Abstract
The diagnosis of male infertility relies largely on conventional semen analysis, and its interpretation has a profound influence on subsequent management of patients. Despite poor correlation between conventional semen parameters and male fertility potential, inclusion of advanced semen quality tests to routine male infertility workup algorithms has not been widely accepted. Oxidative stress is one of the major mediators in various etiologies of male infertility; it has deleterious effects on spermatozoa, including DNA damage. Alleviation of oxidative stress constitutes a potential treatment strategy for male infertility. Measurement of seminal oxidative stress is of crucial role in the identification and monitoring of patients who may benefit from treatments. Various tests including reactive oxygen species (ROS) assay, total antioxidant capacity (TAC) assay or malondialdehyde (MDA) assay used by different laboratories have their own drawbacks. Oxidation-reduction potential (ORP) is a measure of overall balance between oxidants and antioxidants, providing a comprehensive measure of oxidative stress. The MiOXSYS™ System is a novel technology based on a galvanostatic measure of electrons; it presents static ORP (sORP) measures with static referring to the passive or current state of activity between oxidants and antioxidants. Preliminary studies have correlated sORP to poor semen qualities. It is potentially useful in prognostication of assisted reproductive techniques outcomes, screening of antioxidants either in vivo or during IVF cycles, identification of infertile men who may benefit from treatment of oxidative stress, and monitoring of treatment success. The simplified laboratory test requiring a small amount of semen would facilitate clinical application and research in the field. In this paper, we discuss the measurement of ORP by the MiOXSYS System as a real-time assessment of seminal oxidative stress, and argue that it is a potential valuable clinical test that should be incorporated into the male infertility workup and become an important guide to the treatment of oxidative stress-induced male infertility.
Keywords: diagnosis, male infertility, oxidation-reduction potential, oxidative stress, semen, treatment
Introduction
Global rates of male infertility range from 2.5% to 12%. That means at least 30 million men worldwide are infertile. Africa and Eastern Europe have the highest rates [Agarwal et al. 2015a], whereas a calculated percentage showed that 4.5–6% of North American males are infertile [Agarwal et al. 2015a]. In the United States, infertility affects 9.4% of males, according to the Centers for Disease Control and Prevention [Martinez et al. 2012]. Males are found to be solely responsible for 20–30% of infertility cases and contribute to 50% of cases overall [Agarwal et al. 2015a].
Infertility can pose a wide range of sociocultural, emotional, physical and financial problems [Slade et al. 2007; Greil et al. 2010]. The management of male infertility includes assessing and identifying a patient’s potential health problems. Decreased general health status has been associated with lower sperm concentration, lower total testosterone levels and higher follicle-stimulating hormone values [Ventimiglia et al. 2015]. Conditions such as testicular cancer [Raman et al. 2005], colorectal cancer, melanoma and prostate cancer [Walsh et al. 2010; Eisenberg et al. 2013] were found to be more prevalent among infertile men as were other noncancerous disorders [Salonia et al. 2009]. Infertility resulting from hypogonadism has also been associated with a decline in health [Zarotsky et al. 2014; Aversa and Morgentaler, 2015].
Many clinicians rely on conventional semen parameters as a surrogate measure of a man’s ability to father a child [Catanzariti et al. 2013; Esteves, 2014]. However, this approach seems to be an oversimplification of the assessment of male fertility potential due to large inter- and intra-individual variations in conventional semen parameters.
Oxidative stress has been identified as a major mediator in various etiologies of male infertility [Agarwal et al. 2014a]. Treatments of oxidative stress, including oral antioxidants and varicocelectomy, have been studied widely in patients with varicocele-associated male subfertility and unexplained male infertility. Current assays for seminal oxidative stress can measure reactive oxygen species (ROS) directly or indirectly, all of which have their own drawbacks.
Measurement of oxidation-reduction potential (ORP) by the MiOXSYS™ System presents a novel and comprehensive measure of seminal oxidative stress (Aytu BioScience, Inc.). It has a number of advantages over existing semen quality measures. The MiOXSYS System and measurement of ORP represent an invaluable clinical tool that obviates the need for complicated oxidative stress assays. The system facilitates wider application of oxidative stress assays in both clinical and research settings. The incorporation of ORP measurement into the armamentarium of the male infertility specialist will facilitate management of infertile couples by identifying candidates who may benefit from treatment of oxidative stress.
In this paper, we first illustrate the pitfalls of conventional semen analysis in clinical practice. We then discuss the role of oxidative stress in male infertility, potential treatment strategies, and current oxidative stress assays and their drawbacks. Finally, the superiority of ORP measurement using the MiOXSYS System is discussed.
Semen analysis
Semen analysis is the most widely used biomarker of male fertility potential [Esteves et al. 2012]. The results provide information on the functional status of the seminiferous tubules, epididymis and accessory sex glands [Esteves, 2014], which in turn influences the subsequent workup, treatment and outcomes [Baker et al. 2015]. However, semen characteristics that discriminate between infertile and fertile men are not well defined and the clinical use of conventional semen parameters is far from perfect.
Firstly, the results fall within the accepted reference ranges in up to 40% of infertile men [Moghissi and Wallach, 1983; Guzick et al. 2001; van der Steeg et al. 2011]. This is due, in part, to the fact that conventional semen analysis does not assess the diverse array of biological properties that spermatozoa express as eminent specialized cells, or account for putative sperm dysfunctions that can be assessed by sperm DNA damage or seminal oxidative stress tests [Duran et al. 2002; Esteves, 2014; Papillon-Smith et al. 2015].
Secondly, the reference values are derived from a population of fertile men and therefore do not represent the population in question: men who are unable to initiate a pregnancy. The lower reference values for ‘normal’ were set at the fifth percentile of the population distribution – a threshold that has no known correlation with fecundity [Joffe, 2010; Baker et al. 2015].
Thirdly, the World Health Organization (WHO) reference limits do not represent the distribution of fertile men across the globe [Barratt et al. 2011], as the group of studied men consisted of a limited population of individuals from large cities in the northern hemisphere and a small subset of men from Australia [Esteves, 2014; Papillon-Smith et al. 2015].
As the definition of male factor infertility remains less clear, most clinicians continue to rely on these lower reference limits of semen analysis parameters to diagnose, as well as to formulate treatment plans without investigating the underlying etiology/mechanism [Catanzariti et al. 2013; Esteves, 2014]. Up to 30% of men who are unable to father a biological child have a normal male infertility workup, which commonly constitutes one or two semen analyses. The male infertility evaluation must go far beyond a simple semen analysis [Esteves et al. 2011, 2012]. We believe that the assessment of male fertility based on conventional semen analysis alone should not be considered adequate and must be complemented with more reliable, quantifiable, unbiased and universal functional measures of semen quality.
The American Society for Reproductive Medicine (ASRM) has acknowledged the limitations of conventional semen analysis and included sperm function testing in the assessment of infertile men [ASRM: Practice Committee of American Society for Reproductive Medicine, 2008]. The inclusion of advanced semen quality tests for the assessment of seminal oxidative stress to the male infertility workup algorithms has been recommended by experts [Zini and Sigman, 2009; Agarwal et al. 2013].
Oxidative stress
Oxidative stress occurs when ROS and the levels of other free radicals are greatly increased or antioxidant levels are substantially decreased such that the delicate balance between oxidants and antioxidants is disturbed [Sharma and Agarwal, 1996; Agarwal et al. 2012]. In other words, oxidative stress is a condition that reflects an imbalance between ROS and a biological system’s ability to readily detoxify (antioxidant defense) the reactive intermediates or repair the resulting damage [Hampl et al. 2012; Saalu, 2010].
ROS are mostly highly reactive substances with very short halflives (atoms, molecules, or fragments of atom and molecules derived from oxygen, nitrogen, other organic compounds, or ions of transition metals such as Cu2+ and Fe2+). These substances called radicals (or free radicals) contain at least one unpaired valence electron. They pair their unpaired electron with an electron taken from other compounds, causing oxidation. New radicals are formed from originally nonradical molecules with great oxidative ability, thus promulgating radical chain reactions [Durackova, 2014]. ROS and antioxidants significantly interfere with oxidation-reduction processes in cells and organisms, changing the redox (or oxidative) state of the cell; such states can stimulate or inhibit activities of various signal proteins, leading to the alteration of signal pathways. An oxidative milieu can lead to cell destruction by apoptosis or necrosis, and reducing milieu can lead to cell survival [Durackova, 2014].
The sources of ROS in semen are both intrinsic and extrinsic. Activated leukocytes (mainly polymorphonuclear leukocytes and macrophages) resulting from inflammation and infection are significant intrinsic producers of ROS in semen [Wolff, 1995; Whittington and Ford, 1999; Saleh et al. 2002; Potts and Pasqualotto, 2003]. Immature spermatozoa with abnormal head morphology and cytoplasmic retention are another important source [Agarwal et al. 2014b]. Damaged, deficient or abnormal spermatozoa as a result of impaired spermatogenesis can yield excessive ROS as well [Aitken and Clarkson, 1987; Aitken et al. 1989]. Sertoli cells in semen have also been shown to possess the ability to produce ROS [Hipler et al. 2000]. Other intrinsic etiologies include varicocele (higher grade is associated with greater amounts of ROS production), cryptorchidism, testicular torsion and ageing [Shiraishi et al. 2012; Ko et al. 2014]. Extrinsic sources such as cigarette smoking [Lavranos et al. 2012], alcohol consumption [Saalu, 2010], exposure to radiation [Manda et al. 2007; Agarwal et al. 2008a] and other environmental toxins [Ko et al. 2014] have been associated with elevated testicular and/or seminal ROS levels. Common sources of ROS in semen and their adverse effects are illustrated in Figure 1.
Figure 1.
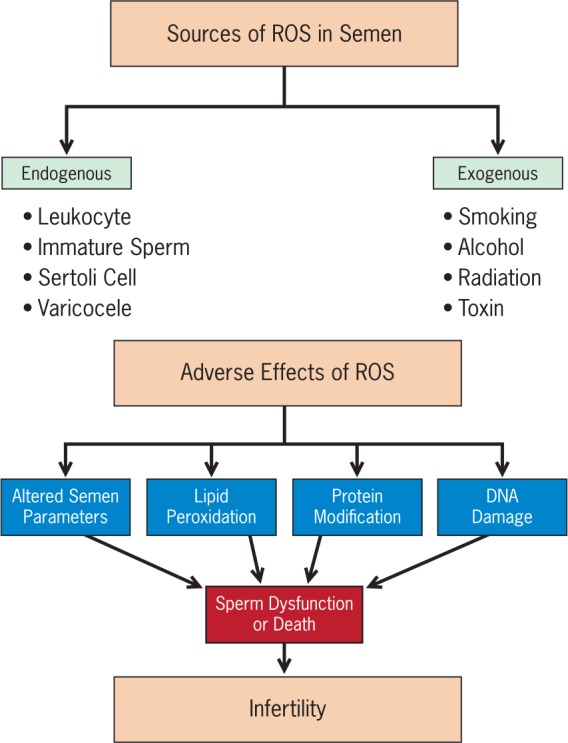
Common sources of excessive reactive oxygen species (ROS) in semen and their deleterious effects.
A delicate balance of reduction and oxidation is required for essential sperm function, including chromatin compaction in maturing spermatozoa during epididymal transit [Wright et al. 2014], capacitation, hyperactivation, acrosome reaction and sperm–oocyte fusion, leading to successful fertilization [de Lamirande and Gagnon, 1993; Aitken et al. 1995; Agarwal and Said, 2004; Kothari et al. 2010; Guthrie and Welch, 2012]. Supraphysiologic ROS levels can affect sperm structural and functional integrity including motility, morphology, count and viability, thereby making it one of the important etiologies of male factor infertility [Jones et al. 1979; Aitken, 1989; Twigg et al. 1998; Whittington et al. 1999; Pasqualotto et al. 2000; Agarwal et al. 2003, 2008b, 2014a, 2014b, 2014c; Aziz et al. 2004; Athayde et al. 2007; Desai et al. 2009; Chen et al. 2013; Vessey et al. 2014].
High ROS concentrations in infertile men have been associated with DNA fragmentation and poor chromatin packing [Kodama et al. 1997; Aitken and Krausz, 2001; Saleh and Agarwal, 2002; Aitken et al. 2003; Moustafa et al. 2004; Cocuzza et al. 2007; Desai et al. 2009; Aitken and De Iuliis, 2010; Novotny et al. 2013]. Sperm DNA damage may decrease fertilization rates, reduce implantation, impair embryonic development, and increase miscarriage/pregnancy loss and the potential for birth defects [Henkel et al. 2003; Ozmen et al. 2007; Tarozzi et al. 2007; Lewis and Simon, 2010; Zini et al. 2010; Agarwal et al. 2012]. ROS affect mitochondrial or nuclear DNA of sperm at an amino acid or molecular level in the form of base modification (especially guanine), attacking the phosphodiester backbones and producing base-free sites, point mutations, polymorphisms, deletions, translocations, strand breaks, and chromatin cross-links, frame shifts and even rearranging chromosomes [Agarwal et al. 2003, 2012; Aitken and Koppers, 2011; Gharagozloo and Aitken, 2011]. Sperm chromatin has a highly condensed and organized structure that helps to protect it from oxidative damage [Schulte et al. 2010], but when compaction is poor and chromatin protamination is incomplete, sperm DNA is more vulnerable to ROS. DNA damage is a contributory factor to apoptosis [Chen et al. 2013], and in cases of more severe damage of spermatozoa, apoptosis results in low sperm counts characteristic of idiopathic male factor infertility [Agarwal et al. 2003].
Alleviation of oxidative stress and potential treatment strategies
Treatment of oxidative stress-associated male infertility has been studied widely in patients with varicocele-associated subfertility and unexplained infertility. In these patients, treatment strategies such as surgical varicocele repair (varicocelectomy) and oral antioxidant therapy have been used.
Varicocelectomy
Current evidence suggests that oxidative stress is the central element contributing to infertility in men with varicocele and that varicocelectomy is beneficial for alleviating oxidative stress-associated infertility. Varicocele accounts for 19–41% of primary male infertility cases and up to 80% of secondary male infertility cases [Goldstein et al. 1992; Witt and Lipshultz, 1993; Madgar et al. 1995]. Studies demonstrate that varicocele repair is a viable option to decrease oxidative stress and restore the antioxidant defense system and, therefore, to improve fertility [Hamada et al. 2013]. An increase in varicocele grade is associated with an increase in seminal ROS levels and a decrease in sperm concentration [Cocuzza et al. 2012].
Several studies suggested that varicocelectomy reduces seminal oxidative stress in infertile men [Mostafa et al. 2001; Hurtado de Catalfo et al. 2007; Chen et al. 2008; Dada et al. 2010; Sakamoto et al. 2008]. Varicocelectomy performed in infertile men with clinical varicocele and high levels of seminal ROS resulted in a rapid decline in free radical levels within 1 month and a slower decline in DNA damage from pre-operative levels [Dada et al. 2010]. Sakamoto and colleagues reported that the excessive oxidative stress in the seminal plasma of infertile men with varicocele is reduced after varicocele repair and the procedure ameliorates sperm DNA damage after 6 months. A significant increase in sperm concentration and a reduction in nitric oxide, superoxide dismutase, 8-hydroxy-2′-deoxyguanosine level and superoxide dismutase activity were noted after varicocele repair. The percentage of apoptosis-positive sperm also decreased significantly after varicocelectomy [Sakamoto et al. 2008].
In subfertile patients with varicocele, varicocelectomy significantly decreased oxidative damage in sperm DNA and increased antioxidant capacity in seminal plasma [Chen et al. 2008]. Sperm motility, morphology and density improved in more than 70% of postvaricocelectomy patients, whereas the incidence of 4977 base pair (bp) deletion of mitochondrial DNA and 8-hydroxy-2′-deoxyguanosine content in sperm DNA (both markers of oxidative stress) was reduced. However, levels of other markers of oxidative stress such as seminal plasma protein thiols and ascorbic acid increased in these patients after varicocelectomy [Chen et al. 2008].
Hurtado de Catalfo and colleagues reported that levels of liposoluble and hydrosoluble antioxidants decreased and activities of the antioxidant defense system enzymes increased in the seminal plasma and spermatozoa of infertile men with unilateral left varicocele after varicocelectomy [Hurtado de Catalfo et al. 2007]. Varicocelectomy was also found to reduce ROS and malondialdehyde (MDA) levels and increase antioxidant activity of seminal plasma in infertile men with varicocele [Mostafa et al. 2001]. MDA is formed as a byproduct as a result of the reaction between oxygen and unsaturated lipids [Ayala et al. 2014]. It appears to be the most mutagenic product of lipid peroxidation [Esterbauer et al. 1990] and is one of the frequently used indicators of overall lipid peroxidation levels. Because of the facile reaction of omega-3 and omega-6 fatty acids with thiobarbituric acid, an intensely colored chromogen fluorescent red adduct is formed and MDA can be quantified to determine the oxidative stress [Pryor, 1989]. In varicocelectomized patients, seminal plasma levels of ROS (hydrogen peroxide, nitric oxide) and MDA were significantly reduced, and levels of four out of six tested antioxidants (superoxide dismutase, catalase, glutathione peroxidase and vitamin C, but not vitamin E, albumin) were significantly increased at both 3 and 6 months postoperatively [Mostafa et al. 2001].
Zini and colleagues suggested that varicocelectomy can improve the disposal of residual sperm cytoplasm by the testis and/or epididymis in infertile men with varicocele. The percentage of spermatozoa with residual cytoplasm decreased, while the percentages of motile spermatozoa and normal forms increased 6 months after varicocelectomy [Zini et al. 1999]. They suggested that varicocelectomy reduces the potential for ROS generation by spermatozoa in infertile patients with varicocele.
Marmar and colleagues reported that spontaneous pregnancy rates were higher in infertile couples where the male partner underwent varicocelectomy than in those where the male partner did not [Marmar et al. 2007]. The meta-analysis indicated that varicocelectomy in infertile men with palpable lesions and at least one abnormal semen parameter improved the odds of spontaneous pregnancy in their female partners.
Onozawa and colleagues observed a higher postoperative/preoperative ratio of sperm density in higher-graded varicocele as well as a higher pregnancy rate in the partners of the varicocelectomized men than in the conservatively treated patients [Onozawa et al. 2002]. Another recent meta-analysis elucidated the impact of surgical varicocele repair on the pregnancy rate [Kim et al. 2013]. In an analysis of all seven trials included in that study, a forest plot using the random effects model showed an odds ratio of 1.90. In a subanalysis of the three studies that included patients with clinical varicocele and abnormal semen parameters, the fixed effects pooled odds ratio was higher, favoring varicocelectomy. The authors concluded that varicocelectomy is effective in men with clinical varicocele and impaired semen quality, and therefore surgical varicocele repair should be offered as the first-line treatment of clinical varicocele in subfertile men [Kim et al. 2013].
However, Baazeem and colleagues could not demonstrate any positive impact on spontaneous pregnancy rates after varicocele repair [Baazeem et al. 2011]. However, they did note that the procedure led to a reduction in sperm DNA damage and seminal oxidative stress, along with improvement in semen parameters (count and motility). Another recent review and meta-analytical study indicated that performing varicocelectomy in infertile patients with clinical varicocele prior to intracytoplasmic sperm injection (ICSI) was associated with improved pregnancy outcomes [Esteves et al. 2015]. Clinical pregnancy rates and live birth rates were higher in the varicocelectomized men than in the men without previous varicocelectomy. However, it has been suggested that the beneficial effect of varicocelectomy is time dependent, with greater results being achieved 6 months after surgery [Hamada et al. 2013]. Therefore, the contrasting results might be caused by differences in the timing of postoperative marker measurement.
Oral antioxidant therapy
In spite of the conflicting data, many urologists prescribe oral antioxidant therapy for men with clinical subfertility with or without varicocele [Esteves and Agarwal, 2011]. The rationale for recommending oral antioxidant therapy is based on the premise that seminal oxidative stress is due in part to a deficiency in seminal antioxidants and the lack of serious side effects related to antioxidant therapy. Ideally, an oral antioxidant should reach high concentrations in the reproductive tract and restore vital elements important for spermatogenesis. Additionally, the antioxidant supplement should augment the scavenging capacity of seminal plasma and reduce levels of seminal ROS [Zini et al. 2009].
Oral treatment with antioxidants such as vitamins E, C, A, B complex, coenzyme Q10 (CoQ10), ubiquinol, glutathione, l-carnitine, lactoferrin, β-carotene, lycopene, pantothenic acid, α-lipoic acid, N-acetyl-cysteine, selenium, zinc, copper or supplements containing a combination of these antioxidants have been used with success to varying degrees [Hughes et al. 1998; Balercia et al. 2004; Greco et al. 2005; Piomboni et al. 2008; Ghanem et al. 2010; Wang et al. 2010; Zini et al. 2010; Moslemi and Tavanbakhsh, 2011; Chen et al. 2012; Safarinejad, 2012; Walczak-Jedrzejowska et al. 2013; Durairajanayagam et al. 2014; Haghighian et al. 2015; Thakur et al. 2015]. In a randomized, triple-blind, placebo-controlled clinical trial, Haghighian and colleagues observed that antioxidant therapy in the form of α-lipoic acid supplementation in infertile men improved semen parameters (sperm count, concentration and motility) and seminal levels of total antioxidant capacity (TAC) and MDA [Haghighian et al. 2015]. In infertile men, oral antioxidant therapy with Carni-Q-Nol (440 mg l-carnitine fumarate + 30 mg ubiquinol + 75 IU vitamin E + 12 mg vitamin C) softules twice or thrice daily decreased sperm pathology after 3 months, improved sperm density after 3 and 6 months, increased (ubiquinone + ubiquinol) and α-tocopherol, and decreased oxidative stress levels [Gvozdjakova et al. 2015]. Apart from improving sperm parameters, the supplementary therapy with Carni-Q-Nol resulted in a 45% pregnancy rate.
Festa and colleagues reported that oral supplementation with CoQ10 improved semen parameters and the antioxidant capacity of seminal plasma in infertile men with low-grade varicocele [Festa et al. 2014]. In another randomized double-blind, placebo-controlled trial of idiopathic oligoasthenoteratozoospermic men, oral CoQ10 supplementation increased seminal plasma TAC levels and thus reduced oxidative stress, but the semen parameters remained unaffected [Nadjarzadeh et al. 2011]. A number of other studies assessing the effects of oral antioxidant therapy in infertile men reported improved conventional semen parameters and sperm dysfunction, as well as decreased DNA fragmentation and damage [Gupta and Kumar, 2002; Greco et al. 2005; Chi et al. 2008; Piomboni et al. 2008; Gil-Villa et al. 2009; Zini et al. 2009; Ghanem et al. 2010; Wang et al. 2010; Agarwal and Sekhon, 2011; Moslemi and Tavanbakhsh, 2011; Chen et al. 2012; Safarinejad, 2012; Durairajanayagam et al. 2014; Kumalic and Pinter, 2014].
However, some contrasting reports exist in the literature [Donnelly et al. 1999; Menezo et al. 2007; Giustarini et al. 2008]. The methodological and clinical heterogeneity concerning the studied population as well as type, dosage and duration of antioxidant therapy make it difficult to compare the results and draw an unambiguous conclusion as to the optimal active dose and duration of any specific oral supplement therapy.
Moreover, antioxidant supplements are not free from potential side effects [Ko and Sabanegh, 2012; Walczak-Jedrzejowska et al. 2013; Ko et al. 2014]. Uncontrolled antioxidant therapy can be harmful to the patient as it may lead the system towards the reduced status. This paradoxical effect of antioxidants is called the ‘antioxidant paradox’ wherein a certain amount of antioxidants is essential for normal cell function (because cells generally function in a reduced state) on one hand, and a certain limited and localized level of ROS is also essential for sperm cell function on the other [Halliwell, 2000; Kothari et al. 2010]. For now, oral antioxidant supplementation continues to be a reasonable treatment option before proceeding with other more expensive treatment strategies [Ko and Sabanegh, 2014]. Further placebo-controlled, dietary-controlled, double-blind, randomized-controlled, prospective studies with standardized supplement regimens are needed to elucidate the role of antioxidant therapy in the treatment of oxidative stress and management of male infertility [Ko et al. 2014].
Measurement of seminal oxidative stress
The differences between the studies’ results concerning the role of oxidative stress in the pathogenesis of male infertility may be partly explained by the heterogeneous test methods used to measure oxidative stress. These tests are broadly divided into two categories based on their ability to directly or indirectly measure ROS [Hamada et al. 2013].
The ROS assay is the most widely utilized direct method [Zorn et al. 2003; Pons-Rejraji et al. 2009; Svobodova et al. 2009; Agarwal et al. 2014d], whereas measurement of antioxidant levels or activities using the TAC assay is less commonly used [Benedetti et al. 2012; Haghighian et al. 2015; Macanovic et al. 2015; Roychoudhury et al. 2016]. Post hoc damage is often measured as an indirect measure of oxidative stress. The most frequently used indirect test detects the presence of MDA, which indicates that lipid peroxidation has occurred as a result of excessive oxidative stress [Hosen et al. 2015; Moazamian et al. 2015].
The chemiluminescent ROS assay is based on the reaction between luminol and oxidizing compounds. The semen sample is incubated with a luminol reagent and the amount of light emitted is measured using a luminometer. The final chemiluminescent signal is the integrated sum of the partial signals generated by every spermatozoon [Agarwal et al. 2004]. It is measured in semen and the data are presented as relative light units per second per 106 sperm (RLU/s/106 sperm) [Agarwal et al. 2015a]. Several studies have found that ROS levels may be able to distinguish poor quality samples from good quality ones. A recent study by our group established an optimal cutoff value of 102.2 RLU/s/106 sperm ROS to differentiate between controls and infertile men with 76.4% sensitivity, 53.3% specificity, an 82.1% positive predictive value and a 44.5% negative predictive value [Agarwal et al. 2015a]. In another study, ROS levels were used by our laboratory to predict teratozoospermia-positive semen samples, achieving 63.9% sensitivity, 65.1% specificity and 61.4% area under curve [Agarwal et al. 2014b]. Using the same dataset, it is not surprising that morphology outpredicted the ROS cutoff, but motility also had a respectable specificity of 77.8%. An earlier study compared semen from proven donors with those that were infertile [Desai et al. 2009]. Again, an ROS cutoff was established, achieving 77.8% sensitivity and 82.4% specificity. These values are comparable with what has been found for predicting fertility based strictly on motility and morphology [Agarwal et al. 2014a]. Thus, measuring ROS might be a helpful, albeit time-consuming, way of identifying poor semen samples.
The TAC test assesses the cumulative effect of all antioxidants present within the semen based on their ability to scavenge free radicals with any specific or nonspecific mechanism(s) available [Agarwal et al. 2006, 2014a; Muller et al. 2013]. Multiple tests are available to measure TAC, although the 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) equivalent antioxidant capacity (TEAC) is the most widely used test for seminal TAC currently. This test is also called the ABTS (2,20-azino-di-[3-ethylbenzthiazoline sulfonate]) assay, which is based on the inhibition of oxidation of ABTS to ABTS+. The capacity of the antioxidants in a given sample to prevent ABTS oxidation is proportional to their concentration. The reaction is colorimetric and data are converted into comparative units using a standard curve. A stable blue-green color is formed and the standard curve can be based on the activity of Trolox, a water-soluble vitamin E compound [Mahfouz et al. 2009]. It is measured in seminal plasma and the results are reported as micromoles of Trolox equivalent.
TAC levels are also used to distinguish between poor and good quality semen samples. Our laboratory standardized a colorimetric TAC assay in a kit form that was used successfully with seminal plasma [Mahfouz et al. 2009]. In a recent study, we established a new diagnostic cutoff TAC value of 1947 µm in seminal plasma with 63.0% specificity and 59.5% sensitivity to distinguish the prevalence of oxidative stress in infertile patients compared with healthy men [Roychoudhury et al. 2016]. At this cutoff value the sensitivity was 59.5% and the specificity was 63.0%.
Another measure of oxidative stress known as the ROS-TAC score can better differentiate fertile from infertile men than ROS or TAC alone [Sharma et al. 1999, 2001; Pasqualotto et al. 2008]. The ROS and TAC values from controls are used to create an index of these two variables using controls as a reference point. Infertile men who were able to initiate pregnancies had higher ROS-TAC scores than those who failed to initiate a pregnancy [Sharma et al. 1999].
Lipid peroxidation is a measure of the damage done to proximate lipids by free radicals. Lipid peroxidation is largely represented by the presence of a byproduct, MDA. MDA is also a mutagenic compound, so MDA-based assays can also act as indirect and post hoc indicators of DNA damage in semen samples [Marnett, 1999; Martinez-Alfaro et al. 2006]. Similar to other assays, the MDA assay is based on a color change measured by a microplate reader and converted using a standard curve.
The advantages and disadvantages of commonly used tests to measure seminal oxidative stress are presented in Table 1.
Table 1.
Advantages and disadvantages of commonly used techniques to measure seminal oxidative stress.
| Assay | Advantages | Disadvantages | Reference |
|---|---|---|---|
| ROS by chemiluminescence | ● Chemiluminescence is robust ● High sensitivity and specificity ● Luminol measures global ROS levels – both extracellular and intracellular (superoxide anion, hydrogen peroxide, hydroxyl radical) |
● Time-consuming method ● Requires large and expensive equipment ● Variables such as semen age, volume, repeated centrifugation, temperature control and background luminescence may interfere with measurement |
Agarwal et al. [2004, 2015b]; Desai et al. [2009]; Kashou et al. [2013]; Vessey et al. [2014] |
| TAC | ● Rapid colorimetric method ● Measures total antioxidants in seminal plasma |
● Does not measure enzymatic antioxidants ● Length of the inhibition time is a critical aspect of the test ● Requires expensive microplate readers |
Miller et al. [1993]; Mahfouz et al. [2009]; Roychoudhury et al. [2016] |
| ROS-TAC score | ● Better predictor compared with ROS and TAC alone | ● Requires statistical modeling ● Not a direct measure of ROS or TAC, rather a prediction of oxidative stress |
Sharma et al. [2001]; Pasqualotto et al. [2008] |
| MDA (TBARS adduct by colorimetry or fluoroscopy) | ● Measures lipid peroxidation ● Detects MDA-TBA adduct by colorimetry or fluoroscopy |
● Rigorous controls required ● Non-specific test providing post hoc measure only |
Chirico et al. [1993]; Marnett, [1999]; Martinez-Alfaro et al. [2006]; Grotto et al. [2007] |
| ORP | ● Provides redox balance in real time ● Measures all known and unknown oxidants and antioxidants ● Less time-consuming and requires less expertise ● Can be measured in semen and seminal plasma, including frozen specimens |
● Affected by viscosity of the sample | Shapiro, [1972]; Rael et al. [2007]; Rael and Bar-Or [2014]; Agarwal et al. [2015b, 2016a, 2016b] |
MDA, malondialdehyde; ROS, reactive oxygen species; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances.
Oxidation-reduction potential: measurement and clinical utility
With the numerous ways in which ‘oxidative stress’ is implied by partitioned measures, it is not unreasonable to question its role in male infertility. Therefore, it is essential to develop an unbiased and universal measure of semen quality that represents the overall oxidant and antioxidant activity in a given sample.
ORP, also known as the redox potential, is a measure of the potential for electrons to move from one chemical species to another [McCord, 2000; Costantini and Verhulst, 2009]. To quench the damaging effects of oxidants, antioxidants work by donating electrons to the oxidants, thereby reducing the chances of oxidants to acquire electrons from other nearby structures and cause damage. ORP is a measure of this relationship between oxidants and antioxidants, providing a comprehensive measure of oxidative stress. Higher ORP values indicate an imbalance in the activity of oxidants relative to antioxidants, thus differentiating the degree of oxidative stress-induced male factor infertility. Monitoring ORP levels may help predict treatment efficacy in such patients as higher ORP levels are indicative of the progression of infertility. ORP represents the balance among all known and unknown contributors of oxidative stress and is not limited to only a specific constituent as in ROS or TAC assays. Recently, a novel technology based on a galvanostatic measure of electrons has been developed that easily and readily measures ORP – the MiOXSYS System (Figure 2). This system has been used to assess changes in oxidative stress in trauma patients and as a function of extreme exercise [Rael et al. 2007, 2009; Stagos et al. 2015a, 2015b]. Data from the MiOXSYS System are presented in static ORP measures (sORP), with static referring to the passive or current state of activity between oxidants and antioxidants.
Figure 2.
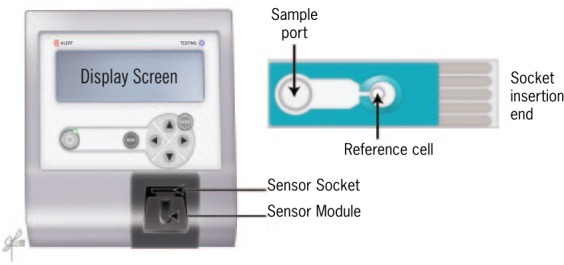
MiOXSYS System for measurement of oxidation-reduction potential (ORP): MiOXSYS analyzer and sensor.
In the MiOXSYS System, a small volume (~30 µl) of liquefied neat semen is added to the pre-inserted sensor. The sample is wicked through the membrane to the measuring electrodes at the end. The MiOXSYS analyzer applies a low voltage current and the electron activity is measured in millivolts (mV). The entire process takes less than 4 minutes; the only preparation needed is to liquefy the semen sample – a step that is required by all oxidative stress assays. Similar to previous assays, the data are relative. Higher sORP values indicate a higher state of oxidative stress. Using sORP, our data [Agarwal et al. 2016a, 2016b] reinforce the impression that oxidative stress is related to poor semen quality [Zorn et al. 2003; Pons-Rejraji et al. 2009; Benedetti et al. 2012; Agarwal et al. 2014e; Macanovic et al. 2015]. sORP measured in semen from the male partners of infertile couples suggest that values are significantly lower in samples that have normal individual semen parameters (Figure 3). For example, semen samples that have more normal sperm morphology have significantly lower sORP values than those that have more abnormal morphology. When conventional semen parameters and sORP were analyzed in 366 men, sORP values (mean ± standard error of the mean, SEM) were lower (p < 0.0001) in the samples with normal semen parameters than in those with semen parameters outside the normal range (Figure 3). Volume was the only parameter that did not affect sORP values, similar to the ROS assay [Zorn et al. 2003]. This clearly establishes the positive association of sORP values with conventional semen parameters.
Figure 3.
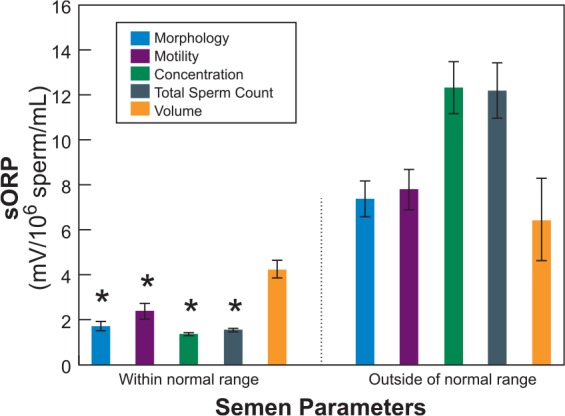
Comparison of sORP (mean ± SEM) measured in semen samples with semen parameters (sperm morphology, motility, concentration, total count and semen volume) within the normal range and those outside according to 2010 WHO criteria.
Total sample size, n = 366: morphology (within range n = 183, outside range n = 183), motility (within range n = 219, outside range n = 147), concentration (within range n = 260, outside range n = 106), total sperm count (within range n = 264, outside range n = 102), volume (within range n = 313, outside range n = 53).
Data analyzed by individual parameter using Student’s t-test.
*Differences were statistically significant at a p < 0.0001.
SEM, standard error of the mean; sORP, static oxidation-reduction potential; WHO, World Health Organization.
Semen samples from oligoasthenoteratozoospermic (OAT) patients have higher levels of isoprostane (a byproduct of arachidonic acid peroxidation), lower levels of catalase and lower TAC levels [Khosrowbeygi and Zarghami, 2007a, 2007b]. sORP data from 218 normozoospermic men and 69 OAT patients are presented in Figure 4; the data confirm the state of oxidative stress as the OAT patients have significantly higher (p < 0.0001) sORP values (mean ± SEM) than the normozoospermic men who had normal concentration, motility and morphology; this is supported by previous findings [Cavallini, 2006]. Furthermore, sORP measurements are not affected by the age of semen or seminal plasma for up to 2 hours.
Figure 4.
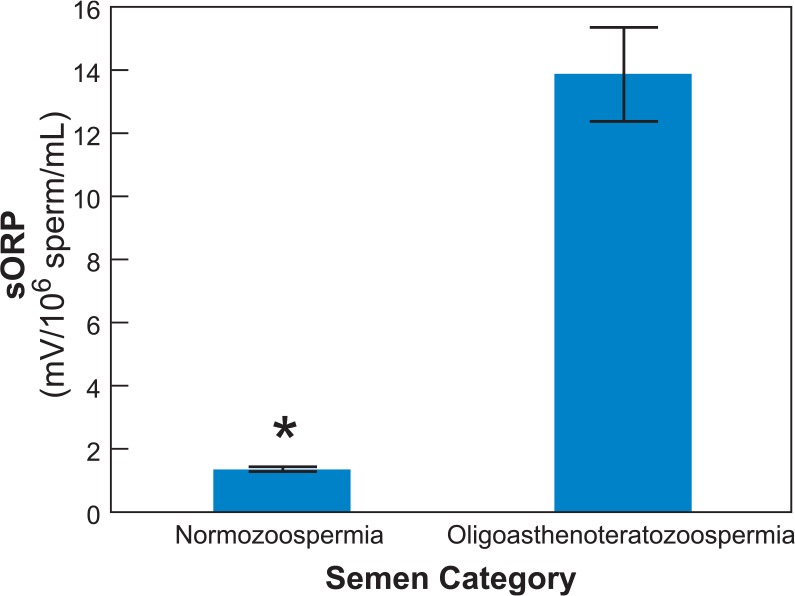
Comparison of sORP (mean ± SEM) in semen samples from normozoospermic and oligoasthenoteratozoospermic men. Normozoospermic semen (n = 149) have significantly lower sORP values than oligoasthenoteratozoospermic semen (n = 69).
Data were analyzed by Student’s t-test.
*Differences were statistically significant at a p < 0.0001.
SEM, standard error of the mean; sORP, static oxidation-reduction potential.
Application of sORP measurement in clinical practice is expected to benefit the treatment of infertile men with varicocele, infection, inflammation, spinal cord injury and severe oligozoospermia [Agarwal et al. 2016a]. sORP can be measured in cryopreserved semen samples as a single marker for oxidants and available antioxidant reserve, which is important in predicting the success of assisted reproductive techniques [Agarwal et al. 2016b]. sORP values in a given sample can be used as an initial screening tool to identify samples that are more likely to benefit from various sophisticated tests and further treatment [Agarwal et al. 2016b]. Assessing changes in seminal oxidative stress over time would be helpful to monitor the effect of antioxidant therapies and define effective doses and durations. Most measures of oxidative stress are inefficient and provide a single faceted view. We suggest that sORP would be a better alternative, as it is more easily applicable and comprehensive in the measurement of the overall oxidative stress in a sample.
Conclusion
ORP provides a comprehensive measure of oxidative stress by analyzing all known and unknown oxidants and antioxidants with a high sensitivity and specificity [Agarwal et al. 2016a, 2016b]. Compared with existing semen oxidative stress tests, sORP measurements using the portable MiOXSYS System can assess seminal oxidative stress quickly in small volumes of semen samples, and even in cryopreserved semen samples. It facilitates the wider application of oxidative stress measurement in clinical and research settings. Measurement of seminal oxidative stress is an invaluable tool to identify patients who will potentially benefit from treatment. Progress can be readily monitored and studied with the ready-to-use ORP assay kit. The measurement of sORP by the MiOXSYS System as an advanced and independent test for semen quality should find its place in the male infertility workup algorithm.
Footnotes
Funding: The stay of Dr Shubhadeep Roychoudhury at the Cleveland Clinic was supported by the Department of Biotechnology, Government of India.
Conflict of interest statement: Kimberly B. Bjugstad is a paid employee of Aytu BioScience, Inc.
Contributor Information
Ashok Agarwal, Director, Andrology Center and American Center for Reproductive Medicine, Lerner College of Medicine, Cleveland Clinic, 10681 Carnegie Avenue, Desk X11, Cleveland, OH 44195, USA.
Shubhadeep Roychoudhury, American Center for Reproductive Medicine, Cleveland Clinic, Cleveland, OH, USA Department of Life Science and Bioinformatics, Assam University, Silchar, India.
Kimberly B. Bjugstad, Aytu BioScience, Inc. Englewood, CO, USA
Chak-Lam Cho, Department of Surgery, Kwong Wah Hospital, Hong Kong.
References
- Agarwal A., Ahmad G., Sharma R. (2015a) Reference values of reactive oxygen species in seminal ejaculates using chemiluminescence assay. J Assist Reprod Genet 32: 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Allamaneni S., Said T. (2004) Chemiluminescence technique for measuring reactive oxygen species. Reprod Biomed Online 9: 466–468. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Deepinder F., Sharma R., Ranga G., Li J. (2008a) Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril 89: 124–128. [DOI] [PubMed] [Google Scholar]
- Agarwal A., du Plessis S., Sharma R., Samanta L., Harlev A., Ahmad G., et al. (2015b) Establishing the oxidation-reduction potential in semen and seminal plasma. In: 71st Annual Meeting of the American Society for Reproductive Medicine, Baltimore, MD, 18–22 October 2015, poster P-116. [Google Scholar]
- Agarwal A., Hamada A., Esteves S. (2012) Insight into oxidative stress in varicocele-associated male infertility: part 1. Nat Rev Urol 9: 678–690. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Makker K., Sharma R. (2008b) Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 59: 2–11. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Mulgund A., Alshahrani S., Assidi M., Abuzenadah A., Sharma R., et al. (2014e) Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol 12: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Mulgund A., Hamada A., Chyatte M. (2015a) A unique view on male infertility around the globe. Reprod Biol Endocrinol 13: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Mulgund A., Sharma R., Sabanegh E. (2014d) Mechanisms of oligozoospermia: an oxidative stress perspective. Syst Biol Reprod Med 60: 206–216. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Said T. (2004) Carnitines and male infertility. Reprod Biomed Online 8: 376–384. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Said T., Bedaiwy M., Banerjee J., Alvarez J. (2006) Oxidative stress in an assisted reproductive techniques setting. Fertil Steril 86: 503–512. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Saleh R., Bedaiwy M. (2003) Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 79: 829–843. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Sekhon L. (2011) Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: is it justified? Indian J Urol 27: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Sharma R., du Plessis S., Sabanegh E. (2016a) MiOXSYS – a novel method of measuring oxidation-reduction potential in semen and seminal plasma. Fertil Steril. In press. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Sharma R., du Plessis S., Sikka S., Sharda Y., Speyer C., et al. (2016b) Validation of oxidation-reduction potential in fresh and frozen samples with MiOXSYS System. Fertil Steril. In press. [Google Scholar]
- Agarwal A., Sharma R., Sharma R., Assidi M., Abuzenadah A., Alshahrani S., et al. (2014c) Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod Biol Endocrinol 12: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Tvrda E., Sharma R. (2014b) Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol 12: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Virk G., Ong C., du Plessis S. (2014a) Effect of oxidative stress on male reproduction. World J Mens Health 32: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Zini A., Sigman M. (2013) Is sperm DNA integrity assessment useful? J Urol 190: 1645–1647. [DOI] [PubMed] [Google Scholar]
- Aitken R. (1989) The role of free oxygen radicals and sperm function. Int J Androl 12: 95–97. [DOI] [PubMed] [Google Scholar]
- Aitken R., Baker M., Sawyer D. (2003) Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reprod Biomed Online 7: 65–70. [DOI] [PubMed] [Google Scholar]
- Aitken R., Clarkson J. (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 81: 459–469. [DOI] [PubMed] [Google Scholar]
- Aitken R., Clarkson J., Fishel S. (1989) Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod 41: 183–197. [DOI] [PubMed] [Google Scholar]
- Aitken R., De Iuliis G. (2010) On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod 16: 3–13. [DOI] [PubMed] [Google Scholar]
- Aitken R., Koppers A. (2011) Apoptosis and DNA damage in human spermatozoa. Asian J Androl 13: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken R., Krausz C. (2001) Oxidative stress, DNA damage and the Y chromosome. Reproduction 122: 497–506. [DOI] [PubMed] [Google Scholar]
- Aitken R., Paterson M., Fisher H., Buckingham D., van Duin M. (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 108: 2017–2025. [DOI] [PubMed] [Google Scholar]
- ASRM: Practice Committee of American Society for Reproductive Medicine (2008) Report on varicocele and infertility. Fertil Steril 90(Suppl. 5): S247–S249. [DOI] [PubMed] [Google Scholar]
- Athayde K., Cocuzza M., Agarwal A., Krajcir N., Lucon A., Srougi M., et al. (2007) Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl 28: 613–620. [DOI] [PubMed] [Google Scholar]
- Aversa A., Morgentaler A. (2015) The practical management of testosterone deficiency in men. Nat Rev Urol 12: 641–650. [DOI] [PubMed] [Google Scholar]
- Ayala A., Munoz M., Arguelles S. (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-noneal. Oxid Med Cell Longev. Article ID: 360438. DOI: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Saleh R., Sharma R., Lewis-Jones I., Esfandiari N., Thomas A., Jr., et al. (2004) Novel association between sperm reactive oxygen species production, sperm morphological defects, and the sperm deformity index. Fertil Steril 81: 349–354. [DOI] [PubMed] [Google Scholar]
- Baazeem A., Belzile E., Ciampi A., Dohle G., Jarvi K., Salonia A., et al. (2011) Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol 60: 796–808. [DOI] [PubMed] [Google Scholar]
- Baker K., Li J., Sabanegh E., Jr. (2015) Analysis of semen parameters in male referrals: impact of reference limits, stratification by fertility categories, predictors of change, and comparison of normal semen parameters in subfertile couples. Fertil Steril 103: 59–65. [DOI] [PubMed] [Google Scholar]
- Balercia G., Mosca F., Mantero F., Boscaro M., Mancini A., Ricciardo-Lamonica G., et al. (2004) Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril 81: 93–98. [DOI] [PubMed] [Google Scholar]
- Barratt C., Mansell S., Beaton C., Tardif S., Oxenham S. (2011) Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl 13: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti S., Tagliamonte M., Catalani S., Primiterra M., Canestrari F., De Stefani S., et al. (2012) Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online 25: 300–306. [DOI] [PubMed] [Google Scholar]
- Catanzariti F., Cantoro U., Lacetera V., Muzzonigro G., Polito M. (2013) Comparison between WHO (World Health Organization) 2010 and WHO 1999 parameters for semen analysis - interpretation of 529 consecutive samples. Arch Ital Urol Androl 85: 125–129. [DOI] [PubMed] [Google Scholar]
- Cavallini G. (2006) Male idiopathic oligoasthenoteratozoospermia. Asian J Androl 8: 143–157. [DOI] [PubMed] [Google Scholar]
- Chen S., Allam J., Duan Y., Haidl G. (2013) Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 288: 191–199. [DOI] [PubMed] [Google Scholar]
- Chen S., Huang W., Chang L., Wei Y. (2008) Attenuation of oxidative stress after varicocelectomy in subfertile patients with varicocele. J Urol 179: 639–642. [DOI] [PubMed] [Google Scholar]
- Chen X., Li Z., Ping P., Dai J., Zhang F., Shang X. (2012) Efficacy of natural vitamin E on oligospermia and asthenospermia: a prospective multi-centered randomized controlled study of 106 cases. Zhonghua Nan Ke Xue 18: 428–431. [PubMed] [Google Scholar]
- Chi H., Kim J., Ryu C., Lee J., Park J., Chung D., et al. (2008) Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum Reprod 23: 1023–1028. [DOI] [PubMed] [Google Scholar]
- Chirico S., Smith C., Marchant C., Mitchinson M., Halliwell B. (1993) Lipid peroxidation in hyperlipidaemic patients. A study of plasma using an HPLC-based thiobarbituric acid test. Free Radic Res Commun 19: 51–57. [DOI] [PubMed] [Google Scholar]
- Cocuzza M., Athayde K., Alvarenga C., Srougi M., Hallak J. (2012) Grade 3 varicocele in fertile men: a different entity. J Urol 187: 1363–1368. [DOI] [PubMed] [Google Scholar]
- Cocuzza M., Sikka S., Athayde K., Agarwal A. (2007) Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol 33: 603–621. [DOI] [PubMed] [Google Scholar]
- Costantini D., Verhulst S. (2009) Does high antioxidant capacity indicate low oxidative stress? Funct Ecol 23: 506–509. [Google Scholar]
- Dada R., Shamsi M., Venkatesh S., Gupta N., Kumar R. (2010) Attenuation of oxidative stress & DNA damage in varicocelectomy: implications in infertility management. Indian J Med Res 132: 728–730. [PMC free article] [PubMed] [Google Scholar]
- De Lamirande E., Gagnon C. (1993) Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med 14: 157–166. [DOI] [PubMed] [Google Scholar]
- Desai N., Sharma R., Makker K., Sabanegh E., Agarwal A. (2009) Physiologic and pathologic levels of reactive oxygen species in neat semen of infertile men. Fertil Steril 92: 1626–1631. [DOI] [PubMed] [Google Scholar]
- Donnelly E., McClure N., Lewis S. (1999) Antioxidant supplementation in vitro does not improve human sperm motility. Fertil Steril 72: 484–495. [DOI] [PubMed] [Google Scholar]
- Durackova Z. (2014) Free radicals and antioxidants for non-experts. In: Laher I. (ed.), Systems Biology of Free Radicals and Antioxidants. Berlin: Springer Verlag. [Google Scholar]
- Durairajanayagam D., Agarwal A., Ong C., Prashast P. (2014) Lycopene and male infertility. Asian J Androl 16: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran E., Morshedi M., Taylor S., Oehninger S. (2002) Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod 17: 3122–3128. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Betts P., Herder D., Lamb D., Lipshultz L. (2013) Increased risk of cancer among azoospermic men. Fertil Steril 100: 681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Eckl P., Ortner A. (1990) Possible mutagens derived from lipids and lipid precursors. Mutat Res 238: 223–233. [DOI] [PubMed] [Google Scholar]
- Esteves S. (2014) Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 40: 443–453. [DOI] [PubMed] [Google Scholar]
- Esteves S., Agarwal A. (2011) Novel concepts in male infertility. Int Braz J Urol 37: 5–15. [DOI] [PubMed] [Google Scholar]
- Esteves S., Hamada A., Kondray V., Pitchika A., Agarwal A. (2012) What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet 286: 217–229. [DOI] [PubMed] [Google Scholar]
- Esteves S., Miyaoka R., Agarwal A. (2011) An update on the clinical assessment of the infertile male. [corrected]. Clinics (Sao Paulo) 66: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves S., Roque M., Agarwal A. (2015) Outcome of assisted reproductive technology in men with treated and untreated varicocele: systematic review and meta-analysis. Asian J Androl 18: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa R., Giacchi E., Raimondo S., Tiano L., Zuccarelli P., Silvestrini A., et al. (2014) Coenzyme Q10 supplementation in infertile men with low-grade varicocele: an open, uncontrolled pilot study. Andrologia 46: 805–807. [DOI] [PubMed] [Google Scholar]
- Ghanem H., Shaeer O., El-Segini A. (2010) Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril 93: 2232–2235. [DOI] [PubMed] [Google Scholar]
- Gharagozloo P., Aitken R. (2011) The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 26: 1628–1640. [DOI] [PubMed] [Google Scholar]
- Gil-Villa A., Cardona-Maya W., Agarwal A., Sharma R., Cadavid A. (2009) Role of male factor in early recurrent embryo loss: do antioxidants have any effect? Fertil Steril 92: 565–571. [DOI] [PubMed] [Google Scholar]
- Giustarini D., Dalle-Donne I., Colombo R., Milzani A., Rossi R. (2008) Is ascorbate able to reduce disulfide bridges? A cautionary note. Nitric Oxide 19: 252–258. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Gilbert B., Dicker A., Dwosh J., Gnecco C. (1992) Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol 148: 1808–1811. [DOI] [PubMed] [Google Scholar]
- Greco E., Romano S., Iacobelli M., Ferrero S., Baroni E., Minasi M., et al. (2005) ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod 20: 2590–2594. [DOI] [PubMed] [Google Scholar]
- Greil A., Slauson-Blevins K., McQuillan J. (2010) The experience of infertility: a review of recent literature. Sociol Health Illn 32: 140–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotto D., Santa Maria L., Boeira S., Valentini J., Charao M., Moro A., et al. (2007) Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J Pharm Biomed Anal 43: 619–624. [DOI] [PubMed] [Google Scholar]
- Gupta N., Kumar R. (2002) Lycopene therapy in idiopathic male infertility – a preliminary report. Int Urol Nephrol 34: 369–372. [DOI] [PubMed] [Google Scholar]
- Guthrie H., Welch G. (2012) Effects of reactive oxygen species on sperm function. Theriogenology 78: 1700–1708. [DOI] [PubMed] [Google Scholar]
- Guzick D., Overstreet J., Factor-Litvak P., Brazil C., Nakajima S., Coutifaris C., et al. (2001) Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 345: 1388–1393. [DOI] [PubMed] [Google Scholar]
- Gvozdjakova A., Kucharska J., Dubravicky J., Mojto V., Singh R. (2015) Coenzyme Q10, α-tocopherol, and oxidative stress could be important metabolic biomarkers of male infertility. Dis Markers. DOI: 10.1155/2015/827941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighian H., Haidari F., Mohammadi-Asl J., Dadfar M. (2015) Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril 104: 318–324. [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2000) The antioxidant paradox. Lancet 355: 1179–1180. [DOI] [PubMed] [Google Scholar]
- Hamada A., Esteves S., Agarwal A. (2013) Insight into oxidative stress in varicocele-associated male infertility: part 2. Nat Rev Urol 10: 26–37. [DOI] [PubMed] [Google Scholar]
- Hampl R., Drabkova P., Kandar R., Stepan J. (2012) Impact of oxidative stress on male infertility. Ceska Gynekol 77: 241–245. [PubMed] [Google Scholar]
- Henkel R., Kierspel E., Hajimohammad M., Stalf T., Hoogendijk C., Mehnert C., et al. (2003) DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online 7: 477–484. [DOI] [PubMed] [Google Scholar]
- Hipler U., Gornig M., Hipler B., Romer W., Schreiber G. (2000) Stimulation and scavestrogen-induced inhibition of reactive oxygen species generated by rat sertoli cells. Arch Androl 44:147–154. [DOI] [PubMed] [Google Scholar]
- Hosen M., Islam M., Begum F., Kabir Y., Howlader M. (2015) Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran J Reprod Med 13: 525–532. [PMC free article] [PubMed] [Google Scholar]
- Hughes C., Lewis S., McKelvey-Martin V., Thompson W. (1998) The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum Reprod 13: 1240–1247. [DOI] [PubMed] [Google Scholar]
- Hurtado de Catalfo G., Ranieri-Casilla A., Marra F., de Alaniz M., Marra C. (2007) Oxidative stress biomarkers and hormonal profile in human patients undergoing varicocelectomy. Int J Androl 30: 519–530. [DOI] [PubMed] [Google Scholar]
- Joffe M. (2010) Semen quality analysis and the idea of normal fertility. Asian J Androl 12: 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Mann T., Sherins R. (1979) Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril 31: 531–537. [DOI] [PubMed] [Google Scholar]
- Kashou A., Sharma R., Agarwal A. (2013) Assessment of oxidative stress in sperm and semen. Methods Mol Biol 927: 351–361. [DOI] [PubMed] [Google Scholar]
- Khosrowbeygi A., Zarghami N. (2007a) Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot Essent Fatty Acids 77: 117–121. [DOI] [PubMed] [Google Scholar]
- Khosrowbeygi A., Zarghami N. (2007b) Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin Pathol 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Lee J., Kang D., Lee H., Seo J., Cho K. (2013) Impact of surgical varicocele repair on pregnancy rate in subfertile men with clinical varicocele and impaired semen quality: a meta-analysis of randomized clinical trials. Korean J Urol 54: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E., Sabanegh E. (2012) The role of over-the-counter supplements for the treatment of male infertility–fact or fiction? J Androl 33: 292–308. [DOI] [PubMed] [Google Scholar]
- Ko E., Sabanegh E. (2014) The role of nutraceuticals in male fertility. Urol Clin North Am 41: 181–193. [DOI] [PubMed] [Google Scholar]
- Ko E., Sabanegh E., Jr., Agarwal A. (2014) Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril 102: 1518–1527. [DOI] [PubMed] [Google Scholar]
- Kodama H., Yamaguchi R., Fukuda J., Kasai H., Tanaka T. (1997) Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 68: 519–524. [DOI] [PubMed] [Google Scholar]
- Kothari S., Thompson A., Agarwal A., du Plessis S. (2010) Free radicals: their beneficial and detrimental effects on sperm function. Indian J Exp Biol 48: 425–435. [PubMed] [Google Scholar]
- Kumalic S., Pinter B. (2014) Review of clinical trials on effects of oral antioxidants on basic semen and other parameters in idiopathic oligoasthenoteratozoospermia. Biomed Res Int. DOI: 10.1155/2014/426951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavranos G., Balla M., Tzortzopoulou A., Syriou V., Angelopoulou R. (2012) Investigating ROS sources in male infertility: a common end for numerous pathways. Reprod Toxicol 34: 298–307. [DOI] [PubMed] [Google Scholar]
- Lewis S., Simon L. (2010) Clinical implications of sperm DNA damage. Hum Fertil 13: 201–207. [DOI] [PubMed] [Google Scholar]
- Macanovic B., Vucetic M., Jankovic A., Stancic A., Buzadzic B., Garalejic E., et al. (2015) Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: a pilot study. Dis Markers. DOI: 10.1155/2015/436236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madgar I., Weissenberg R., Lunenfeld B., Karasik A., Goldwasser B. (1995) Controlled trial of high spermatic vein ligation for varicocele in infertile men. Fertil Steril 63: 120–124. [DOI] [PubMed] [Google Scholar]
- Mahfouz R., Sharma R., Sharma D., Sabanegh E., Agarwal A. (2009) Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril 91: 805–811. [DOI] [PubMed] [Google Scholar]
- Manda K., Ueno M., Moritake T., Anzai K. (2007) alpha-Lipoic acid attenuates X-irradiation-induced oxidative stress in mice. Cell Biol Toxicol 23: 129–137. [DOI] [PubMed] [Google Scholar]
- Marmar J., Agarwal A., Prabakaran S., Agarwal R., Short R., Benoff S., et al. (2007) Reassessing the value of varicocelectomy as a treatment for male subfertility with a new meta-analysis. Fertil Steril 88: 639–648. [DOI] [PubMed] [Google Scholar]
- Marnett L. (1999) Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res 424: 83–95. [DOI] [PubMed] [Google Scholar]
- Martinez G., Daniels K., Chandra A. (2012) Fertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010. National Health Statistics Reports, Number 51. Hyattsville, MD: Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- Martinez-Alfaro M., Palma-Tirado L., Sandoval-Zapata F., Carabez-Trejo A. (2006) Correlation between formamidopyrimidine DNA glycosylase (FPG)-sensitive sites determined by a comet assay, increased MDA, and decreased glutathione during long exposure to thinner inhalation. Toxicol Lett 163: 198–205. [DOI] [PubMed] [Google Scholar]
- McCord J. (2000) The evolution of free radicals and oxidative stress. Am J Med 108: 652–659. [DOI] [PubMed] [Google Scholar]
- Menezo Y., Hazout A., Panteix G., Robert F., Rollet J., Cohen-Bacrie P., et al. (2007) Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online 14: 418–421. [DOI] [PubMed] [Google Scholar]
- Miller N., Rice-Evans C., Davies M., Gopinathan V., Milner A. (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 84: 407–412. [DOI] [PubMed] [Google Scholar]
- Moazamian R., Polhemus A., Connaughton H., Fraser B., Whiting S., Gharagozloo P., et al. (2015) Oxidative stress and human spermatozoa: diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod 21: 502–515. [DOI] [PubMed] [Google Scholar]
- Moghissi K., Wallach E. (1983) Unexplained infertility. Fertil Steril 39: 5–21. [DOI] [PubMed] [Google Scholar]
- Moslemi M., Tavanbakhsh S. (2011) Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med 4: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa T., Anis T., El-Nashar A., Imam H., Othman I. (2001) Varicocelectomy reduces reactive oxygen species levels and increases antioxidant activity of seminal plasma from infertile men with varicocele. Int J Androl 24: 261–265. [DOI] [PubMed] [Google Scholar]
- Moustafa M., Sharma R., Thornton J., Mascha E., Abdel-Hafez M., Thomas A., Jr., et al. (2004) Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod 19: 129–138. [DOI] [PubMed] [Google Scholar]
- Muller C., Lee T., Montano M. (2013) Improved chemiluminescence assay for measuring antioxidant capacity of seminal plasma. Methods Mol Biol 927: 363–376. [DOI] [PubMed] [Google Scholar]
- Nadjarzadeh A., Sadeghi M., Amirjannati N., Vafa M., Motevalian S., Gohari M., et al. (2011) Coenzyme Q10 improves seminal oxidative defense but does not affect on semen parameters in idiopathic oligoasthenoteratozoospermia: a randomized double-blind, placebo controlled trial. J Endocrinol Invest 34: e224–e228. [DOI] [PubMed] [Google Scholar]
- Novotny J., Aziz N., Rybar R., Brezinova J., Kopecka V., Filipcikova R., et al. (2013) Relationship between reactive oxygen species production in human semen and sperm DNA damage assessed by Sperm Chromatin Structure Assay. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 157: 383–386. [DOI] [PubMed] [Google Scholar]
- Onozawa M., Endo F., Suetomi T., Takeshima H., Akaza H. (2002) Clinical study of varicocele: statistical analysis and the results of long-term follow-up. Int J Urol 9: 455–461. [DOI] [PubMed] [Google Scholar]
- Ozmen B., Koutlaki N., Youssry M., Diedrich K., Al-Hasani S. (2007) DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online 14: 384–395. [DOI] [PubMed] [Google Scholar]
- Papillon-Smith J., Baker S., Agbo C., Dahan M. (2015) Pregnancy rates with intrauterine insemination: comparing 1999 and 2010 World Health Organization semen analysis norms. Reprod Biomed Online 30: 392–400. [DOI] [PubMed] [Google Scholar]
- Pasqualotto F., Sharma R., Nelson D., Thomas A., Agarwal A. (2000) Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil Steril 73: 459–464. [DOI] [PubMed] [Google Scholar]
- Pasqualotto F., Sundaram A., Sharma R., Borges E., Jr., Pasqualotto E., Agarwal A. (2008) Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil Steril 89: 602–607. [DOI] [PubMed] [Google Scholar]
- Piomboni P., Gambera L., Serafini F., Campanella G., Morgante G., De Leo V. (2008) Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl 10: 201–206. [DOI] [PubMed] [Google Scholar]
- Pons-Rejraji H., Sion B., Saez F., Brugnon F., Janny L., Grizard G. (2009) Role of reactive oxygen species (ROS) on human spermatozoa and male infertility. Gynecol Obstet Fertil 37: 529–535. [DOI] [PubMed] [Google Scholar]
- Potts J., Pasqualotto F. (2003) Seminal oxidative stress in patients with chronic prostatitis. Andrologia 35: 304–308. [PubMed] [Google Scholar]
- Pryor W. (1989) On the detection of lipid hydroperoxides in biological samples. Free Radic Biol Med 7: 177–178. [DOI] [PubMed] [Google Scholar]
- Rael L., Bar-Or R. (2014) Method and apparatus for measuring oxidation-reduction potential. US Patent 8,641,888. [Google Scholar]
- Rael L., Bar-Or R., Aumann R., Slone D., Mains C., Bar-Or D. (2007) Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem Biophys Res Commun 361: 561–565. [DOI] [PubMed] [Google Scholar]
- Rael L., Bar-Or R., Mains C., Slone D., Levy A., Bar-Or D. (2009) Plasma oxidation-reduction potential and protein oxidation in traumatic brain injury. J Neurotrauma 26: 1203–1211. [DOI] [PubMed] [Google Scholar]
- Raman J., Nobert C., Goldstein M. (2005) Increased incidence of testicular cancer in men presenting with infertility and abnormal semen analysis. J Urol 174: 1819–1822; discussion 1822. [DOI] [PubMed] [Google Scholar]
- Roychoudhury S., Sharma R., Sikka S., Agarwal A. (2016) Diagnostic application of total antioxidant capacity in seminal plasma to assess oxidative stress in male factor infertility. J Asst Reprod Genet. DOI: 10.1007/s10815-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalu L. (2010) The incriminating role of reactive oxygen species in idiopathic male infertility: an evidence based evaluation. Pak J Biol Sci 13: 413–422. [DOI] [PubMed] [Google Scholar]
- Safarinejad M. (2012) The effect of coenzyme Q10 supplementation on partner pregnancy rate in infertile men with idiopathic oligoasthenoteratozoospermia: an open-label prospective study. Int Urol Nephrol 44: 689–700. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Ishikawa T., Kondo Y., Yamaguchi K., Fujisawa M. (2008) The assessment of oxidative stress in infertile patients with varicocele. BJU Int 101: 1547–1552. [DOI] [PubMed] [Google Scholar]
- Saleh R., Agarwal A. (2002) Oxidative stress and male infertility: from research bench to clinical practice. J Androl 23: 737–752. [PubMed] [Google Scholar]
- Saleh R., Agarwal A., Kandirali E., Sharma R., Thomas A., Nada E., et al. (2002) Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril 78: 1215–1224. [DOI] [PubMed] [Google Scholar]
- Salonia A., Matloob R., Gallina A., Abdollah F., Sacca A., Briganti A., et al. (2009) Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol 56: 1025–1031. [DOI] [PubMed] [Google Scholar]
- Schulte R., Ohl D., Sigman M., Smith G. (2010) Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet 27: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H. (1972) Redox balance in the body: an approach to quantitation. J Surg Res 13: 138–152. [DOI] [PubMed] [Google Scholar]
- Sharma R., Agarwal A. (1996) Role of reactive oxygen species in male infertility. Urology 48: 835–850. [DOI] [PubMed] [Google Scholar]
- Sharma R., Pasqualotto F., Nelson D., Thomas A., Jr., Agarwal A. (1999) The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum Reprod 14: 2801–2807. [DOI] [PubMed] [Google Scholar]
- Sharma R., Pasqualotto A., Nelson D., Thomas A., Jr., Agarwal A. (2001) Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J Androl 22: 575–583. [PubMed] [Google Scholar]
- Shiraishi K., Matsuyama H., Takihara H. (2012) Pathophysiology of varicocele in male infertility in the era of assisted reproductive technology. Int J Urol 19: 538–550. [DOI] [PubMed] [Google Scholar]
- Slade P., O’Neill C., Simpson A., Lashen H. (2007) The relationship between perceived stigma, disclosure patterns, support and distress in new attendees at an infertility clinic. Hum Reprod 22: 2309–2317. [DOI] [PubMed] [Google Scholar]
- Stagos D., Goutzourelas N., Bar-Or D., Ntontou A., Bella E., Becker A., et al. (2015a) Application of a new oxidation-reduction potential assessment method in strenuous exercise-induced oxidative stress. Redox Rep 20: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagos D., Goutzourelas N., Ntontou A.M., Kafantaris I., Deli C.K., Poulios A., et al. (2015b) Assessment of eccentric exercise-induced oxidative stress using oxidation-reduction potential markers. Oxid Med Cell Longev. DOI: 10.1155/2015/204615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svobodova M., Oborna I., Fingerova H., Novotny J., Brezinova J., Radova L., et al. (2009) Comparison of reactive oxygen species production in neat semen and washed spermatozoa. Ceska Gynekol 74: 399–403. [PubMed] [Google Scholar]
- Tarozzi N., Bizzaro D., Flamigni C., Borini A. (2007) Clinical relevance of sperm DNA damage in assisted reproduction. Reprod Biomed Online 14: 746–757. [DOI] [PubMed] [Google Scholar]
- Thakur A., Littarru G., Funahashi I., Painkara U., Dange N., Chauhan P. (2015) Effect of ubiquinol therapy on sperm parameters and serum testosterone levels in oligoasthenozoospermic infertile men. J Clin Diagn Res 9: BC01–BC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg J., Fulton N., Gomez E., Irvine D., Aitken R. (1998) Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod 13: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Van der Steeg J., Steures P., Eijkemans M., Habbema J., Hompes P., Kremer J., et al. (2011) Role of semen analysis in subfertile couples. Fertil Steril 95: 1013–1019. [DOI] [PubMed] [Google Scholar]
- Ventimiglia E., Capogrosso P., Boeri L., Serino A., Colicchia M., Ippolito S., et al. (2015) Infertility as a proxy of general male health: results of a cross-sectional survey. Fertil Steril 104: 48–55. [DOI] [PubMed] [Google Scholar]
- Vessey W., Perez-Miranda A., Macfarquhar R., Agarwal A., Homa S. (2014) Reactive oxygen species in human semen: validation and qualification of a chemiluminescence assay. Fertil Steril 102: 1576–1583. [DOI] [PubMed] [Google Scholar]
- Walczak-Jedrzejowska R., Wolski J., Slowikowska-Hilczer J. (2013) The role of oxidative stress and antioxidants in male fertility. Cent European J Urol 66: 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T., Schembri M., Turek P., Chan J., Carroll P., Smith J., et al. (2010) Increased risk of high-grade prostate cancer among infertile men. Cancer 116: 2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang S., Qu C., Huo H., Li W., Li J., et al. (2010) L-carnitine: safe and effective for asthenozoospermia. Zhonghua Nan Ke Xue 16: 420–422. [PubMed] [Google Scholar]
- Whittington K., Ford W. (1999) Relative contribution of leukocytes and of spermatozoa to reactive oxygen species production in human sperm suspensions. Int J Androl 22: 229–235. [DOI] [PubMed] [Google Scholar]
- Whittington K., Harrison S., Williams K., Day J., McLaughlin E., Hull M., et al. (1999) Reactive oxygen species (ROS) production and the outcome of diagnostic tests of sperm function. Int J Androl 22: 236–242. [DOI] [PubMed] [Google Scholar]
- Witt M., Lipshultz L. (1993) Varicocele: a progressive or static lesion? Urology 42: 541–543. [DOI] [PubMed] [Google Scholar]
- Wolff H. (1995) The biologic significance of white blood cells in semen. Fertil Steril 63: 1143–1157. [DOI] [PubMed] [Google Scholar]
- Wright C., Milne S., Leeson H. (2014) Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online 28: 684–703. [DOI] [PubMed] [Google Scholar]
- Zarotsky V., Huang M., Carman W., Morgentaler A., Singhal P., Coffin D., et al. (2014) Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology 2: 819–834. [DOI] [PubMed] [Google Scholar]
- Zini A., Buckspan M., Jamal M., Jarvi K. (1999) Effect of varicocelectomy on the abnormal retention of residual cytoplasm by human spermatozoa. Hum Reprod 14: 1791–1793. [DOI] [PubMed] [Google Scholar]
- Zini A., San Gabriel M., Baazeem A. (2009) Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet 26: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zini A., San Gabriel M., Libman J. (2010) Lycopene supplementation in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Fertil Steril 94: 1033–1036. [DOI] [PubMed] [Google Scholar]
- Zini A., Sigman M. (2009) Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl 30: 219–229. [DOI] [PubMed] [Google Scholar]
- Zorn B., Vidmar G., Meden-Vrtovec H. (2003) Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl 26: 279–285. [DOI] [PubMed] [Google Scholar]


