Translocations involving the KMT2A (MLL) gene, located at 11q23, and two different partner genes, ELL and MLLT1, located at 19p13.1 and 19p13.3, respectively, have been reported in both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).1,2 However, there are limited data describing clinical and molecular differences between adult patients with t(11;19)(q23;p13.1) and those with t(11;19)(q23;p13.3). Neither the Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer3 nor large studies correlating chromosome abnormalities with clinical outcome4 distinguish the two types of t(11;19); instead they combine both into one cytogenetic group. Herein, we report the largest, to our knowledge, series of 35 adults with acute leukemia and t(11;19)(q23;p13.1) or t(11;19)(q23;p13.3) who were treated on Cancer and Leukemia Group B (CALGB)/Alliance for Clinical Trials in Oncology (Alliance) trials. Our study is also the largest to describe the clinical features and disease outcomes of these patients as well as to provide mutational analyses, and gene- and microRNA (miR)-expression patterns, thereby providing the rationale to identify each as separate entities.
We identified and clinically characterized 19 adults with t(11;19) (q23;p13.1) and AML and 16 with t(11;19)(q23;p13.3), 12 with AML, three with ALL and one with ambiguous lineage acute leukemia (ALAL), who received AML-directed therapy and is grouped with AML patients, enrolled onto a prospective cytogenetics companion trial, CALGB 8461. We also performed RNA, microRNA and targeted DNA-sequencing studies on subsets of patients in each cytogenetic group. See Supplementary Information for methods details.
Translocation (11;19)(q23;p13.1) was found exclusively in AML patients; however, t(11;19)(q23;p13.3) was detected in both AML and ALL patients. The only significant clinical differences between AML patients with either translocation were the higher marrow (P = 0.003) and blood (P = 0.004) blast percentages in patients with t(11;19)(q23;p13.3) (Table 1). Among AML patients, there were no major differences between the two cytogenetic groups with respect to complete remission (CR) rates, disease-free survival or overall survival. Both translocations were associated with a poor prognosis, with a median overall survival of 8.2 months for t(11;19) (q23;p13.1) patients and 10.0 months for t(11;19)(q23;p13.3) AML patients (Table 1, Supplementary Figure S1). The median follow-up for the four patients alive and without an event is 6.9 years.
Table 1.
Comparison of clinical characteristics of patients with t(11;19) (q23;p13.1) and those with t(11;19)(q23;p13.3)
| Characteristic | t(11;19) (q23;p13.1) AML (n = 19) |
t(11;19) (q23;p13.3) AML (n = 13)a |
P-valueb | t(11;19) (q23;p13.3) ALL (n=3) |
|---|---|---|---|---|
| Age, y | 0.42 | |||
| Median | 54 | 38 | 79 | |
| Range | 20–70 | 25–84 | 30–79 | |
| Age group, n (%) | 1.00 | |||
| <60 y | 13 (68) | 9 (69) | 1 (33) | |
| ≥60 y | 6 (32) | 4 (31) | 2 (67) | |
| Female sex, n (%) | 11 (58) | 9 (69) | 0.71 | 3 (100) |
| Race, n (%) | 1.00 | |||
| White | 15 (83) | 11 (85) | 2 (67) | |
| Non-white | 3 (17) | 2 (15) | 1 (33) | |
| Hemoglobin, g/dl | 0.51 | |||
| Median | 9.1 | 9.5 | 12.5 | |
| Range | 2.8–15.8 | 6.2–12.0 | 7.7–13.8 | |
| Platelet count, × 109/l | 1.00 | |||
| Median | 49 | 56 | 66 | |
| Range | 16–163 | 20–184 | 11–165 | |
| WBC, × 109/l | 0.14 | |||
| Median | 29.4 | 10.0 | 92.5 | |
| Range | 2.1–97.5 | 2.3–80.7 | 11.8–231.9 | |
| Bone marrow blasts, % | 0.003 | |||
| Median | 57 | 87 | 90 | |
| Range | 26–93 | 45–96 | 65–94 | |
| Blood blasts, % | 0.004 | |||
| Median | 10 | 58 | 46 | |
| Range | 0–75 | 2–86 | 10–98 | |
| Therapy-related leukemia, n (%) |
0.20 | |||
| Yes | 0 (0) | 2 (15) | 0 (0) | |
| No | 19 (100) | 11 (85) | 3 (100) | |
| FAB (centrally reviewed), n (%) |
— | NR | ||
| M0 | 0 (0) | 1 (9) | ||
| M1 | 1 (6) | 1 (9) | ||
| M2 | 3 (17) | 0 (0) | ||
| M4 | 7 (39) | 0 (0) | ||
| M5 | 7 (39) | 6 (55) | ||
| M6 | 0 (0) | 0 (0) | ||
| Other | 0 (0) | 3 (27) | ||
| Extramedullary involvement, n (%) |
5 (29) | 3 (27) | 1.00 | 0 (0) |
| Transplantation in CR1, n (%) |
1.00 | |||
| Allo | 2 (11) | 1 (8) | 1 (33) | |
| Auto | 2 (11) | 1 (8) | 0 (0) | |
| None | 15 (78) | 11 (85) | 2 (67) | |
| Translocation (11;19), n (%) |
0.07 | |||
| Occurring as a sole abnormality |
14 (74) | 5 (38) | 2 (67) | |
| With additional abnormalities |
5 (26) | 8 (62) | 1 (33) | |
| Complete remission, n (%) |
9 (47) | 8 (62) | 0.49 | 2 (67) |
| Disease-free survival | 0.78 | 2.1 and 7.9 mo | ||
| Median, months | 5.6 | 9.7 | ||
| % Disease-free at 12 months, (95% CI) |
33 (8–62) | 25 (4–56) | ||
| % Disease-free at 60 months, (95% CI) |
22 (3–51) | 25 (4–56) | ||
| Overall survival | 0.59 | 0.8, 5.2 and 8.8 mo |
||
| Median, months | 8.2 | 10.0 | ||
| % Alive at 12 months, % (95% CI) |
21 (7–41) | 46 (19–70) | ||
| % Alive at 60 months, % (95% CI) |
11 (2–28) | 15 (2–39) |
Abbreviations: allo, allogeneic hematopoietic stem-cell transplantation; auto, autologous hematopoietic stem-cell transplantation; CI, confidence interval; CR1, first complete remission, FAB, French-American-British classification; mo, months; n, number; NR, not reported; WBC, white blood count; y, years.
Includes one patient with ambiguous lineage acute leukemia.
P-values for categorical variables are from Fisher’s exact test, P-values for continuous variables are from Wilcoxon rank sum test for comparisons between AML patients with t(11;19)(q23;p13.1) and those with t(11;19)(q23;p13.3).
Four (21%) t(11;19)(q23;p13.1) patients underwent either autologous (patients no. 3, 16) or allogeneic (no. 5, 6) hematopoietic stem-cell transplantation (HSCT) in CR1. Two (15%) AML patients with t(11;19)(q23;p13.3) received autologous (no. 30) or allogeneic (no. 25) HSCT in CR1. Three of these six patients remain alive and disease-free at last follow-ups of 38.4 months (no. 6), 90.7 months (no. 25) and 134.3 months (no. 30) after diagnosis. Clinical and cytogenetic data for individual patients are provided in Supplementary Tables S1–S3.
We compared our findings with the literature and found them mostly concordant (Supplementary Tables S4 and S5). Others have also found t(11;19)(q23;p13.1) exclusively in AML patients, who were primarily female and had M4/M5 marrow morphology. However, t(11;19)(q23;p13.3) patients in the literature were mainly male, whereas two-thirds of CALGB/Alliance patients with t(11;19) (q23;p13.3) were female.
Cytogenetically, t(11;19)(q23;p13.3) AML patients tended to have a higher incidence of secondary chromosome abnormalities at diagnosis than AML patients with t(11;19)(q23;p13.1) (62% vs 26%, P=0.07). In most t(11;19)(q23;p13.1) patients (74%), the translocation was detected as an isolated chromosome abnormality; however, five patients had additional clonal abnormalities including trisomy 8 (+8), trisomy 21, paracentric inversion within 12p, deletion of 20q and a complex karyotype. The most common secondary abnormality was +8, found in six AML patients, but its incidence was higher among patients with t(11;19)(q23;p13.3) than those with t(11;19)(q23;p13.1) (83% vs 17%) (Supplementary Tables S1 and S2). In six of the seven patients for whom cytogenetic data were available at the time of relapse, the translocation present at diagnosis was also found at relapse.
When we combined our data with the literature, secondary abnormalities occurred significantly more often in t(11;19) (q23;p13.3) patients (55% vs 21%, P < 0.001). Our data and the literature identified unbalanced structural abnormalities of 12p as novel recurrent secondary rearrangements. These 12p rearrangements, invariably leading to loss of 12p genetic material, were present at diagnosis in four patients (no. 29 and 30, cases reported by Ninomiya et al.5 and Cerveira et al.6), all with t(11;19)(q23; p13.3). A fifth patient with t(11;19)(q23;p13.3)7 and one patient with t(11;19)(q23;p13.1) (no. 17) acquired similar unbalanced 12p abnormalities at relapse. Notably, among adult patients, unbalanced 12p abnormalities were detected exclusively in patients diagnosed with AML.3
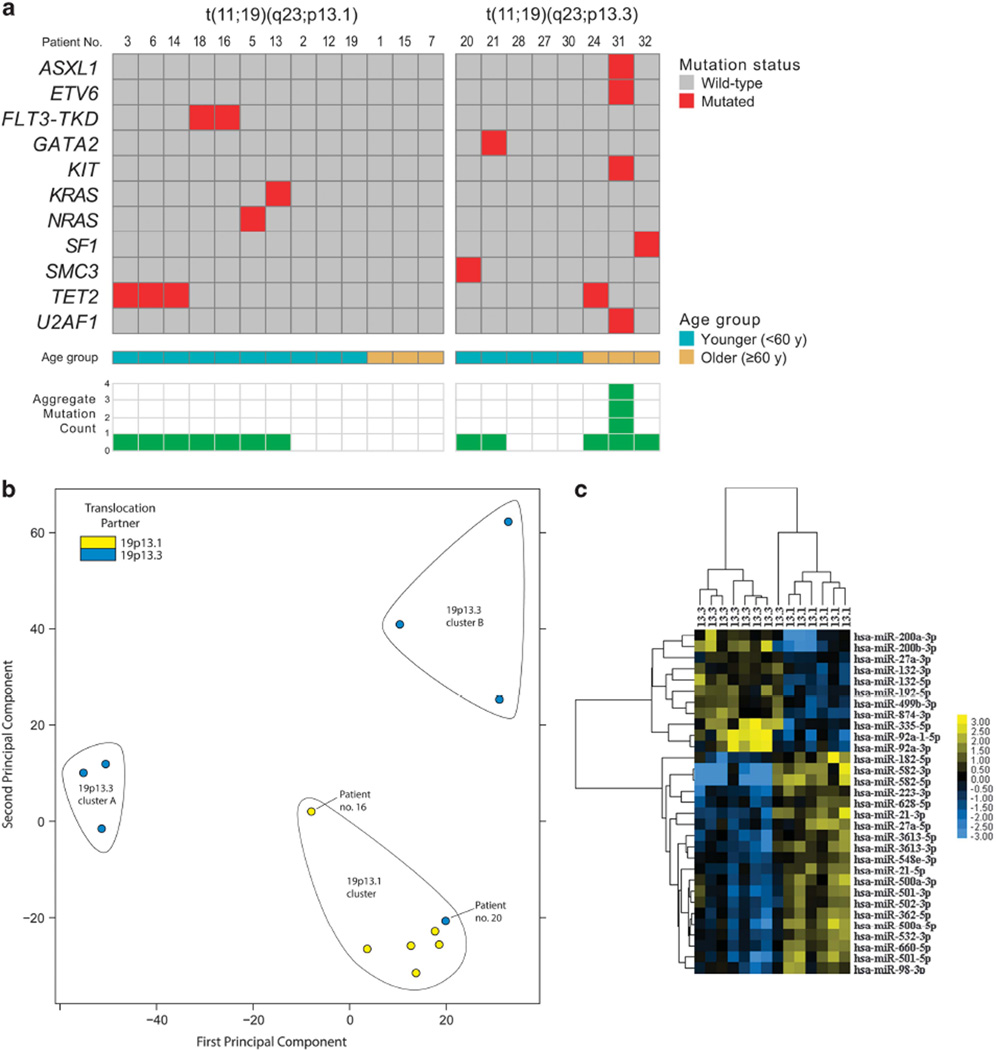
We next performed targeted DNA-sequencing studies in 13 AML patients with t(11;19)(q23;p13.1) and eight patients with t(11;19)(q23;p13.3) (seven with AML and one with ALAL) to search for molecular differences between the two cytogenetic groups of AML patients (Figure 1a and Supplementary Table S6). In general, the mutation rate was low in both patient subsets using a variant allele frequency cutoff of 0.3 (Figure 1a). Whereas FLT3-TKD and mutations in KRAS, NRAS, CEBPA (single mutation) and TET2 were the only mutations observed in the t(11;19)(q23;p13.1) group, patients with t(11;19)(q23;p13.3) had a wider mutational spectrum. Specifically, t(11;19)(q23;p13.3) patients harbored mutations in ASXL1, ETV6, GATA2, KIT and TET2. Notably, we found no recurrent AML-associated mutations such as double CEBPA mutations or mutations in DNMT3A, FLT3-ITD, IDH1, IDH2, NPM1, TP53 or WT1 in either group. The relatively low number of gene mutations found in CALGB/Alliance patients is consistent with the findings reported by The Cancer Genome Atlas Research Network,8 in which patients with KMT2A fusions had the fewest number of recurrent mutations (with a mean of approximately two mutations in the coding regions of the genome (tier 1 mutations)) among 10 AML categories analyzed in that study. Our findings thus support the suggestion that gene fusions involving KMT2A, such as KMT2A-MLLT1 and KMT2A-ELL, require a smaller number of cooperating mutations than other AML-initiating genetic alterations.8
Figure 1.
(a) Oncoprint of pre-treatment gene mutations detected in t(11;19)(q23;p13.1) AML patients and t(11;19)(q23;p13.3) AML patients plus one with ambiguous lineage acute leukemia (ALAL) using a variant allele frequency cutoff of 0.3. Not marked is a single CEBPA mutation found in patient no. 13. (b) Principle component analysis plot depicting relative gene-expression profiles of six AML patients with t(11;19)(q23; p13.1), and six patients with AML plus one with ALAL (no. 20) harboring t(11;19)(q23;p13.3). Each dot represents one patient and is color-coded by translocation partner. X and Y axes: Euclidean distance between gene-expression profiles in arbitrary units. (c) Heat map of the microRNA-expression patterns of t(11;19)(q23;p13.1) AML patients versus those of AML patients, plus one with ALAL, with t(11;19)(q23;p13.3). The heat map shows expression levels of 31 miRNAs found differentially expressed between t(11;19)(q23;p13.1) versus t(11;19)(q23;p13.3) cases. Rows represent probe sets and columns individual patients. Patients are grouped according to t(11;19)(q23;p13.1) versus t(11;19)(q23; p13.3), and genes are ordered by hierarchical cluster analysis. Expression values of the probe sets are represented by color: blue represents expression less than the median value for the given probe set, and yellow, expression greater than the median value for the given probe set.
Stranded mRNA sequencing was performed on six AML patient samples with t(11;19)(q23;p13.1) (no. 5, 6, 14, 15, 16, 18) and seven with t(11;19)(q23;p13.3) (six with AML (no. 21, 24, 27, 28, 30, 31) and one with ALAL (no. 20)). Gene-expression profiles of t(11;19) (q23;p13.1) patients clustered tightly, whereas expression profiles of t(11;19)(q23;p13.3) patients were more diffuse and may represent two distinct subgroups (Figure 1b, Supplementary Figure S3). Of note, the outlier case (no. 20) was reconfirmed by cytogenetics and RNA-sequencing to harbor t(11;19)(q23;p13.3) (Supplementary Table S8).
Because the expression profiles of the t(11;19)(q23;p13.1) cases were very similar, a large number of protein-coding genes were found to be differentially expressed between cytogenetic groups at the 1% false discovery rate (q-value) level. To further enhance this, we eliminated the two cases clustering apart from their cytogenetic group (no. 16 and 20). Using a q-value cutoff of 0.01, a total of 690 genes were differentially expressed between the cytogenetic groups (Supplementary Table S9). We examined these 690 distinguishing genes with Gene Set Enrichment Analysis9,10 to probe for biological differences between the two groups and observed that patients with t(11;19)(q23;p13.3) had a molecular profile with a more immature myeloid gene-expression pattern than patients with t(11;19)(q23;p13.1)11 (Supplementary Figure S2).
In the context of disease response, 54 genes were differentially expressed between patients who achieved CR and those who did not, with 37 genes up- and 17 genes downregulated in patients who did not attain CR (Supplementary Table S10A). A repetition of this analysis after controlling for the translocation partner yielded a longer list of 72 genes (Supplementary Table S10B). Genes associated with failure to achieve CR were enriched for genes encoding zinc-finger transcription factors, many of which reside on the 19q arm. The biology of these, and other genes associated with CR attainment, should be explored in future studies. Consistent with prior reports,12,13 MECOM (EVI1) expression was observed in both our KMT2A-ELL and KTM2A-MLLT1 AML cases. None of five patients with MECOM overexpression achieved a CR, whereas six of eight patients with low MECOM expression did.
We also performed small RNA sequencing on six AML patient samples with t(11;19)(q23;p13.1) and eight with t(11;19)(q23; p13.3) (seven with AML and one with ALAL). Again, these groups displayed distinct expression profiles; miR-expression profiling revealed 31 specific miRs that were differentially expressed between the cytogenetic groups. As with gene-expression, the miR-expression profiles clustered well overall (Figure 1c).
Lavallée et al.14 recently explored the transcriptomes of four patients with KMT2A-MLLT1 (MLL-ENL) and three with KMT2A-ELL (MLL-ELL), and reported differing gene-expression patterns between patients with and without KMT2A gene rearrangements. With our larger number of t(11;19) cases we were able to directly compare the transcriptional profiles of AML with KMT2A-MLLT1 and KMT2A-ELL fusions. Our results and those of Lavallée et al.14 support further exploration of biological differences between patients with KMT2A-MLLT1 and those with KMT2A-ELL.
In conclusion, our results add to the limited literature pertaining to t(11;19) in adults with acute leukemia and demonstrate that t(11;19)(q23;p13.1) and t(11;19)(q23;p13.3) are indeed two distinct rearrangements associated with unique clinical and cytogenetic features. Both translocations, similar to the majority of other 11q23/KMT2A rearrangements, carry a poor prognosis, and both our study and published reports15 suggest that these patients should receive allogeneic HSCT in first remission. RNA-sequencing studies showed distinct gene- and miR-expression patterns between AML patients with t(11;19)(q23;p13.1) and those with t(11;19)(q23;p13.3) supporting the recognition of these two translocations as separate entities.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180861, U10CA180850, U24CA114725, CA140158, CA16058, the Coleman Leukemia Research Foundation, the Pelotonia Fellowship Program (A-KE), the NCCN Foundation (JSB) and by an allocation of computing resources from The Ohio Supercomputer Center. The content, opinions, findings and conclusions are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, the National Comprehensive Cancer Network (NCCN) or the NCCN Foundation.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
BB, JSB, JK, A-KE, SV, KM, JCB and CDB contributed to the design and analysis of the study and the writing of the manuscript; A-KE performed laboratory-based research; JSB, JK, SV and DN performed statistical analyses; AJC, AWB, JEK, RMS, KM and CDB were involved directly or indirectly in the care of patients and/or sample procurement. All authors read and agreed on the final version of the manuscript.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Contributor Information
B Bhatnagar, Email: bhavana.bhatnagar@osumc.edu.
CD Bloomfield, Email: clara.bloomfield@osumc.edu.
REFERENCES
- 1.Huret JL, Brizard A, Slater R, Charrin C, Bertheas MF, Guilhot F, et al. Cytogenetic heterogeneity in t(11;19) acute leukemia: clinical, hematological and cytogenetic analyses of 48 patients--updated published cases and 16 new observations. Leukemia. 1993;7:152–160. [PubMed] [Google Scholar]
- 2.Moorman AV, Hagemeijer A, Charrin C, Rieder H, Secker-Walker LM. The translocations, t(11;19)(q23;p13.1) and t(11;19)(q23;p13.3): a cytogenetic and clinical profile of 53 patients. Leukemia. 1998;12:805–810. doi: 10.1038/sj.leu.2401016. [DOI] [PubMed] [Google Scholar]
- 3.Mitelman F, Johansson B, Mertens F, editors. Mitelman database of chromosome aberrations and gene fusions in cancer, August 2015 update. [Accessed 19 August 2015]; http://cgap.nci.nih.gov/Chromosomes/Mitelman.
- 4.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trial. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 5.Ninomiya M, Abe A, Yokozawa T, Ozeki K, Yamamoto K, Ito M, et al. Establishment of a myeloid leukemia cell line, TRL-01, with MLL-ENL fusion gene. Cancer Genet Cytogenet. 2006;169:1–11. doi: 10.1016/j.cancergencyto.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Cerveira N, Lisboa S, Correia C, Bizarro S, Santos J, Torres L, et al. Genetic and clinical characterization of 45 acute leukemia patients with MLL gene rearrangements from a single institution. Mol Oncol. 2012;6:553–564. doi: 10.1016/j.molonc.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vendrame-Goloni CB, Varella-Garcia M, Carvalho-Salles AB, Ruiz MA, Junior OR, Fett-Conte AC. Translocation (11;19)(q23;p13.3) associated with a novel t(5;16) (q13;q22) in a patient with acute myelocytic leukemia. Cancer Genet Cytogenet. 2003;141:71–74. doi: 10.1016/s0165-4608(02)00646-5. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gal H, Amariglio N, Trakhtenbrot L, Jacob-Hirsh J, Margalit O, Avigdor A, et al. Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia. 2006;20:2147–2154. doi: 10.1038/sj.leu.2404401. [DOI] [PubMed] [Google Scholar]
- 12.Grossmann V, Schnittger S, Poetzinger F, Kohlmann A, Stiel A, Eder C, et al. High incidence of RAS signalling pathway mutations in MLL-rearranged acute myeloid leukemia. Leukemia. 2013;27:1933–1936. doi: 10.1038/leu.2013.90. [DOI] [PubMed] [Google Scholar]
- 13.Gröschel S, Schlenk RF, Engelmann J, Rockova V, Teleanu V, Kühn MWM, et al. Deregulated expression of EVI1 defines a poor prognostic subset of MLL-rearranged acute myeloid leukemias: a study of the German-Austrian Acute Myeloid Leukemia Study Group and the Dutch-Belgian-Swiss HOVON/SAKK Cooperative Group. J Clin Oncol. 2012;31:95–103. doi: 10.1200/JCO.2011.41.5505. [DOI] [PubMed] [Google Scholar]
- 14.Lavallée VP, Baccelli, Krosl J, Wilhelm B, Barabé F, Gendron P, et al. The transcriptomic landscape and directed chemical interrogation of MLL-rearranged acute myeloid leukemias. Nat Genet. 2015;47:1030–1037. doi: 10.1038/ng.3371. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Kantarjian H, Pierce S, Faderl S, O'Brien S, Qiao W, et al. Prognostic significance of 11q23 aberrations in adult acute myeloid leukemia and the role of allogeneic stem cell transplantation. Leukemia. 2013;27:836–842. doi: 10.1038/leu.2012.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.