Abstract
Tight junctions play a key role in restricting or regulating passage of liquids, ions and large solutes through various biological barriers by the paracellular route. Changes in paracellular permeation indicate alteration of the tight junction. However, it is very difficult to obtain the structural change information by measuring paracellular flux based on transepithelial electrical resistance or using fluorescein-labeled dextrans. Here we show that the BSA and GSH stabilized gold nanoclusters exhibit marginal cytotoxicity and pass through the MDCK monolayer exclusively through the paracellular pathway. We propose a double fluorescence probe strategy, the combination of a proven paracellular indicator (europium complex) with fluorescent gold nanoclusters. We calculate changes of structural parameters in tight junctions based on determination of the diffusion coefficients of the probes. Two different types of tight junction openers are used to validate our strategy. Results show that EDTA disrupts tight junction structures and induces large and smooth paracellular pore paths with an average radius of 17 nm, but vanadyl complexes induce paths with the radius of 6 nm. The work suggests that the double fluorescence probe strategy is a useful and convenient approach for in vitro investigation of tight junction structural alternations caused by pharmacological or pathological events.
The tight junction (TJ), located at the apical-most portion of the intercellular junctional complex, composes over a hundred TJ proteins arranged in order and fabricates a complex signaling network involving trans-membrane proteins, cytoskeletal proteins, scaffolding components, regulatory and signaling molecules, etc1,2. TJ plays a key role in restricting or regulating passage of liquids, ions, and large solutes through the paracellular pathway3,4,5. TJ structures can be altered under various physiological and/or pathological events, to name a few, the salivary gland and some endothelial cell secretion6,7, inflammatory mediator-induced changes in paracellular permeability8,9 and toxicity of metal ions10,11. Moreover, the use of TJ opening reagents12,13,14,15,16,17, e.g. chitosan18,19,20,21, has been an important biopharmaceutical strategy for the delivery of highly hydrophilic macromolecular drugs (e.g. peptide/protein drugs). Thence, methods that help to characterize the properties of paracellular pathways would be of significance for elucidating the TJ architecture and mechanism of regulation.
The TJ structure can be visualized by electron microscopy22,23 or fluorescence microscopy with genetically encoded fluorescent proteins24,25, however, these approaches are not feasible to study the alteration of the tight junction upon the treatment with drugs and/or stimuli. This TJ alteration is readily indicated by the changes of the trans-epithelial electrical resistance (TEER)26,27 or the apparent permeability coefficient (Papp) of a paracellular permeation probe/indicator28,29, e.g. manitol30,31, inulin32,33, cimetidine34,35 and hydrophilic fluorophores such as Eu-DTPA complexes36,37, lucifer yellow38,39 and different sized fluorescein isothiocyanate-labeled dextrans (MW 3∼40 kDa, e.g. FD4)40,41. However, TEER cannot provide any information about the TJ pore size or charge selectivity; while the previous approaches using paracellular probes/markers would either provided very limited information and/or require abundant of work. It is a great challenge to develop new methods that can be easily and conveniently implemented to sense the TJ changes associated with the physiological and/or pathological events.
Previous studies using the freeze-fracture technique and probes of different sizes revealed that the TJ structure had several pore pathways allowing flux of solutes with radii ranging from about 4 Å to 60 Å42. This inspires us to postulate that the diffusion of solutes in the paracellular routes could be similar to that in the porous medium. Therefore, it is possible to calculate the pore size (r) and retention parameters of tight junction channels by measuring the diffusion coefficients (Dp) of two or more probes of known molecular sizes and following the Fick’s law of diffusion and the amended Knudsen equation.
In the present work, we developed a novel method for investigating the dynamic change of the TJ pore size (r) and the channel retention property. Two sized fluorescent gold nanoclusters (AuNCs), BSA-templated AuNCs (AuNC@BSA) and GSH-protected AuNCs (AuNC@GSH), were first validated as novel paracellular permeability indicators. Then by combining the proven paracellular indicator, Eu-DTPA complex, with one of the AuNCs, the change of the TJ pore size (r) and retention parameters upon treatment of two different TJ openers was calculated.
Results and Discussion
Synthesis and characterization of AuNCs
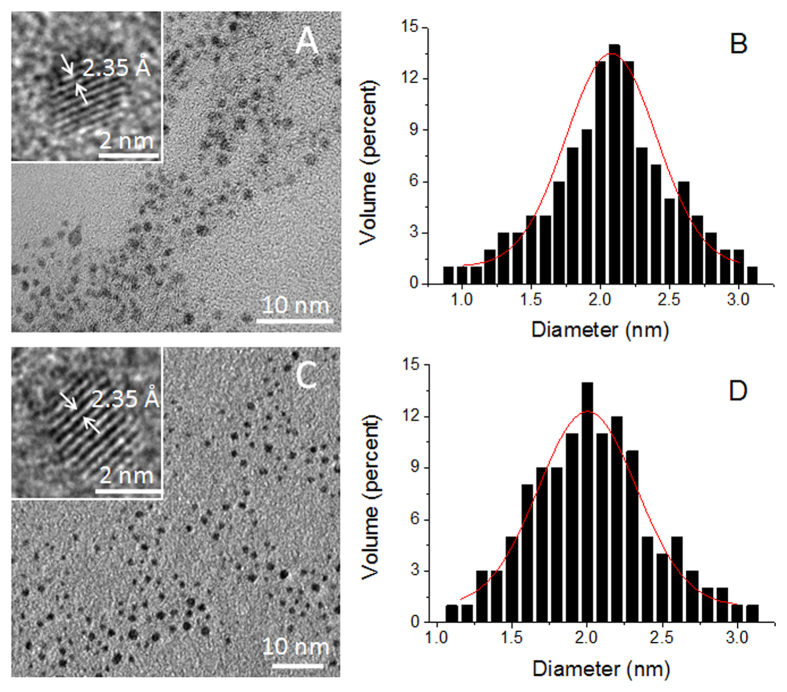
AuNC@GSH43 and AuNC@BSA44 were prepared according to the published procedure. Characterization using UV-Vis, fluorescence, infrared, X-ray photoelectron spectroscopies and DLS (Fig. S1–S5) demonstrated that the synthesis resulted in desired products. TEM measurements indicated that the core size for AuNC@GSH and AuNC@BSA were 2.07 ± 0.45 nm (n = 120) and 2.04 ± 0.40 nm (n = 120), respectively (Fig. 1). Insets in TEM images show lattice plane parameters match the (111) lattice spacing of the face-centered cubic Au45. In DMEM cell culture media, AuNC@GSH and AuNC@BSA (Fig. S5) showed the volume weighted hydrodynamic sizes of 6.0 ± 1.0 nm and 12.1 ± 0.3 nm, respectively. Both AuNCs had negative surface charges (Table 1).
Figure 1.
TEM images of AuNC@GSH (A) and AuNC@BSA (C). Insets display thelattice fringe of an individual nanocluster. Histograms of (B) and (D) show the size distribution of AuNC@GSH and AuNC@BSA obtained from corresponding TEM image, respectively.
Table 1. Some physicochemical parameters of Eu-DTPA and AuNC probes.
| Parameters | Eu-DTPA | AuNC@GSH | AuNC@BSA |
|---|---|---|---|
| MA (g/mol) | 560.54 | 1.4 × 104 | 7.1 × 104 |
| Particle core diameter (nm) (TEM) | — | 2.1 ± 0.5 | 2.0 ± 0.4 |
| Volume weighted hydrodynamic diameter (nm) | 0.902 | 6.0 ± 1.03 | 12.1 ± 0.33 |
| ζ potential (mV) | — | −1.47 ± 4.96 | −7.89 ± 4.38 |
| DAB (m2/S)1 | 3.36 × 10−10 | 1.15 × 10−10 | 6.68 × 10−11 |
1Parameters calculated using the Polson’s equation: 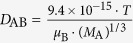 whereVA is the volume of solute probe; μB and MB are the viscosity and molar mass of the solvent water, respectively; T is the absolute temperature.
whereVA is the volume of solute probe; μB and MB are the viscosity and molar mass of the solvent water, respectively; T is the absolute temperature.
2The diameter of Eu-DTPA was calculated using HyperChem software.
3Data were from DLS measurement (Figure S5).
Cytotoxicity of AuNCs on MDCK cells
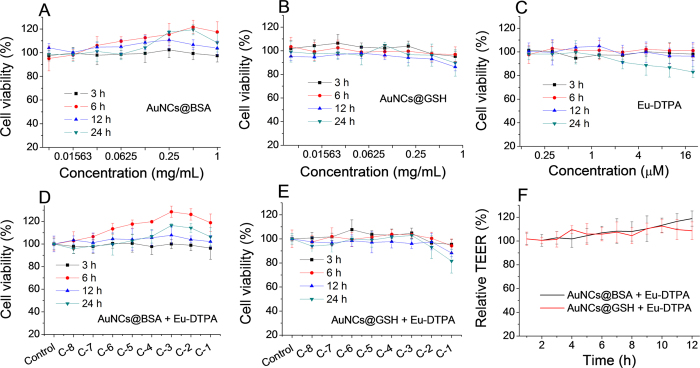
The cytotoxicity of AuNCs and Eu-DTPA complexes on MDCK cell was assessed by MTS assays and TEER measurements. Results (Fig. 2) showed that AuNCs or Eu-DTPA complexes or their combination had no significant effects on cell viability in the test concentration ranges. The cell viability greater than 100% upon treatment with high concentrations of AuNC@BSA (Fig. 2A,D) was an artifact caused by the excessive amount of BSA in AuNC@BSA preparation. The alternation of TEER is normally more sensitive than MTT/MTS assessments10, however, it was observed that AuNCs or Eu-DTPA did not cause any decline of TEER over 24-h incubation (Fig. 2F). These results indicated that AuNCs or the combination with Eu-DTPA was not toxic to MDCK cells.
Figure 2. MTS assays of MDCK cells upon treatment with AuNCs and Eu-DTPA.
(A) AuNC@BSA. (B) AuNC@GSH. (C) Eu-DTPA. (D) AuNC@BSA + Eu-DTPA. (E) AuNC@GSH + Eu-DTPA. C-1 denotes combination of 20 μmol/L Eu-DTPA with 1 mg/mL AuNC@BSA or 0.8 mg/mL AuNC@GSH, and C-2∼C-8 denote proportional dilution of C-1. (F) TEER of MDCK cell monolayer after treated with AuNCs and Eu-DTPA combination at C-1 concentration. All data were the mean ± SD of four replicates.
Mechanism of AuNCs permeation through MDCK monolayer
For calculation of TJ parameter changes, two different size paracellular pathway probes are needed. The Eu-DTPA complex is selected as the small size probe due to good biocompatibility and correlation of Papp with TEER46,47. In addition, the long life fluorescence of Eu3+ allows the use of time-resolved fluorescence measurements to achieve a high sensitivity with the interference-free background without separation from the second fluorescent probe. The gold nanoclusters (AuNCs) are considered as the large size probes due to their tunable particle size, excellent biocompatibility and desirable photostability44,48,49. In addition, if successful, it may open new opportunities for biological application of fluorescent nanomaterials.
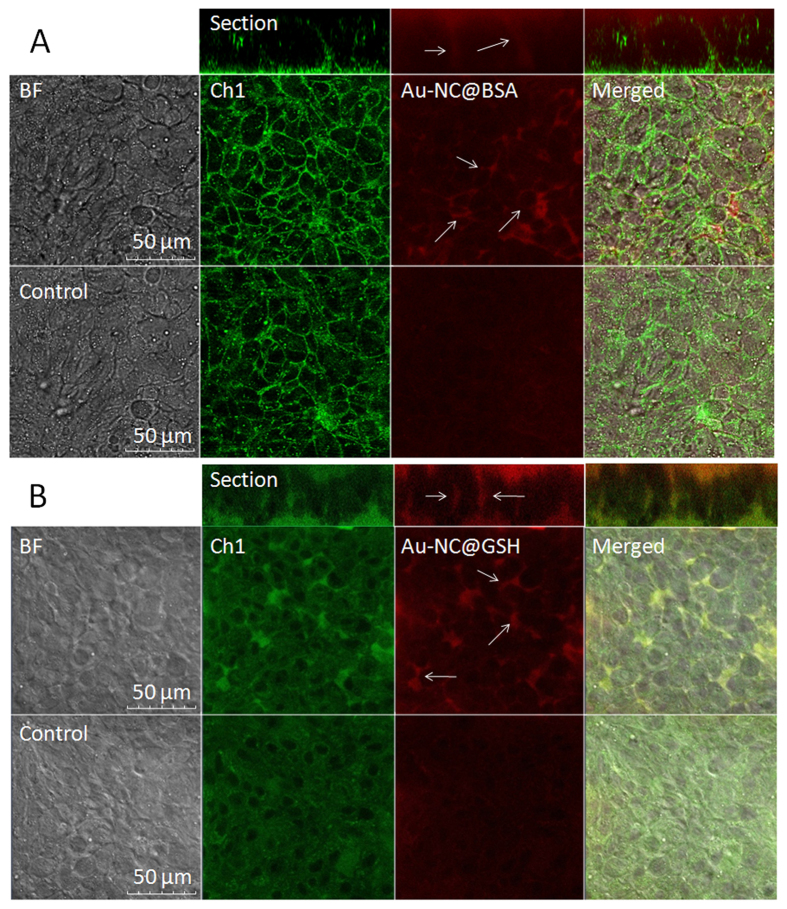
The criteria determining the mechanism of membrane permeation of a solute include the value of the apparent permeability coefficient (Papp)(less than 10−6 cm/s for paracellular diffusion)50 and the rate of cellular accumulation. The Papp of AuNC@BSA and AuNC@GSH were determined to be 1.99 × 10−7 cm/s and 3.66 × 10−7 cm/s, respectively. ICP-MS assays indicated (Fig. 3) that cellular accumulation of both AuNCs (<0.5%) were marginal. Moreover, under the confocal fluorescence microscope, AuNCs were only observed in the intercellular space (Fig. 4) upon opening TJ with EDTA. All the evidence indicated the transport of AuNC@BSA across MDCK monolayer exclusively through the paracellular pathway.
Figure 3. Rates of cellular accumulation of AuNCs in MDCK cells.
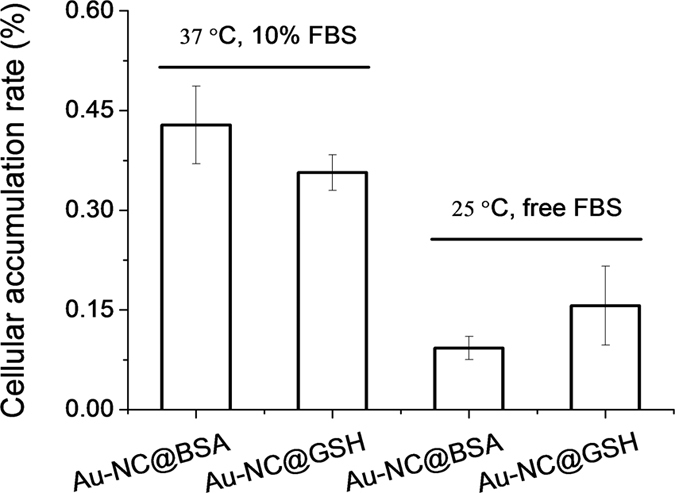
The incubated time was 6 h at 37 °C (10% FBS) and 25 °C (free FBS), respectively.
Figure 4. Confocal images of AuNCs in TJs of MDCK layers after EDTA treatment (0.5 mmol/L) for 30 min at 25 °C.
The concentrations of AuNCs were 1 mg/mL for AuNC@BSA (A) and 0.8 mg/mL for AuNC@GSH (B). Ch1 denote cell autofluorescence with λEx/Em of 488/520 nm.
Changes of P app of the Eu-DTPA-AuNCs double probes upon TJ opening
The paracellular permeability changes upon TJ opening in response to a variety of physiological and/or toxicological factors, e.g. cytokines, stress factors, dextrans, metal ions and metal chelators. Herein, we tested the effect of two different TJ openers, EDTA and vandylacetylacetonate (VO(acac)2). EDTA causes reversible TJ opening by depleting Ca2+ and Mg2+ that maintains the structure and conformation of TJ proteins51,52; while, vanadium complexes can increase paracellular permeability through induction of oxidative stress, which was regarded as one of the major mechanism for the toxicity of these anti-diabetic agents10.
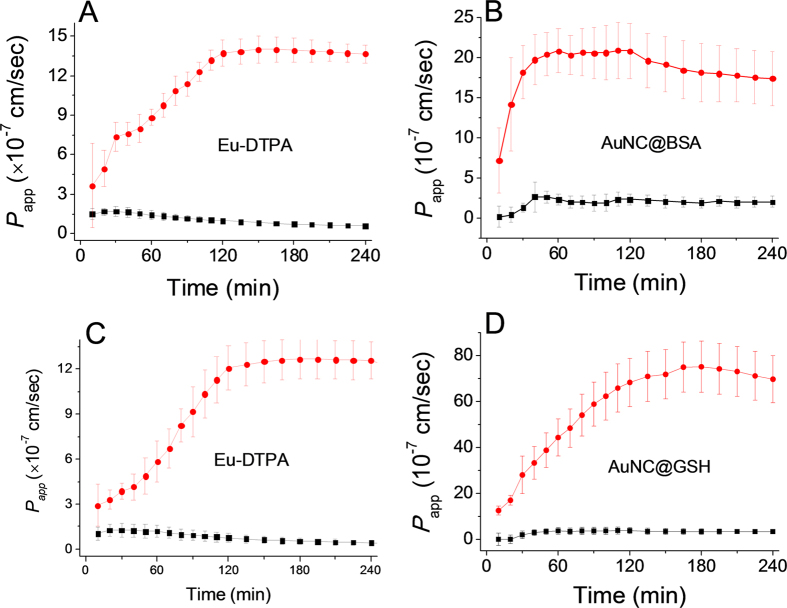
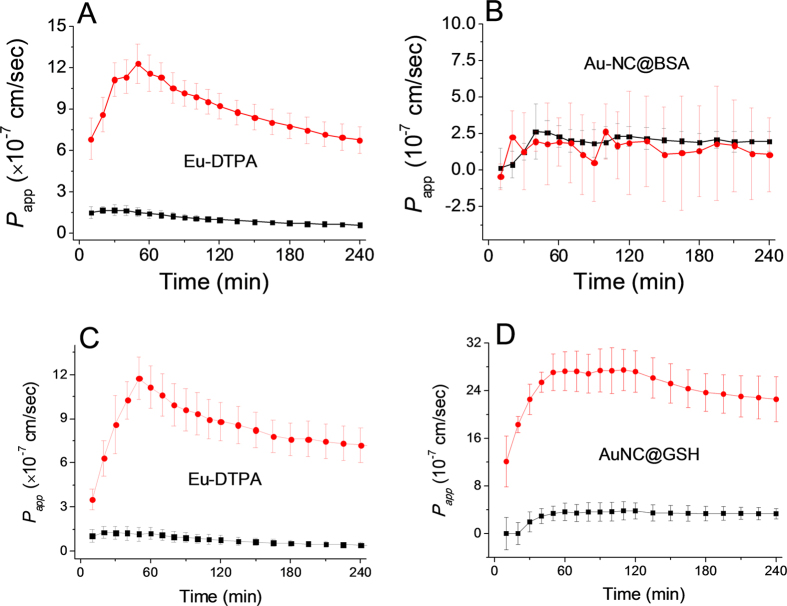
As shown in Fig. 5, upon addition of EDTA (0.5 mmol/L), the paracellular permeability of the probes increased significantly. Upon treatment with vanadyl complexes(80 μmol/L, Fig. 6), the permeability of Eu-DTPA and AuNC@GSH increased rapidly in the first hour and then gradually decreased, suggesting partial recovery of the TJ structure in the later hours. Treatment with vanadyl complexes did not elevate the permeability of AuNC@BSA. These results indicated that EDTA and vanadyl complexes caused different patterns of TJ alternation in both the extent of opening and the TJ architecture.
Figure 5. The time course of membrane permeation of double fluorescent probes in absence (black line) and presence (red line) of EDTA treatment (0.5 mmol/L).
A and B are the permeability of Eu-DTPA (A) and AuNC@BSA (B) in the Eu-DTPA (20 μmol/L)−AuNC@BSA (1 mg/mL) probe system, respectively; C and D are the permeability of Eu-DTPA (C) and AuNC@GSH (D) in the Eu-DTPA (20 μmol/L)−AuNC@GSH (0.8 mg/mL) probe system, respectively. Data were the mean ± SD of three replicates.
Figure 6. The time course of membrane permeation of double fluorescent probes in absence (black line) and presence (red line) of vanadyl complexes (80 μmol/L).
A and B are the permeability of Eu-DTPA (A) and AuNC@BSA (B) in the Eu-DTPA (20 μmol/L)−AuNC@BSA (1 mg/mL) probe system, respectively; C and D are the permeability of Eu-DTPA (C) and AulNC@GSH (D) in the Eu-DTPA (20 μmol/L)−AuNC@GSH (0.8 mg/mL) probe system, respectively. Data were the mean ± SD of three replicates.
Alternations of TJ pore size and retention capacity upon TJ opening
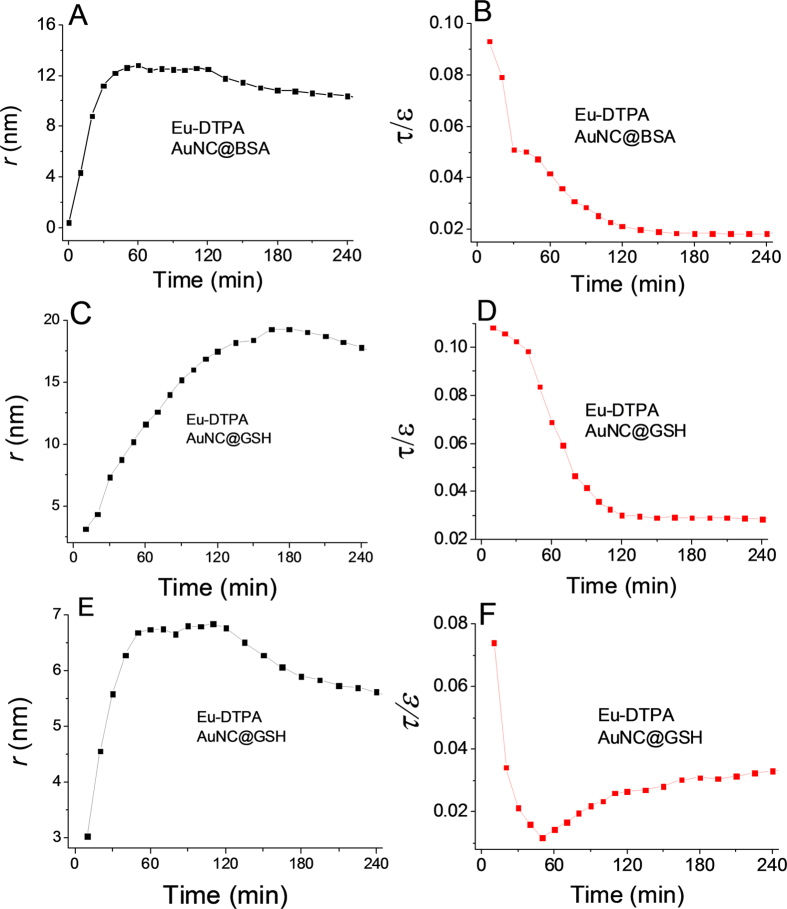
By using the Papp data in Figs 5 and 6, the pore size (r) and retention capacity values (ε/τ) of the tight junction of MDCK cell monolayer upon treatment with EDTA or VO(acac)2 were estimated accordingly (Fig. 7).Several important conclusions can be drawn from the results. First, the EDTA treatment (Fig. 7) caused two major changes: the TJ pore size (r) significantly increased to ∼16 nm while the TJ retention capacity (ε/τ) decreased monotonously. This suggests that depletion of Ca2+ by EDTA induced large and smooth pores in the intercellular space possibly by directly disrupting the TJ protein connection. The calculation using two sets of probes fundamentally gave a similar result. Second, as the permeability of AuNC@BSA did not change for the vanadyl complex treatment, the calculation was achieved on the Eu-DTPA -AuNC@GSH probe set. Results (Fig. 7E,F) indicated that the vanadium treatment increased TJ pore size (r) up to ∼6 nm while the retention capacity (ε/τ) was observed to rapidly decrease at first but then recovered partially in the following hour of incubation, which agrees with the change of the permeability of Eu-DTPA probe (Fig. 6A,C). Overall, the results suggested that vanadium may alter the TJ structure possibly by re-arranging the TJ architecture, leading to domination of the large pore path (∼60 Å)42 in the intercellular space. Nevertheless, the details of the mechanism need to be further investigated by biological and structural methods. Third, it is noted that the ε/τ response of AuNC@BSA upon EDTA treatment showed an interrupt point at ∼40 min and the increase of pore size was suspended afterward at ∼12 nm (Fig. 7A,B). While AuNC@BSA assay (Fig. 7C,D) showed a continual increase of pore size up to 17 nm until 180 min of treatment. We postulated that AuNC@BSA, due to the interaction of the template albumin with the pathway molecules, might stay somewhere in the paracellular path and thus restrict further increase of TJ pore. Probably for the same reason, the Papp of AuNC@BSA did not response to the vanadium treatment (Fig. 6B). The present results were consistent with our previous observation that a BSA-Eu-DTPA complex did not show Papp correlation to the treatment of vanadyl complexes53.Therefore, AuNC@GSH may better reflect alteration of the TJ structure than AuNC@BSA. In addition, these results may suggest that albumin might play a role in repairing TJ damage and as well address the role of protein corona on regulating the biological properties of nanoparticles54,55. Additionally, although TEER assays indicate <20% change in TJ upon the treatment of Caco-2 or MDCK cells with vanadium compounds herein and in our previous studies, the pore size caused by vanadium compounds was found up to 6 nm, which should allow flux of most biological substances. Therefore, more attention should be paid to the metal toxicity on TJ of biological barriers in further toxicological studies of vanadium compounds.
Figure 7.
Alteration of tight junction pore size, r (left column) and retention capacity, ε/τ (right column) calculated by use of Eu-DTPA/AuNC@BSA probes (A,B) and Eu-DTPA/AuNC@GSH (C,D) probes upon EDTA treatment, and Eu-DTPA/AuNC@GSH (E,F) probe upon vanadium treatment.
Conclusions
In summary, we have demonstrated that a double fluorescent probe strategy can be used to study the alteration of TJ structure. Our work first confirms that AuNCs pass through the MDCK monolayer exclusively through the paracellular pathway. Then, we successfully investigated the kinetic properties of TJ structure changes for the first time by employing the double fluorescence probes of Eu-DTPA and AuNCs following treatment with two different TJ openers. The calculation suggests that EDTA treatment induces large and smooth pore path with the pore size of ∼17 nm, while vanadyl complexes cause TJ structure re-arrangement and induce TJ pores with pore size of ∼6 nm. Overall, our work indicates that combination of Eu-DTPA and AuNCs probes may provide new and convenient tools to investigate TJ structural alternations in vitro in pharmacological and/or pathological processes.
Methods
Synthesis of gold nanoclusters (AuNCs)
GSH-protected gold nanoclusters (denoted as AuNC@GSH) were synthesized according to the previous method43. Briefly, 10 mL of HAuCl4 solution (4 mmol/L) was mixed with 10 mL of glutathione solution (6 mmol/L) under vigorously stirring. Then the mixture was heated up to 70 °C and incubated for 24 h under continuous gentle stirring. The excessive amounts of glutathione and unreacted-HAuCl4 were removed by dialysis and the concentration of product AuNC@GSH was quantified using ICP-MS(NexlON 300X, Perkin Elmer, USA).
BSA-stabilized AuNCs (denoted as AuNC@BSA) were prepared according to the previously reported procedure44. Briefly, a solution of HAuCl4 (10 mmol/L) was added to an equal volume of BSA solution (50 mg/mL). The mixture was vigorously stirred for 2 min, followed by adding 1/10 volume of 1 M NaOH. The resulting mixture was incubated at 37 °C under nitrogen purging and continuous gentle stirring (ca. 200 rpm) for 12 h. The concentration of AuNC@BSA solution was quantified using ICP-MS (NexlON 300X, Perkin Elmer, USA) after purification by dialysis and stored at 4 °C.
Preparation of Eu-DTPA probe
Eu-DTPA complexes were prepared using the previous method56. Briefly, 0.01 mol/L EuCl3 solution (prepared by dissolving 0.2760 g Eu2O3 in 5 mL of 3 mol/L HCl and diluting to 100 mL with double-distilled H2O) was added dropwise into 0.01 mol/L DTPA solution in Hank’s balanced salt solution (HBSS; pH 7.0) until the appearance of a white cloudy sediment, then the supernatant (Eu-DTPA) was collected after centrifuge (3 min, 10000×g).
Preparation of non-fluorescence DMEM medium (NF-DMEM)
To prevent the interference of auto-fluorescence background in DMEM media, some amino acids (tryptophan, Tyrosine, and Phenylalanine) and vitamins (folic acid, pyridoxine hydrochloride, and riboflavin etc.) were excluded referring to GIBCOTM catalogue media formulations when preparing the non-fluorescence DMEM media (NF-DMEM). The fluorescence-free DMEM media did not show any influences on MDCK cell viability or tight junction formation of MDCK cell monolayer.
MDCK cell culture
MDCK cells were cultured in high glucose DMEM supplied with 10% FBS and penicillin-streptomycin (100 μg/mL) and maintained in a humidified atmosphere containing 5% CO2 at 37 °C in 25 cm2 plastic flasks. The medium was refreshed every 2 days. Cells were passaged at 70–90% confluency using 0.25% (w/v) trypsin-0.02% (w/v) ethylenediaminetetraacetic acid (EDTA) solution. The MDCK cells used in this study were under passage 50.
Cytotoxicity assay
Toxicity of AuNCs on MDCK cells was estimated by MTS assay using a Cell Titer 96® A Queous One Solution Cell Proliferation Assay kit (Promega Inc., Madison, WI). In brief, MDCK cells (3∼5 × 103 cells/mL) were seeded in 96-well plates (200 μL per well). After attaching on the wall of plates, the cells were incubated with various concentrations of AuNCs in DMEM for 3, 6, 12, and 24 h. Then the cells were rinsed with DMEM and incubated with MTS solution for another 3 h at 37 °C. The absorbance of the color development in AuNCs-treated and -untreated cells was measured in a Bio-Rad Microplate reader.
Transport experiment and P app calculation
Cells were seeded onto Transwell filters (aperture, 3 μm; diameter, 12 mm) at a density of 1 × 105 cells per well and were allowed to grow and differentiate for about 7 days. Cell monolayers were used when the net trans-epithelial electrical resistance (TEER) exceeded 200 Ω∙cm2.
For the trans-epithelial transport experiments10, the MDCK monolayers in the transwells insert were rinsed twice with pre-warmed NF-DMEM media. Then different concentrations of Eu-DTPA and/or AuNCs in NF-DMEM media were added to the apical chamber and samples were withdrawn from the basolateral chamber at different time intervals. The apparent permeability coefficients (Papp) of Eu-DTPA/AuNCs were calculated by the formula Papp = (ΔQ/Δt)/(AC0), where A is the surface area of the cell monolayers (1.13 cm2 in this study), C0 is the initial concentration of AuNCs (mg/mL) in the apical chamber.
The concentration of Eu-DTPA was determined by a time-resolved fluorescence assay. Briefly, the sample were mixed with two volumes of fluorescence enhancement solution containing 30 μmol/L β-NTA, 10 mmol/L TOPO, 0.2% Triton X-100, and 0.1 mol/L potassium hydrogen phthalate buffer (pH 3.0). After the mixture was sit for 1 h at room temperature, the fluorescence intensity was measured on a Flexstation 3 microplate reader with λEx/Em of 340/616 nm and a measurement window from 600 to 1000 ms.
The concentration of AuNCs was determined by fluorescence assays. The fluorescence spectroscopy of AuNCs was obtained with a Hitachi F4600 spectrophotometer (Hitachi, Japan). The fluorescent intensity of AuNC samples from the basolateral chamber were detected on a Flexstation 3 microplate reader with λEx/Em of 488/630 nm for AuNC@BSA and 405/608 nm for AuNC@GSH.
Treatment of MDCK monolayers with EDTA or vanadium complexes
For the paracellular diffusion, permeability of solutes would be greatly improved when the TJ structure was opened or destroyed. EDTA could disrupt TJ proteins by depleting Ca2+ and Mg2+ ions57,58 and vanadium complexes can damage TJ through inducing oxidative stress10,59,60. Hereby, the influence of EDTA and vanadium complexes on the permeability of AuNCs and Eu-DTPA across MDCK cell monolayer were investigated by including 0.5 mmol/L of EDTA or 80 μmol/L of VO(acac)2 in the Ca2+ and Mg2+ free NF-DMEM in the transport experiments described above.
Calculation of the pore size and the retention capacity of tight junction upon TJ opening
Given that the diffusion of the probes in tight junction channels is similar to that in porous medium and there is no specific interaction among the solutes, solvents and tight junction channels, diffusion of the probes could be described by the amended Knudsen equation:
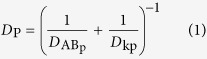 |
in which
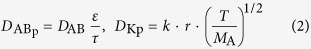 |
where DAB is the diffusion coefficient of probes in solvent B; ε and τ represent porosity and tortuosity of the porous medium, respectively; r is the radius of the pore; MA is the molecular mass of the solute. The diffusion coefficient of solutes (Dp) in the paracellular pore pathways can be estimated through the Papp values (see Supplementary Materials). The ε/τ is in overall reflecting the retention capacity of the porous medium on the solutes. Considering the size exclusion effect, ε/τ is thus different among the Eu-DTPA and AuNCs, we assume that the large AuNC probes only pass through the large pores and their ε/τ is assigned as the intrinsic one. Therefore, the ε/τ factor for Eu-DTPA would be described by
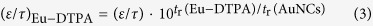 |
where the retention time (tr) of the probes was measured on a size exclusion gel column (e.g. Sephadex G25, see Fig. S6). Then, the Knudsen equations for the three fluorescent probes were obtained as:
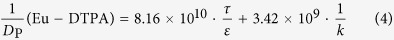 |
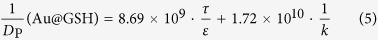 |
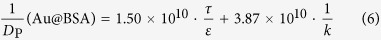 |
By assigning the size (r∼4 Å) of primary TJ pores42 as the average size of TJ pores, the calibration constant was calculated to be 0.088 ± 0.008. Then, the change of pore size (r) and retention capacity (ε/τ) could be calculated using the following two sets of equations:
• When using Eu-DTPA + AuNC@GSH:
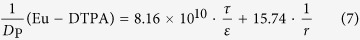 |
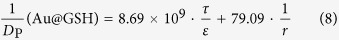 |
• When using Eu-DTPA + AuNC@BSA:
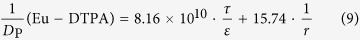 |
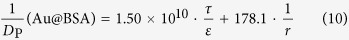 |
Confocal Imaging of Transport of AuNCs by the Paracellular Pathway
Briefly, MDCK cells cultured on the glass bottom cell culture dish were rinsed three times and incubated three hours with NF-DMEM media before assay. The detection began after addition of AuNC probes and the TJ opener, EDTA (0.5 mmol/L), in NF-DMEM. The fluorescence was observed with a Zeiss LSM 760 laser scanning confocal microscope (Zeiss, Germany).
ICP-MS quantifying cellular uptake
When the cell growth reached nearly 90% confluence (in the 6-well microplate), the Eu-DTPA and AuNC probe solution was added into the cultivation media at the desired concentrations at 37 °C (10% FBS) or 25 °C (free FBS). After 6 hours of incubation, the cells were washed, trypsinized and collected. The samples were treated with aqua regia overnight to dissolve the cells and the Au particles. Then the samples were analyzed on an ICP-MS (NexlON 300X, PerkinElmer, USA) to measure the amount of gold atoms per cell.
Statistics
All the experiments were repeated at least three times. Results are expressed as the means ± SD. All results were analyzed by t-test or one-way analysis of variance (ANOVA). A P-value less than 0.05 was considered as statistically significant.
Additional Information
How to cite this article: Wang, X. et al. Exploring tight junction alteration using double fluorescent probe combination of lanthanide complex with gold nanoclusters. Sci. Rep. 6, 32218; doi: 10.1038/srep32218 (2016).
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 21271012; 31571025).
Footnotes
Author Contributions X.W. and N.W. carried out most of the experiments and completed the manuscript writing. X.W. and N.W. contributed equally to this work. L.Y. provided guidance and assistance with the confocal images. N. L. contributed to the preparation of gold nanoclusters. J.W. gave some suggestion on project planning. X.Y. supervised the whole project as the academic advisor.
References
- Tsukita S., Furuse M. & Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Bio 2, 285–293 (2001). [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Betanzos A., Nava P. & Jaramillo B. E. Tight junction proteins. Prog Biophys Mol Biol 81, 1–44 (2003). [DOI] [PubMed] [Google Scholar]
- Madara J. L. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 60, 143–159 (1998). [DOI] [PubMed] [Google Scholar]
- Wolburg H. & Lippoldt A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vascul Pharmacol 38, 323–337 (2002). [DOI] [PubMed] [Google Scholar]
- Shin K., Fogg V. C. & Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22, 207–235 (2006). [DOI] [PubMed] [Google Scholar]
- Nguyen D. A. D. & Neville M. C. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia 3, 233–246 (1998). [DOI] [PubMed] [Google Scholar]
- Ewert P. et al. Disruption of tight junction structure in salivary glands from sjogren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum 62, 1280–1289 (2010). [DOI] [PubMed] [Google Scholar]
- Woywodt A. et al. Mucosal cytokine expression, cellular markers and adhesion molecules in inflammatory bowel disease. Eur J Gastroenterol Hepatol 11, 267–276 (1999). [DOI] [PubMed] [Google Scholar]
- Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 91, 151–175 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang X. D., Wang K. & Crans D. C. The permeability and cytotoxicity of insulin-mimetic vanadium (III, IV, V)-dipicolinate complexes. J Inorg Biochem 100, 80–87 (2006). [DOI] [PubMed] [Google Scholar]
- Calabro A. R., Gazarian D. I. & Barile F. A. Effect of metals on beta-actin and total protein synthesis in cultured human intestinal epithelial cells. J Pharmacol Toxicol Methods 63, 47–58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst B. J. Intestinal permeation enhancers. J Pharm Sci 89, 429–442 (2000). [DOI] [PubMed] [Google Scholar]
- Tamai I. & Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci 89, 1371–1388 (2000). [DOI] [PubMed] [Google Scholar]
- Salama N. N., Eddington N. D. & Fasano A. Tight junction modulation and its relationship to drug delivery. Adv Drug Del Rev 58, 15–28 (2006). [DOI] [PubMed] [Google Scholar]
- Neuwelt E. et al. Strategies to advance translational research into brain barriers. Lancet Neurol 7, 84–96 (2008). [DOI] [PubMed] [Google Scholar]
- Chen Y. & Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Del Rev 64, 640–665 (2012). [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32, 1959–1972 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonaje K. et al. Opening of epithelial tight junctions and enhancement of paracellular permeation by chitosan: microscopic, ultrastructural, and computed-tomographic observations. Mol Pharm 9, 1271–1279 (2012). [DOI] [PubMed] [Google Scholar]
- Hsu L. W. et al. Elucidating the signaling mechanism of an epithelial tight-junction opening induced by chitosan. Biomaterials 33, 6254–6263 (2012). [DOI] [PubMed] [Google Scholar]
- van der Lubben I. M., Verhoef J. C., Borchard G. & Junginger H. E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur J Pharm Sci 14, 201–207 (2001). [DOI] [PubMed] [Google Scholar]
- Amidi M., Mastrobattista E., Jiskoot W. & Hennink W. E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Del Rev 62, 59–82 (2010). [DOI] [PubMed] [Google Scholar]
- Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2, doi: 10.1101/cshperspect.a002907 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci 108 (Pt 11), 3443–3449 (1995). [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Furuse M. & Furutani-Seiki M. In vivo imaging of tight junctions using claudin-EGFP transgenic medaka. Methods Mol Biol 762, 171–178 (2011). [DOI] [PubMed] [Google Scholar]
- Matsuda M., Kubo A., Furuse M. & Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci 117, 1247–1257 (2004). [DOI] [PubMed] [Google Scholar]
- Smith J., Wood E. & Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharm Res 21, 43–49 (2004). [DOI] [PubMed] [Google Scholar]
- Tang V. W. & Goodenough D. A. Paracellular ion channel at the tight junction. Biophys J 84, 1660–1673 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 344, 304–307 (2014). [DOI] [PubMed] [Google Scholar]
- Madara J. L. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol 60, 143–159 (1998). [DOI] [PubMed] [Google Scholar]
- Karlsson P. C., Hughes R., Rafter J. J. & Bruce W. R. Polyethylene glycol reduces inflammation and aberrant crypt foci in carcinogen-initiated rats. Cancer Lett 223, 203–209 (2005). [DOI] [PubMed] [Google Scholar]
- Joh T. et al. The protective effect of rebamipide on paracellular permeability of rat gastric epithelial cells. Aliment Pharmacol Ther 18 Suppl 1, 133–138 (2003). [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Trummer E. & Krell H. Increase in biliary permeability subsequent to intrahepatic cholestasis by estradiol valerate in rats. Gastroenterology 93, 533–538 (1987). [DOI] [PubMed] [Google Scholar]
- Behrens I., Stenberg P., Artursson P. & Kissel T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells.Pharm Res 18, 1138–1145 (2001). [DOI] [PubMed] [Google Scholar]
- Pade V. & Stavchansky S. Estimation of the relative contribution of the transcellular and paracellular pathway to the transport of passively absorbed drugs in the Caco-2 cell culture model. Pharm Res 14, 1210–1215 (1997). [DOI] [PubMed] [Google Scholar]
- Nagahara N., Tavelin S. & Artursson P. Contribution of the paracellular route to the pH-dependent epithelial permeability to cationic drugs. J Pharm Sci 93, 2972–2984 (2004). [DOI] [PubMed] [Google Scholar]
- Jorgensen V. L., Nielsen S. L., Espersen K. & Perner A. Increased colorectal permeability in patients with severe sepsis and septic shock. Intensive Care Med 32, 1790–1796 (2006). [DOI] [PubMed] [Google Scholar]
- Hulsmann A. R. et al. Permeability of human isolated airways increases after hydrogen peroxide and poly-L-arginine. Am J Respir Crit Care Med 153, 841–846 (1996). [DOI] [PubMed] [Google Scholar]
- Del Vecchio G. et al. Sodium caprate transiently opens claudin-5-containing barriers at tight junctions of epithelial and endothelial cells. Mol Pharm 9, 2523–2533 (2012). [DOI] [PubMed] [Google Scholar]
- Akbari P. et al. Galacto-oligosaccharides Protect the Intestinal Barrier by Maintaining the Tight Junction Network and Modulating the Inflammatory Responses after a Challenge with the Mycotoxin Deoxynivalenol in Human Caco-2 Cell Monolayers and B6C3F1 Mice. J Nutr 145, 1604–1613 (2015). [DOI] [PubMed] [Google Scholar]
- Makhlof A., Werle M., Tozuka Y. & Takeuchi H. A mucoadhesive nanoparticulate system for the simultaneous delivery of macromolecules and permeation enhancers to the intestinal mucosa. J Control Release 149, 81–88 (2011). [DOI] [PubMed] [Google Scholar]
- Benediktsdottir B. E., Gudjonsson T., Baldursson O. & Masson M. N-alkylation of highly quaternized chitosan derivatives affects the paracellular permeation enhancement in bronchial epithelia in vitro. Eur J Pharm Biopharm 86, 55–63 (2014). [DOI] [PubMed] [Google Scholar]
- Shen L., Weber C. R., Raleigh D. R., Yu D. & Tumer J. R. in Annu Rev Physiol 283–309 (Annual Reviews, Palo Alto, 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z. T. et al. From Aggregation-Induced Emission of Au(I)-Thiolate Complexes to Ultrabright Au(0)@Au(I)-Thiolate Core-Shell Nanoclusters. J Am Chem Soc 134, 16662–16670 (2012). [DOI] [PubMed] [Google Scholar]
- Xie J. P., Zheng Y. G. & Ying J. Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J Am Chem Soc 131, 888–889 (2009). [DOI] [PubMed] [Google Scholar]
- Wang C., Hu Y. J., Lieber C. M. & Sun S. H. Ultrathin Au nanowires and their transport properties. J Am Chem Soc 130, 8902–8903 (2008). [DOI] [PubMed] [Google Scholar]
- Clausen A. E., Kast C. E. & Bernkop-Schnurch A. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm Res 19, 602–608 (2002). [DOI] [PubMed] [Google Scholar]
- Brayden D. J., Bzik V. A., Lewis A. L. & Illum L. CriticalSorb promotes permeation of flux markers across isolated rat intestinal mucosae and Caco-2 monolayers. Pharm Res 29, 2543–2554 (2012). [DOI] [PubMed] [Google Scholar]
- Zheng J., Nicovich P. R. & Dickson R. M. In Annu Rev Phys Chem 409–431 (Annual Reviews, Palo Alto, 2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akola J., Walter M., Whetten R. L., Hakkinen H. & Gronbeck H. On the structure of thiolate-protected Au-25. J Am Chem Soc 130, 3756–3757 (2008). [DOI] [PubMed] [Google Scholar]
- Gao J. et al. Current protocols in pharmacology. John Wiley & Sons, Inc. New York, 7.2.1–7.2.23 (2000).
- Liang X. L. et al. Transport properties of puerarin and effect of Radix Angelicae Dahuricae extract on the transport of puerarin in Caco-2 cell model. J Ethnopharmacol 144, 677–682 (2012). [DOI] [PubMed] [Google Scholar]
- Lam C. H., Hansen E. A., Janson C., Bryan A. & Hubel A. The characterization of arachnoid cell transport II: paracellular transport and blood-cerebrospinal fluid barrier formation. Neuroscience 222, 228–238 (2012). [DOI] [PubMed] [Google Scholar]
- Xu Z. H., Zhang C. Y., Zhang Y. & Yang X. D. Europium Complexes as Novel Indicators of Paracellular Diffusion. Chem biodivers 9, 1916–1922 (2012). [DOI] [PubMed] [Google Scholar]
- Monopoli M. P., Aberg C., Salvati A. & Dawson K. A. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 7, 779–786 (2012). [DOI] [PubMed] [Google Scholar]
- Walkey C. D. & Chan W. C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev 41, 2780–2799 (2012). [DOI] [PubMed] [Google Scholar]
- Xu Z. H., Zhang C. Y., Zhang Y. & Yang X. D. Europium Complexes as Novel Indicators of Paracellular Diffusion. Chem Biodivers 9, 1916–1922 (2012). [DOI] [PubMed] [Google Scholar]
- Quan Y. S. et al. Effectiveness and toxicity screening of various absorption enhancers using Caco-2 cell monolayers. Biol Pharm Bull 21, 615–620 (1998). [DOI] [PubMed] [Google Scholar]
- Rege B. D., Yu L. X., Husain A. S. & Polli J. E. Effect of common excipients on Caco-2 transport of low-permeability drugs. J Pharm Sci 90, 1776–1786 (2001). [DOI] [PubMed] [Google Scholar]
- Xu Z., Zhang C., Zhang Y. & Yang X. Europium Complexes as Novel Indicators of Paracellular Diffusion. Chem Biodivers 9, 1916–1922 (2012). [DOI] [PubMed] [Google Scholar]
- Yang X. G., Yang X. D., Yuan L., Wang K. & Crans D. C. The permeability and cytotoxicity of insulin-mimetic vanadium compounds. Pharm Res 21, 1026–1033 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.