and thalamus were more likely to be managed by biopsy. We did not exclude these patients as the a priori intention of the present study was to investigate the near-randomized patient distribution between a management by initial surgery or biopsy irrespective of tumor size or location. Patients initially managed by biopsy were more likely to receive early adjuvant therapy creating a certain heterogeneity of both groups.
Conclusion
According to our study, the treatment option of choice for patients with a first diagnosis of DLGG is a resection to the maximum possible extent. If a complete or near-complete tumor volume reduction is not feasible, surgery does not provide a survival benefit but exposes patients to the risk of neurological injury. In these cases, biopsy should be the initial management. Potentially, neoadjuvant chemotherapy may render tumors safely operable to a prognostically valuable extent. Further investigations are needed to precisely predict the attainable RTV to improve selection of surgical candidates.
Additional Information
How to cite this article: Roelz, R. et al. Residual Tumor Volume as Best Outcome Predictor in Low Grade Glioma – A Nine-Years Near-Randomized Survey of Surgery vs. Biopsy. Sci. Rep. 6, 32286; doi: 10.1038/srep32286 (2016).
Supplementary Material
Footnotes
Author Contributions R.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: R.R. and P.C.R. Acquisition of data: R.R., D.S. and P.C.R. Analysis and interpretation of data: R.R., P.C.R., D.S., R.J., R.K. and K.E. Drafting of the manuscript: R.R. and P.C.R. Critical revision of the manuscript for important intellectual content: R.J., K.E., A.W. and V.A.C. Statistical analysis: R.R. and R.K. Administrative, technical, or material support: None. Study supervision: P.C.R. and V.A.C. and A.W.
References
- Louis D. N. et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. (Berl.) 114, 97–109 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouratian N. & Schiff D. Management of low-grade glioma. Curr. Neurol. Neurosci. Rep. 10, 224–231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouratian N., Asthagiri A., Jagannathan J., Shaffrey M. E. & Schiff D. Surgery Insight: the role of surgery in the management of low-grade gliomas. Nat. Clin. Pract. Neurol. 3, 628–639 (2007). [DOI] [PubMed] [Google Scholar]
- Recht L. D. & Bernstein M. Low-grade gliomas. Neurol. Clin. 13, 847–859 (1995). [PubMed] [Google Scholar]
- Rees J. H. Low-grade gliomas in adults. Curr. Opin. Neurol. 15, 657–661 (2002). [DOI] [PubMed] [Google Scholar]
- Schiff D., Brown P. D. & Giannini C. Outcome in adult low-grade glioma: the impact of prognostic factors and treatment. Neurology 69, 1366–1373 (2007). [DOI] [PubMed] [Google Scholar]
- Shaw E. G. & Wisoff J. H. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro-Oncol. 5, 153–160 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffietti R. et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 17, 1124–1133 (2010). [DOI] [PubMed] [Google Scholar]
- Trautmann T. G. & Shaw E. G. Supratentorial low-grade glioma: is there a role for radiation therapy? Ann. Acad. Med. Singapore 25, 392–396 (1996). [PubMed] [Google Scholar]
- Duffau H. & Taillandier L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro-Oncol. 17, 332–342 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeravagu A. et al. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database Syst. Rev. 4, CD009319 (2013). [DOI] [PubMed] [Google Scholar]
- Duffau H. A new philosophy in surgery for diffuse low-grade glioma (DLGG): oncological and functional outcomes. Neurochirurgie. 59, 2–8 (2013). [DOI] [PubMed] [Google Scholar]
- Pallud J. & Duffau H. Is a prospective trial necessary to suggest a clinical relevance? Neuro-Oncol. 16, 1295–1296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakola A. S. et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308, 1881–1888 (2012). [DOI] [PubMed] [Google Scholar]
- Babu R., Bagley J. H., Park J. G., Friedman A. H. & Adamson C. Low-grade astrocytomas: the prognostic value of fibrillary, gemistocytic, and protoplasmic tumor histology. J. Neurosurg. 119, 434–441 (2013). [DOI] [PubMed] [Google Scholar]
- Jung T.-Y. et al. Early prognostic factors related to progression and malignant transformation of low-grade gliomas. Clin. Neurol. Neurosurg. 113, 752–757 (2011). [DOI] [PubMed] [Google Scholar]
- Charlson M. E., Pompei P., Ales K. L. & MacKenzie C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- Pignatti F. et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 20, 2076–2084 (2002). [DOI] [PubMed] [Google Scholar]
- Sawaya R. et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 42, 1044–1055, discussion 1055–1056 (1998). [DOI] [PubMed] [Google Scholar]
- Shakal A. A. S. & Mokbel E. A. H. Hemorrhage after stereotactic biopsy from intra-axial brain lesions: incidence and avoidance. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 75, 177–182 (2014). [DOI] [PubMed] [Google Scholar]
- Capelle L. et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases: clinical article. J. Neurosurg. 118, 1157–1168 (2013). [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 372, 2481–2498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Passow J. E. et al. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N. Engl. J. Med. 372, 2499–2508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod S. et al. Molecular stereotactic biopsy technique improves diagnostic accuracy and enables personalized treatment strategies in glioma patients. Acta Neurochir. (Wien) 156, 1427–1440 (2014). [DOI] [PubMed] [Google Scholar]
- Jackson R. J. et al. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-Oncol. 3, 193–200 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirt M. J., Villavicencio A. T., Bulsara K. R. & Friedman A. H. MRI-guided stereotactic biopsy in the diagnosis of glioma: comparison of biopsy and surgical resection specimen. Surg. Neurol. 59, 277–281, discussion 281–282 (2003). [DOI] [PubMed] [Google Scholar]
- Woodworth G. et al. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurol. Res. 27, 358–362 (2005). [DOI] [PubMed] [Google Scholar]
- Gousias K., Schramm J. & Simon M. Extent of resection and survival in supratentorial infiltrative low-grade gliomas: analysis of and adjustment for treatment bias. Acta Neurochir. (Wien) 156, 327–337 (2014). [DOI] [PubMed] [Google Scholar]
- Hervey-Jumper S. L. & Berger M. S. Role of surgical resection in low- and high-grade gliomas. Curr. Treat. Options Neurol. 16, 284 (2014). [DOI] [PubMed] [Google Scholar]
- Majchrzak K. et al. The assessment of prognostic factors in surgical treatment of low-grade gliomas: a prospective study. Clin. Neurol. Neurosurg. 114, 1135–1144 (2012). [DOI] [PubMed] [Google Scholar]
- Sanai N. & Berger M. S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 62, 753–764, discussion 264–266 (2008). [DOI] [PubMed] [Google Scholar]
- Smith J. S. et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 26, 1338–1345 (2008). [DOI] [PubMed] [Google Scholar]
- Snyder L. A. et al. The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J. Neurosurg. 120, 309–314 (2014). [DOI] [PubMed] [Google Scholar]
- Blonski M. et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J. Neurooncol. 106, 353–366 (2012). [DOI] [PubMed] [Google Scholar]
- Blonski M. et al. Neoadjuvant chemotherapy may optimize the extent of resection of World Health Organization grade II gliomas: a case series of 17 patients. J. Neurooncol. 113, 267–275 (2013). [DOI] [PubMed] [Google Scholar]
- Cordier D. et al. A better surgical resectability of WHO grade II gliomas is independent of favorable molecular markers. J. Neurooncol. 121, 185–193 (2015). [DOI] [PubMed] [Google Scholar]
- Cordier D., Schädelin S. & Duffau H. Influence of 1p19q status and Ki67 index to predict extent of resection in WHO grade II gliomas: a virtual patient model. J. Neurooncol. 123, 317–318 (2015). [DOI] [PubMed] [Google Scholar]
- Mandonnet E. et al. Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro-Oncol. 9, 63–69 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


