Abstract
Neglecting tree size and stand structure dynamics might bias the interpretation of the diversity-productivity relationship in forests. Here we show evidence that complementarity is contingent on tree size across large-scale climatic gradients in Europe. We compiled growth data of the 14 most dominant tree species in 32,628 permanent plots covering boreal, temperate and Mediterranean forest biomes. Niche complementarity is expected to result in significant growth increments of trees surrounded by a larger proportion of functionally dissimilar neighbours. Functional dissimilarity at the tree level was assessed using four functional types: i.e. broad-leaved deciduous, broad-leaved evergreen, needle-leaved deciduous and needle-leaved evergreen. Using Linear Mixed Models we show that, complementarity effects depend on tree size along an energy availability gradient across Europe. Specifically: (i) complementarity effects at low and intermediate positions of the gradient (coldest-temperate areas) were stronger for small than for large trees; (ii) in contrast, at the upper end of the gradient (warmer regions), complementarity is more widespread in larger than smaller trees, which in turn showed negative growth responses to increased functional dissimilarity. Our findings suggest that the outcome of species mixing on stand productivity might critically depend on individual size distribution structure along gradients of environmental variation.
Growing evidence supporting a causal relationship between increased tree functional diversity and above-ground wood production1,2,3,4 reflects a major role of biodiversity on ecosystem functioning in forests5,6,7,8. Forests cover almost one third of the emerged lands and represent strategic resources for human societies so current species loss rates raise major challenges for human well being on Earth9. Consequences of species loss on forest productivity are however uncertain since diversity effects and the interactions with associated abiotic and biotic determinants are still poorly understood10. In particular, the role of population and individual tree level features such as size or age on the diversity-productivity relationship remain unclear11.
Classical experiments, mostly conducted on short-lived communities (i.e. grasslands, arthropod communities and microbial microcosms), point to niche complementarity, and the greater potential for species packing, as a chief mechanism by which the productivity of species mixtures is enhanced compared to the respective monocultures12 (i.e. overyielding). In forests, empirical evidence of a more intense exploitation of above-13 and below-ground resources14 in species mixtures also suggests a major role of niche complementarity. However, recent studies based on the use of different diversity metrics2,7,15 and modelling experiments16 are inconclusive as to where and when niche complementarity is a relevant mechanism driving diversity-productivity relations17.
To understand the functioning of long-lived communities an appreciation of their size structure dynamics is essential18. Trees have a continuous size development, which modulates their growth19, stand productivity20 and within-community interactions21. A different size implies niche differentiation (both between conspecifics or heterospecifics22) for resource uptake above- and belowground and so it could determine idiosyncratic growth responses along climatic and structural gradients23,24 thus driving population and community dynamics via tree-tree interactions25. Experimental evidence from tropical forest plantations has demonstrated that if biodiversity effects can be reduced to a neighbourhood effect, responses to biodiversity will result from the aggregated effects of local neighbourhoods within plots26. Accordingly, a tree-level approach may allow us to properly evaluate complementarity by accounting for individual tree attributes that can be critical in their response to the local environment27. In situations where strong interspecific competition is mostly established aboveground, size stratification combined with disparate light use strategies can lead to improved light-use efficiency and thus increased biomass packing and productivity13. In support to this idea, recent literature shows how tree size inequality in mixed communities enhances light-resource-use efficiency thus promoting positive diversity-productivity relationships through increased above-ground biomass packing28,29. In contrast, tree size inequality in monospecific forests has been shown to exert net negative effects on stand productivity due to strong intraspecific asymmetric competition30. Thus, size-structure dynamics and shifting tree-tree interactions during secondary succession are expected to alter the biodiversity-ecosystem functioning relationship31,32,33. Unfortunately, classical experiments are unsuitable for addressing long-term processes related with community size-structure dynamics at broad spatial scales34,35, and forest experiments are still at an early stage to provide solid evidence of mechanisms underpinning long-term diversity effects36.
Here, we investigated whether complementarity effects are contingent on tree size across the main climatic gradient of Europe from southern Spain to northern Scandinavian forests. Niche complementarity at the tree level would imply improved individual tree growth rates when growing in neighbourhoods surrounded by a larger proportion of functionally dissimilar neighbours. Although tree growth does not necessarily correlate in a one-to-one relationship with stand productivity, forest ecosystem production can, as a first approximation, be evaluated as the collective sum of individual tree productivity as individual tree growth plays a pivotal role in ecosystem functioning19. Specifically, we assessed for each focal tree the stand relative abundance (estimated as the proportion of the stand basal area) of functionally dissimilar tree species (POFT) (i.e. broad-leaved deciduous, broad-leaved evergreen, needle-leaved deciduous, needle-leaved-evergreen). Thereafter, we regressed focal trees growth against interactive effects of abundance of functionally dissimilar neighbours (POFT), focal tree size (SIZE), stand basal area (SBA) and potential evapotranspiration (PET). We evaluated whether positive growth responses to increased neighbourhood dissimilarity are modulated by tree size across stand structure (i.e. SBA as a proxy of the intensity of interactions) and climatic gradients (e.g. PET as a proxy of available energy). We expect that, in absence of strong belowground limitations, size asymmetric competition would favour complementarity effects according to size stratification of early- and late-successional species during secondary succession13,32. Large trees, in turn, may or may not experience significant positive effects from species mixing depending on whether belowground interactions result in facilitation37 or competition38. Recent findings in temperate forests suggest, in accordance with predictions of the Stress Gradient Hypothesis, that increasing frequency of positive plant-plant interactions due to enhanced stressed conditions might play a key role in driving interspecific interactions and thus higher biodiversity might lead to increased productivity37,39. Nonetheless, positive interactions represent only a fraction of complementarity effects and so positive plant-plant interactions might be obscured by pervasive interspecific competition even in less productive environments40.
Results
The backward selection of predictor variables in the Linear Mixed Model suggested that one out of the four possible three-variable interactions (i.e. SIZE × POFT × PET) and two pairwise interactions (e.g. SIZE × SBA and SBA × PET) should be included in the fixed effects model (Table 1, see also parameter estimates in Appendix A Table A1). A random term with two components was included in the best supported model: species was included to affect the intercept and the slopes associated with POFT and SIZE; and plots nested in countries was included to affect the intercept parameter and the main effects of POFT (see random term selection based on information criterion in Table B1, Appendix B). Interestingly, the effects of SBA were not affected by the random structure ‘plots nested in countries’. The analysis of residuals indicates that the main assumptions of linear models were met (see Fig. A1a,b,c in Appendix A), and the pseudo-R2 pointed to a high goodness of fit, with more than 70% of variance explained by the final model (see Table 1).
Table 1. Fixed effects selection using the Bayesian Information Criterion (BIC).
| Fixed effects selection | df | BIC | Delta BIC | R2-m | R2-c |
|---|---|---|---|---|---|
| Full-model (round #1) | 33 | 610912.3 | 0 | ||
| SIZE × SBA × POFT (removed) | 32 | 610889.8 | −12.5 | ||
| SIZE × POFT × PET (removed) | 31 | 610937.1 | 24.8 | ||
| POFT × SBA × PET (removed) | 31 | 610900.8 | −11.5 | ||
| SIZE × SBA × PET (removed) | 31 | 610914.1 | 1.8 | ||
| Full-model (round #2)* | 28 | 610891.0 | 0 | ||
| SIZE × SBA (removed) | 27 | 614430.1 | 3539.1 | ||
| POFT × SBA (removed) | 27 | 610885.3 | −5.7 | ||
| PET × SBA (removed) | 26 | 611043.7 | 152.7 | ||
| Best-supported model** | 23 | 610885.3 | 0 | 0.367 | 0.705 |
| null model (intercept only) | 6 | 719146.1 | 108260.8 |
We used a hierarchical backward selection of fixed terms starting with a full model that included all the possible three-variable interactions between the proportion of other functional types (POFT), tree basal area (SIZE, m2), stand basal area (SBA, m2 ha−1), and potential evapotranspiration (PET, mm). We tested the contribution of each interaction by removing them one at a time. R2-m is the marginal pseudo R2 (fixed effects only) and R2-c is the conditional pseudo R2 (including both fixed and conditional effects).
*The round#2 of the backward selection started with the best supported model obtained in the first round.
**Best supported model: SIZE × POFT × PET+SIZE × SBA + PET × SBA.
Firstly, variable selection pointed to significant interactive effects of PET × SBA and SIZE × SBA. Negative effects of SBA were pervasive along the entire PET gradient (Fig. 1) although they were notably stronger in smaller trees. Interestingly, the effects of POFT on tree growth were independent of SBA as shown by the results of the backward selection round#2, where the interaction SBA × POFT was finally discarded following the Bayesian information criterion (see Table 1).
Figure 1. Predicted tree growth as function of potential evapotranspiration and stand basal area keeping size and the proportion of other functional types constant in mean values.
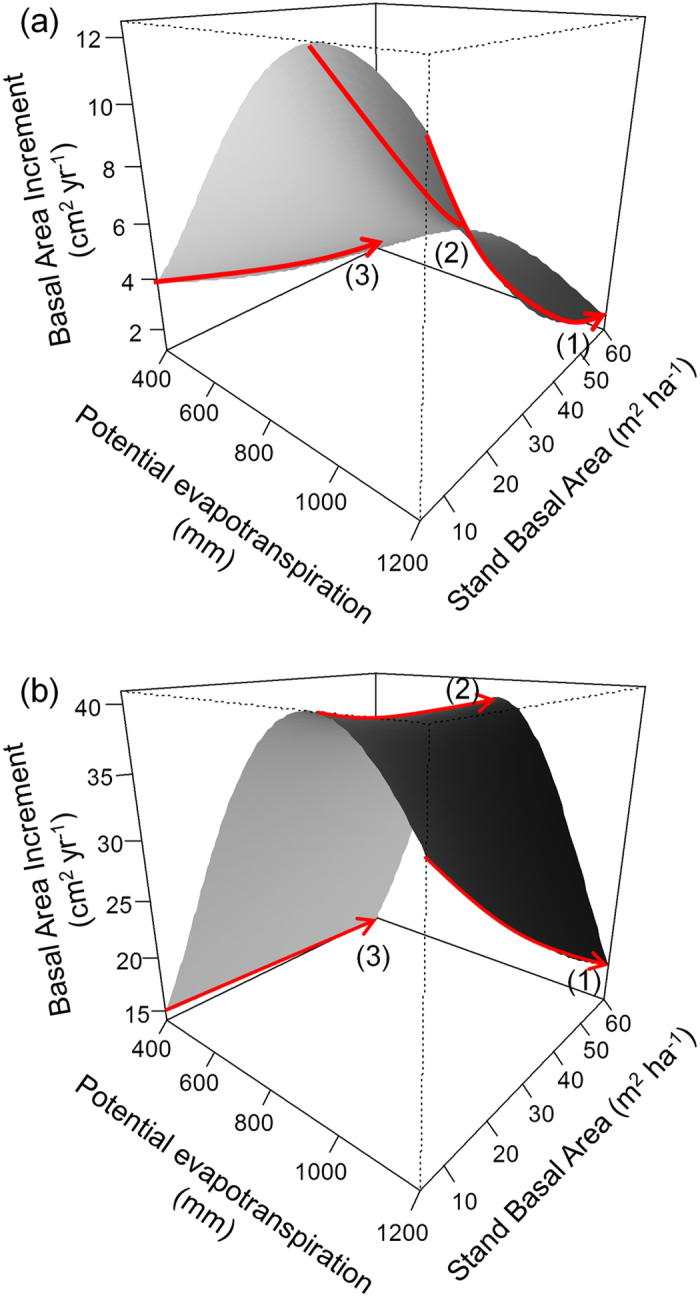
Red arrows indicate the main trends of tree growth at low, intermediate and high PET values.
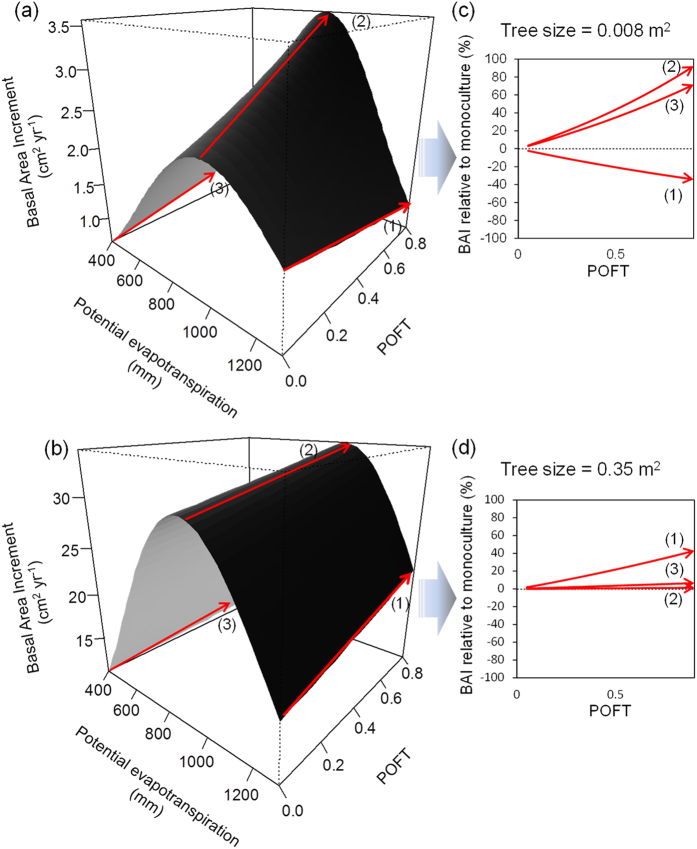
Secondly, we found that POFT effects on tree growth depend on tree size and the intensity and sign of this dependency change along the PET gradient. In particular, in areas of low PET (≈400–500 mm, boreal and alpine biomes), our results indicate complementarity effects on tree growth of small and large trees (Fig. 2a3,b3). Specifically, small focal trees (e.g. 10 cm DBH) surrounded by neighborhoods composed of 50% of other functional types can grow 44.4% more than similar focal trees from monospecific stands. A similar POFT scenario in large trees (e.g. 66 cm DBH) predicted tree growth rates to be only 0.68% higher in mixtures than monospecific stands. Under moderate PET values (i.e. 700–800 mm) the pattern is overall similar to that of boreal areas: i.e. complementarity tended to decrease notably towards sized trees which only grow 3.7% more when surrounded by neighborhoods composed of 50% of other functional types (see Fig. 2a2,b2). In similar conditions, smaller trees grew up to 37% more in mixtures than monospecific stands. Finally, growth responses to a greater POFT in Mediterranean latitudes shifted from net negative to positive as tree size increased (see Fig. 2a1,b1). Thus, a neighborhood composed of 50% of other functional types determines growth rates increments up to 21% compared to monospecific stands in large trees and growth reductions of 24% in small trees.
Figure 2.
Predicted tree growth as function of potential evapotranspiration and the proportion of other functional types for small (a) and large trees (b) (basal area 0.008 m2 and 0.35 m2 respectively) keeping stand basal area in the mean value (25 m2 ha−1). Plots on the right represent predicted growth relative to growth in monoculture (%) for small (c) and large trees (d) keeping stand basal area in the mean value (25 m2 ha−1). Red arrows are indicative of the main trends of tree growth along low, intermediate and high PET values.
Discussion
Tree size and complementarity effects
We found tree size to be a critical factor driving complementarity effects in forests across Europe. This agrees with our expectations associated limiting resources along the large-scale PET gradient. Hence, complementarity effects can emerge from niche separation along spatial/temporal gradients of resource availability and thus tree size plays a pivotal role in determining resource use partitioning. In space, the combination of different light intercepting strategies and size stratification have been reported to significantly enhance resource yielding and biomass packing in mixed forest stands41,42. Partitioning of soil nutrient uptake through different degrees of fine root development can also trigger overyielding among dissimilar tree individuals although this poses major methodological challenges and uncertainties to identify43. Evidence from grassland studies suggests that interspecific differences in leaf phenology might determine temporal segregation of light capture and thus species coexistence44. Decoupled light use patterns associated with a different duration of the growing periods between young and old trees45,46,47 might explain size-dependent overyielding in species mixtures. This is the case of young evergreen trees in boreal-temperate forests where a seasonal decoupling of photosynthetic activity with adult broad-leaved trees might lead to complementary usage of light and thus coexistence48. Thus, differences in Specific Leaf Area, crown architecture and leaf phenology, and the subsequent light use strategies play a pivotal role in forest dynamics and tree development49,50,51. Projected onto a secondary succession scenario in temperate latitudes, species mixing is common in transitional stages when adult individuals of early succesional tree species coincide with juvenile individuals of late-succesional species and thus size stratification allows for biomass packing and overyielding.
Tree size effects on complementarity across the energy availability gradient
In boreo-alpine forests (400–500 mm) our results indicated that strong complementarity effects on small individuals notably weakens with size development. Complementarity in nitrogen uptake43 and bidirectional transferences of soil carbohydrates via mycorrhiza52 have been reported as important mechanisms underpinning the positive diversity-productivity relationship in boreal forests. Interestingly, Cavard and cols53 showed how positive diversity-growth relationships in species mixtures disappear as tree communities mature. Soil nutrient dilapidation during post-fire secondary succession was invoked a plausible explanation for this reduction of complementarity effects in time. Recent findings in Europe suggest a comparatively minor role of functional dispersion on stand growth towards northern latitudes15 which is consistent with this successional scheme if we assume that larger trees have an exponentially higher contribution with stand productivity than smaller ones and thus functional responses of tree communities mostly resemble large-tree responses. Otherwise, a positive diversity-productivity relationship has been extensively reported in European and North American boreal forests in support of the idea that complementarity effects, although comparatively less important than other drivers of tree growth in these latitudes, are important determinants of forest productivity as well.
In temperate forests, complementarity effects depict a similar pattern to the one observed in boreal forests: e.g. strong complementarity effects decrease with tree development. Young broad-leaved deciduous trees are known to benefit from higher light availability when part of the forest overstorey is occupied by early successional conifers32. A recent review, however, highlighted the great number of potential factors that might alter the relative role of niche complementarity driving tree growth in temperate forests54. Moreover, results obtained from permanent plots in central Europe suggest that the lack of overyielding in temperate forests might be attributed to methodological factors associated with specific response variables and methods to compare pure and mixed stands27. In particular, accounting for crown biomass changes has been found to be decisive in detecting overyielding in temperate forests. Thus, forest inventory data may be limited to discern such fine-scale diversity effects.
In Mediterranean forests, our results point to strong complementarity effects in large trees, and contrary, negative effects of functional dissimilarity in small trees. Assuming that larger trees have a comparatively higher contribution to total stand biomass and stand basal area change, this finding is consistent with the existing literature posing significant positive diversity-productivity relationships in Mediterranean forests3,7. Complementarity effects in large trees have been associated with disparate light-use strategies and increased light-use efficiency3 and increased soil fertility55 due to enhanced litter quality. A positive diversity-productivity relationship in water-limited ecosystems has been also discussed in the theoretical framework of the Stress Gradient Hypothesis: e.g. increased abiotic stress conditions lead to increasing frequency and intensity of positive interspecific interactions37. Alternatively, insights from radial growth increments in mixed and monospecific pine-oak stands in Central Spain are consistent with the idea that stressed conditions due to soil water scarcity during intense droughts leave less room for complementarity effects in the Mediterranean38. Yet our results do not allow us to discern which mechanisms underlie the observed reversal, lacking complementarity effects in small trees could be attributed to inevitable trade-offs associated with adaptations to cope simultaneously with shade and drought56. Ecological studies on plant-plant facilitation in arid lands during the last two decades suggest that shade imposes an investment in aboveground parts (e.g. leaves) to increase light interception in the understory at the cost of exacerbated water losses due to higher shoot to root ratio57. Hence, size stratification is not an efficient way to optimize light interception, as in other biomes, because high evapotranspiration regimes expose understory tree individuals to water stress. It is important to note that in warmer Mediterranean areas, the dominant functional types are broad-leaved evergreen tree species and needle-leaved evergreen tree species. This implies that seasonal decoupling of leaf phenology is no longer a plausible complementarity mechanism in time so young/small conifer trees must overcome strong competition for light with adult evergreen broad-leaved trees. On the other hand, many species are resprouters and many of the smaller individuals are ramets which have better developed root systems and carbohydrate storages relative to suppressed saplings in pine stands. Complementarity among adult trees, in contrast, is less dependent on functional trade-offs associated with light and soil water limitations and disparate water-use strategies or root architecture may ameliorate intraspecific competition for soil water.
Concluding remarks and future lines
Neglecting tree size and stand structure and assessing stand growth as an aggregated tree growth measurement might bias the interpretation of the diversity-productivity relationship in forests29 because growth responses of larger trees override those of smaller trees given the exponential relationship between growth and size19. Positive effects of tree diversity on stand growth in the Mediterranean3 and almost neutral-positive effects in temperate and boreal forests14,15 would mask the actual contribution of each individual tree. Thus, it is critical to consider tree size to properly evaluate diversity effects on tree growth throughout the course of tree development in different forest types across climatic gradients29,54,58. Future research efforts are needed to understand ecophysiological processes underpinning large-scale reversals in complementarity due to tree size. It is critical to understand how individual tree features such as size determine tree-tree interactions and how they influence secondary succession dynamics and ecosystem function. This would allow us to better understand the role of positive and negative interactions on size-structured community dynamics, and to establish more efficient conservation and management strategies based on community structure and species interactions rather than on individual species.
Our broad scale approach, however, lacks information on potential drivers of productivity such as soil nutrients, herbivory, disturbances and slight deviations from long-term climatic conditions at each sampling period might affect our findings since all these uncertainties might alter the diversity-productivity relationship. Given the impossibility of long-term experiments for exploring biodiversity-ecosystem functioning relationships in forests (at least for some years to come), we rely on improved large-scale data analyses and modelling. Therefore, further efforts are needed to improve forest inventory protocols and large-scale data harmonization to account for potential confounding drivers of productivity such as soil information, management, secondary succession and herbivory in forest ecosystems.
Methods
National forest inventory data: plot selection and focal species
We used data compiled from the National Forest Inventories (NFIs) of five European countries (Finland, Germany, Spain, Sweden and Belgium -region of Wallonia, see a detailed description of each NFI in Ratcliffe and cols15). Permanent plots with two consecutive surveys and no evidence of tree harvesting before and within consecutive surveys were selected. From those plots, only trees >= 10 cm diameter at breast height (DBH) were included in the dataset to standardise tree selection between the different inventories. The 14 most representative tree species in terms of abundance and distribution were selected as focal trees: Abies alba (needle-leaved evergreen, temperate mountains), Acer pseudoplatanus (broad-leaved deciduous, temperate), Betula pendula (broad-leaved deciduous, boreal-temperate), Betula pubescens (broad-leaved deciduous, boreal-temperate), Carpinus betulus (broad-leaved deciduous, temperate), Castanea sativa (broad-leaved deciduous, temperate), Fagus sylvativa (broad-leaved deciduous, temperate), Juniperus thurifera (needle-leaved evergreen, Mediterranean mountains), Picea abies (needle-leaved evergreen, boreal-alpine), Pinus halepensis (needle-leaved evergreen, Mediterranean), Pinus sylvestris (needle-leaved evergreen, boreal-alpine-temperate), Quercus pyrenaica (broad-leaved deciduous, Mediterranean-temperate transition), Quercus robur (broad-leaved deciduous, temperate), and Quercus ilex (broad-leaved evergreen, Mediterranean). These species represent more than one third of the total stand basal area measured in the whole dataset and are the dominant tree species in at least one of the three principal forest biomes of Europe (i.e. Mediterranean, temperate and boreal). The final number of plots was 32,628 and the number of surviving trees 275,558 after removing dead trees or surviving trees with negative growth rates (7209 trees, 2.6%). Table 2 details the number of plots in the analysis and important plot-level statistics. Figure 3a illustrates the distribution of plots across the continent.
Table 2. Descriptive statistics of plot-level information for each National Forest Inventory (NFI) for the randomly selected populations of trees across Europe.
| Spain | Wallonia | Germany | Sweden | Finland | Total | |
|---|---|---|---|---|---|---|
| No. plots | 18 083 | 90 | 7 649 | 4 621 | 1 619 | 32 628 |
| No. trees | 146 799 | 668 | 38 620 | 48 902 | 15 888 | 275 558 |
| No. species | 11 | 4 | 9 | 5 | 6 | 14 |
| PET (±SD) | 1018.9 ± 163.2 | 715.8 ± 42.1 | 732.9 ± 49.1 | 508.8 ± 57.9 | 505.6 ± 42.5 | 812.2 ± 241.7 |
| SBA (±SD) | 9.4 ± 9.5 | 23.3 ± 12.5 | 25.6 ± 14.8 | 12.6 ± 10.7 | 10.3 ± 8.5 | 20.131 ± 13.0 |
| MTBA (±SD) | 0.06 ± 0.08 | 0.15 ± 0.18 | 0.09 ± 0.09 | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.06 ± 0.08 |
Species ID (alphabetical order): Abies alba (1), Acer pseudoplatanus (2), Betula pendula (3), Betula pubescens (4), Carpinus betulus (5), Castanea sativa (6), Fagus sylvativa (7), Juniperus thurifera (8), Picea abies (9), Pinus halepensis (10), Pinus sylvestris (11), Quercus ilex (12), Quercus pyrenaica (13), Quercus robur (14). PET–Potential Evapotranspiration (mm); SBA–Stand Basal Area (m2 ha−1); MTBA– Mean Tree Basal Area (m2).
Figure 3.
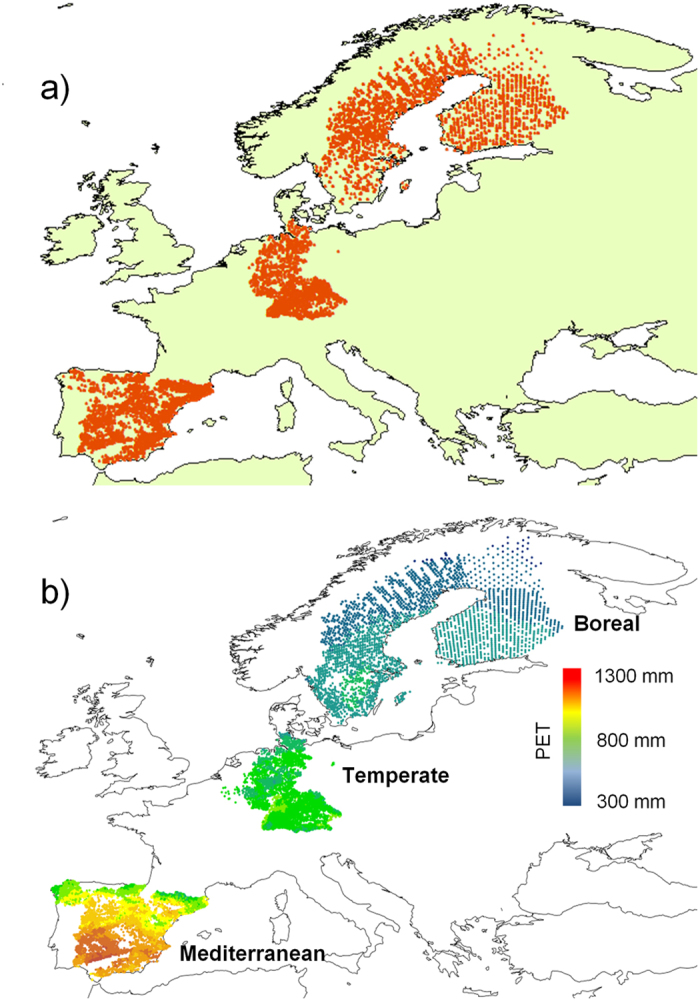
(a) Location of forest inventory plots across the study area (Europe) and (b) potential evapotranspiration (units) throughout the study area. Own preparation based on (a) plot coordinates included in the National Forest Inventories considered, and (b) Potential Evapotranspiration data available in CGIAR-CSI GeoPortal60 using software ArcGIS 13.0 by Esri (license University of Alcalá).
Tree growth and complementarity
We assessed individual tree growth as the basal area increment (BAI) relative to the initial tree basal area and the number of years between consecutive surveys (i.e. annual basal area increment). Tree basal area in each inventory was calculated using dbh measurements as:
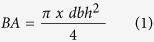 |
where dbh is the diameter at breast height (i.e. 1.3 meters above ground surface) in metres.
Complementarity effects (CE) at the tree level imply that any given tree will grow faster when, keeping biotic and abiotic sources of variability constant, its neighborhood is composed of a larger proportion of functionally dissimilar trees. With this in mind we assessed the proportion of the stand basal area (SBA m2 ha−1) occupied by functional types other than the focal tree (POFT) as follows:
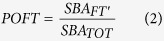 |
where SBAFT′ is the stand basal area of functional types different from the focal tree and SBATOT is the total stand basal area. Positive effects of this index on tree growth will be interpreted as complementarity effects while negative effects will be assumed prevailing interspecific competition. Yet this is of course an elementary approach to the complex world of niche complementarity metrics, its quantitative nature meets the rationale of the aforementioned definition of complementarity from the viewpoint of individual trees. We considered the four most general and divergent functional types regarding leaf phenology and morphology (i.e. broad-leaved deciduous, broad –leaved evergreen, needle-leaved deciduous, needle-leaved evergreen). Specific leaf area has been identified as a critical trait linked to species competitive ability (i.e. broad-leaved species tend to be more tolerant to competition and exhibit stronger competitive effects relative to needle-leaved species49). This classification is also coherent with functional differentiation based on wood density as needle-leaved trees tend to have lower wood density than broad-leaved trees and weaker competitive effects49. In general, differences in crown architecture, leaf phenology, photosynthetic activity, and root architecture between these general functional types have been shown to provide overyielding in boreal and temperate forests14,41,42.
Abiotic and biotic drivers of tree growth
For each individual tree we noted the species identity and calculated the basal area of the tree (SIZE, mm) and the plot basal area (SBA, m2 ha−1) in which the tree resided. Mean climate (i.e. annual mean temperature (MAT, C), annual precipitation (AP, mm) and mean potential evapotranspiration (PET, mm)) for each plot were extracted from the WorldClim database59 and CGIAR-CSI GeoPortal60. Using PET and AP we assessed a water availability index (WAI) as:
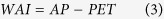 |
where negative values of this index denote water deficit because atmospheric water demand exceeds climatic inputs in rainfall. Because of the high correlation between PET and MAT (r = 0.93) and between PET and WAI (r = −0.71), we selected PET as an integrative variable that summarizes the two main climatic stress gradients in continental Europe, i.e. water deficit towards southern latitudes and low temperature towards northern latitudes and alpine areas (Fig. 3b).
Data analysis
We fitted Linear Mixed Models (LMM) to model tree growth as function of SIZE, SBA, PET and POFT. Plot nested in country and species were included as two independent random terms in the model. Species was considered to affect the intercept parameter and the slopes associated with main effects of both POFT and SIZE under the assumption that (i) growth responses to an increasing proportion of other functional types might vary among the species considered in this study, and (ii) that growth patterns throughout the ontogeny might differ among species. Plot nested in country was considered to affect the intercept parameter and the effects of POFT on tree growth assuming that differences in plot size and sampling methodologies might affect the POFT assessment and thus the sensitivity of tree growth to this index. The model equation takes the form:
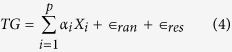 |
where TG is tree growth, 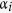 is the set of p parameters associated with the main and interactive effects of
is the set of p parameters associated with the main and interactive effects of 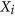 environmental variables, ∈ran is the variance component associated with the random terms, and ∈res is the residual normally-distributed error. We considered a fixed-effect term in the maximal model that included all the potential pair-wise interactions as well as the three variables interactions (i.e. SIZE × SBA × PET, SIZE × SBA × DIV, SIZE × PET × DIV, SBA × PET × DIV). SIZE was included in a log-transformed form and PET as a second order polynomial. Tree growth was log-transformed to meet normality and homogeneity of variance.
environmental variables, ∈ran is the variance component associated with the random terms, and ∈res is the residual normally-distributed error. We considered a fixed-effect term in the maximal model that included all the potential pair-wise interactions as well as the three variables interactions (i.e. SIZE × SBA × PET, SIZE × SBA × DIV, SIZE × PET × DIV, SBA × PET × DIV). SIZE was included in a log-transformed form and PET as a second order polynomial. Tree growth was log-transformed to meet normality and homogeneity of variance.
Model selection was conducted using a backward procedure of predictor variables starting with the maximal model61. We used the Bayesian Information Criterion (BIC) following the rule that net increments lower than 2 units of BIC associated with the elimination of any parameter in the maximal model determined the exclusion of the parameter from the final model61. We started with the selection of the three-variable interactions (round#1) and then tested the pairwise interactions (round#2) and so downwards the main effects of each predictor (round#3). A pseudo-R2 was assessed following Nakagawa and Schielzeth62. This index can be split into marginal and conditional effects, being the marginal R2 the goodness of fit of the fixed effect term only and the conditional R2 the goodness of fit of the whole model including the random term.
Additional Information
How to cite this article: Madrigal-González, J. et al. Complementarity effects on tree growth are contingent on tree size and climatic conditions across Europe. Sci. Rep. 6, 32233; doi: 10.1038/srep32233 (2016).
Acknowledgments
This manuscript was funded by the European Union Seventh Framework Program (FP7/2007–2013) under grant agreement no. 265171, project FUNDIV-Europe, and from project FUNDIVER (MINECO, Spain; CGL2015-69186-C2-2-R). We thank the MAGRAMA for granting access to the Spanish Forest Inventory, the Johann Heinrich von Thünen-Institut for access to the first and second German National Forest Inventories, Natural Resource Institute Finland (LUKE) for making permanent sample plot data from 1985-86 and 1995 available, the Swedish University of Agricultural Sciences for making the Swedish NFI data available, and Hugues Lecomte, from the Walloon Forest Inventory, for access to the Walloon NFI data. J.C. holds a Spanish FPU PhD Grant (FPU12/00575). PR-B was granted by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement No. 265171 (project FunDivEUROPE) and No. PCOFUND-GA-2010-267243 (Plant Fellows).
Footnotes
Author Contributions J.M.-G. and M.A.Z. conceived the idea. P.R.-B., S.R., G.K., J.D., C.W. and A.L. provided data and harmonized the forest database. J.M.-G., P.R.-B. and J.C. conducted the statistical analyses. J.M.-G., M.A.Z. and S.R. wrote the main manuscript and all the coauthors reviewed the manuscript.
References
- Ruiz-Jaen M. C. & Potvin C. Tree diversity explains variation in ecosystem function in a neotropical forest in Panama. Biotropica 42, 638–646 (2010). [Google Scholar]
- Paquette A. & Messier C. The effect of biodiversity on tree productivity: from temperate to boreal forests. Glob. Ecol. Biogeogr. 20, 170–180 (2011). [Google Scholar]
- Vilà M. et al. Species richness and wood production: a positive association in Mediterranean forests. Ecol. Lett. 10, 241–250 (2007). [DOI] [PubMed] [Google Scholar]
- Barrufol M. et al. Biodiversity promotes tree growth during succession in subtropical forest. PloS One 8, e81246 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz S., Hector A. & Wardle D. A. Biodiversity in forest carbon sequestration initiatives: not just a side benefit. Curr. Opin. Environ. Sustain. 1, 55–60 (2009). [Google Scholar]
- Strassburg B. B. et al. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conserv. Lett. 3, 98–105 (2010). [Google Scholar]
- Ruiz-Benito P. et al. Diversity increases carbon storage and tree productivity in Spanish forests. Glob. Ecol. Biogeogr. 23, 311–322 (2014). [Google Scholar]
- Bonan G. B. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320, 1444–1449 (2008). [DOI] [PubMed] [Google Scholar]
- Díaz S., Fargione J., Chapin F. S. III & Tilman D. Biodiversity loss threatens human well-being. PLoS Biol. 4, e277 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer-Lorenzen M., Körner C. & Schulze E. D. (eds). Forest diversity and function: temperate and boreal systems (Vol. 176). (Springer Science & Business, Media 2005). [Google Scholar]
- Nadrowski K., Wirth C. & Scherer-Lorenzen M. Is forest diversity driving ecosystem function and service? Curr. Opin. Environ. Sustain. 2, 75–79 (2010). [Google Scholar]
- Hooper D. U. & Dukes J. S. Overyielding among plant functional groups in a long-term experiment. Ecol. Lett. 7, 95–105 (2004). [Google Scholar]
- Forrester D. I. & Albrecht A. T. Light absorption and light-use efficiency in mixtures of Abies alba and Picea abies along a productivity gradient. Forest Ecol. Manag. 328, 94–102 (2014). [Google Scholar]
- Brassard B. W. et al. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 101, 210–219 (2013). [Google Scholar]
- Ratcliffe S. et al. Modes of functional biodiversity control on tree productivity across the European continent. Glob. Ecol. Biogeogr. (doi: 10.1111/geb.12406) (2016). [DOI] [Google Scholar]
- Morin X., Fahse L., Scherer-Lorenzen M. & Bugmann H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 14, 1211–1219 (2011). [DOI] [PubMed] [Google Scholar]
- Forrester D. I. The spatial and temporal dynamics of species interactions in mixed-species forests: from pattern to process. Forest Ecol. Manag. 312, 282–292 (2014). [Google Scholar]
- West G. B., Enquist B. J. & Brown J. H. A general quantitative theory of forest structure and dynamics. P. Natl. Acad. Sci. 106, 7040–7045 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson N. L. et al. Rate of tree carbon accumulation increases continuously with tree size. Nature 507, 90–93 (2014). [DOI] [PubMed] [Google Scholar]
- Coomes D. A. et al. Wood production response to climate change will depend critically on forest composition and structure. Glob. Change Biol. 20, 3632–3645 (2014). [DOI] [PubMed] [Google Scholar]
- Aakala T., Fraver S., D’Amato A. W. & Palik B. J. Influence of competition and age on tree growth in structurally complex old-growth forests in northern Minnesota, USA. Forest Ecol. Manag. 308, 128–135 (2013). [Google Scholar]
- Niinemets Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 25, 693–714 (2010). [Google Scholar]
- Zang C., Pretzsch H. & Rothe A. Size-dependent responses to summer drought in Scots pine, Norway spruce and common oak. Trees-Struct. Funct. 26, 557–569 (2012). [Google Scholar]
- Madrigal-González J. & Zavala M. A. Competition and tree age modulated last century pine growth responses to high frequency of dry years in a water limited forest ecosystem. Agr. Forest Meteorol. 192, 18–26 (2014). [Google Scholar]
- De Roos A., Persson L. & McCauley E. The influence of size-dependent life-history traits on the structure and dynamics of populations and communities. Ecol. Lett. 6, 473–487 (2003). [Google Scholar]
- Potvin C. & Dutilleull P. Neighborhood effects and size-asymmetric competition in a tree plantation varying in diversity. Ecology 90, 321–327 (2009). [DOI] [PubMed] [Google Scholar]
- Pretzsch H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. Forest Ecol. Manag. 327, 251–264 (2014). [Google Scholar]
- Zhang Y. & Chen H. Y. Individual size inequality links forest diversity and above-ground biomass. J Ecol 103, 1245–1252 (2015). [Google Scholar]
- Dănescu A., Albrecht A. T. & Bauhus J. Structural diversity promotes productivity of mixed, uneven-aged forests in southwestern Germany. Oecologia, doi: 10.1007/s00442-016-3623-4 (2016). [DOI] [PubMed] [Google Scholar]
- Bourdier T. et al. Tree Size Inequality Reduces Forest Productivity: An Analysis Combining Inventory Data for Ten European Species and a Light Competition Model. PLoS ONE 11, e0151852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky J. R. et al. The relationship between tree biodiversity and biomass dynamics changes with tropical forest succession. Ecol. Lett. 17, 1158–1167 (2014). [DOI] [PubMed] [Google Scholar]
- Holzwarth F., Rüger N. & Wirth C. Taking a closer look: disentangling effects of functional diversity on ecosystem functions with a trait-based model across hierarchy and time. Roy. Soc. Open Sci. 2, 140541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin C. & Dutilleul P. Neighborhood effects and size-asymmetric competition in a tree plantation varying in diversity. Ecology 90, 321–327 (2009). [DOI] [PubMed] [Google Scholar]
- Carpenter E. R. Microcosm experiments have little relevance for community and ecosystem Ecology. Ecology 77, 677–680 (1996). [Google Scholar]
- Symstad A. J. et al. Long-term and large-scale perspectives on the relationship between biodiversity and ecosystem functioning. Bioscience 53, 89–98 (2003). [Google Scholar]
- Baeten L. et al. A novel comparative research platform designed to determine the functional significance of tree species diversity in European forests. Perspect. Plant Ecol. 15, 281–291 (2013). [Google Scholar]
- Río M. D., Schütze G. & Pretzsch H. Temporal variation of competition and facilitation in mixed species forests in Central Europe. Plant Biol, 16, 166–176 (2014). [DOI] [PubMed] [Google Scholar]
- Jucker T. et al. Competition for light and water play contrasting roles in driving diversity–productivity relationships in Iberian forests. J. Ecol. 102, 1202–1213 (2014). [Google Scholar]
- Toïgo M. et al. Overyielding in mixed forests decreases with site productivity. J. Ecol. 103, 502–512 (2015). [Google Scholar]
- Condés S. & del Río M. Climate modifies tree interactions in terms of basal area growth and mortality in monospecific and mixed Fagus sylvatica and Pinus sylvestris forests. Eur. J. Forest Res. 134, 1095–1108 (2015). [Google Scholar]
- Ishii H. & Asano S. The role of crown architecture, leaf phenology and photosynthetic activity in promoting complementary use of light among coexisting species in temperate forests. Ecol. Res. 25, 715–722 (2010). [Google Scholar]
- Ishii H., Azuma W. & Nabeshima E. The need for a canopy perspective to understand the importance of phenotypic plasticity for promoting species coexistence and light-use complementarity in forest ecosystems. Ecol. Res. 28, 191–198 (2013). [Google Scholar]
- Houle D., Moore J. D., Quimet R. & Marty C. Tree species partition N uptake by soil depth in boreal forests. Ecology 95, 1127–1133 (2014). [DOI] [PubMed] [Google Scholar]
- Mason N. W., Pipenbaher N., Škornik S. & Kaligarič M. Does complementarity in leaf phenology and inclination promote co-existence in a species-rich meadow? Evidence from functional groups. J. Veg. Sci. 24, 94–100 (2013). [Google Scholar]
- Vieira J., Campelo F. & Nabais C. Age-dependent responses of tree-ring growth and intra-annual density fluctuations of Pinus pinaster to Mediterranean climate. Trees Struct. Funct. 23, 257–265 (2009). [Google Scholar]
- Augspurger C. K. & Bartlett E. A. Differences in leaf phenology between juvenile and adult trees in a temperate deciduous forest. Tree Physiol. 23, 517–525 (2003). [DOI] [PubMed] [Google Scholar]
- Vitasse Y. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytol 198, 149–155 (2013). [DOI] [PubMed] [Google Scholar]
- Miyazawa Y. & Kikuzawa K. Winter photosynthesis by saplings of evergreen broadleaved trees in a deciduous temperate forest. New phytol 165, 857–866 (2005). [DOI] [PubMed] [Google Scholar]
- Kunstler G. et al. Plant functional traits have globally consistent effects on competition. Nature 529, 204–207 (2016). [DOI] [PubMed] [Google Scholar]
- Kohyama T. S. & Takada T. The stratification theory for plant coexistence promoted by one-sided competition. J. Ecol. 97, 463–471 (2009). [Google Scholar]
- Kohyama T. S. & Takada T. One-sided competition for light promotes coexistence of forest trees that share the same adult height. J. Ecol. 100, 1501–1511 (2012). [Google Scholar]
- Simard S. W. et al. Net transfer of carbon between ectomycorrhizal tree species in the field. Nature 388, 579–582 (1997). [Google Scholar]
- Cavard X. et al. Competition and facilitation between tree species change with stand development. Oikos 120, 1683–1695 (2011). [Google Scholar]
- Forrester D. I. & Bauhus J. A Review of Processes Behind Diversity—Productivity Relationships in Forests. Curr. Forest. Rep. 2, 45–61 (2016). [Google Scholar]
- De Marco A., Meola A., Maisto G., Giordano M. & De Santo A. V. Non-additive effects of litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 344, 305–317 (2011). [Google Scholar]
- Holmgren M., Scheffer M. & Huston M. A. The interplay of facilitation and competition in plant communities. Ecology 78, 1966–1975 (1997). [Google Scholar]
- Niinemets Ü. & Valladares F. Tolerance to shade, drought, and waterlogging of temperate Northern Hemisphere trees and shrubs. Ecol Monogr 76, 521–547 (2006). [Google Scholar]
- Pretzsch H. & Schütze G. Effect of tree species mixing on the size structure, density, and yield of forest stands. Eur. J. For. Res. 135, 1–22 (2016). [Google Scholar]
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G. & Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- Trabucco A. & Zomer R. J. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR Consortium for Spatial Information. Published online, available from the CGIAR-CSI GeoPortal at: http://www.csi.cgiar.org/. Date of access: 10/2010 (2009).
- Burnham K. P. & Anderson D. Model selection and multi-model inference. A Pratical informatio-theoric approch (Sringer, 2003). [Google Scholar]
- Nakagawa S. & Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Method. Ecol. Evol. 4, 133–142 (2013). [Google Scholar]