Abstract
Until recently, most clinicians and scientists believed that the experience of pain is perceptually proportional to the amount of incoming peripheral nociceptive drive due to injury or inflammation in the area perceived to be painful. However, many cases of chronic pain have defied this logic, leaving clinicians perplexed as to how patients are experiencing pain with no obvious signs of injury in the periphery. Conversely, there are patients who have a peripheral injury and/or inflammation but little or no pain. What makes some individuals experience intense pain with minimal peripheral nociceptive stimulation and others experience minimal pain with serious injury? It is increasingly well accepted in the scientific community that pain can be generated and maintained or, through other mechanisms, suppressed by changes in the central nervous system, creating a complete mismatch between peripheral nociceptive drive and perceived pain. In fact, there is no known chronic pain condition where the observed extent of peripheral damage reproducibly engenders the same level of pain across individuals. Temporomandibular disorders (TMDs) are no exception. This review focuses on the idea that TMD patients range on a continuum—from those whose pain is generated peripherally to those whose pain is centralized (i.e., generated, exacerbated, and/or maintained by central nervous system mechanisms). This article uses other centralized chronic pain conditions as a guide, and it suggests that the mechanistic variability in TMD pain etiology has prevented us from adequately treating many individuals who are diagnosed with the condition. As the field moves forward, it will be imperative to understand each person’s pain from its own mechanistic standpoint, which will enable clinicians to deliver personalized medicine to TMD patients and eventually provide relief in even the most recalcitrant cases.
Keywords: orofacial pain/TMD, treatment planning, psychosocial factors, neuroscience/ neurobiology, evidence-based dentistry/health care, multisensory perception
Introduction
The idea that chronic pain should be treated according to its underlying mechanisms, which can differ across individuals with the same diagnosis, was first proposed around the turn of the century (Max 2000). At that time, the author stated that this would be possible in the future, but due to the scientific progress in understanding the mechanisms behind chronic pain over the last 2 decades, mechanism-based treatments are now possible, at least to some extent.
Temporomandibular disorder (TMD) refers to a family of symptoms characterized chiefly by pain in the temporomandibular joint and/or surrounding muscle. Many clinicians consider persistent pain in the general orofacial region not clearly identifiable as headache to be TMD. However, it is quite clear that in many patients diagnosed with TMD, the pain and other symptoms involve much more than pathology of the temporomandibular joint disorders (TMJ) and/or surrounding structures. Comorbid (i.e., non-TMD) pain is extremely common, with >50% of TMD patients reporting headache/migraine, neck pain, joint pain, and low back pain, while only 17% report pain isolated in the face and jaw (Plesh et al. 2011). Nonetheless, TMD patients do not necessarily exhibit widespread pain, and they tend to have somewhat lower rates of comorbid syndromes than do other idiopathic pain conditions. For example, approximately 24% of TMD patients meet criteria for fibromyalgia (FM) versus 41% for irritable bowel syndrome (Yunus 2012). In aggregate, TMD patients tend to display hyperalgesia (i.e., increased pain sensitivity) and other sensory anomalies as compared with healthy subjects on experimental measures, but some studies have failed to find significant differences on these measures. One explanation for the discrepant results that have been obtained in the TMD literature is that different mechanisms produce symptoms that meet the criteria for TMD diagnosis and many previous studies have failed to parse them out. Over the last decade, Maixner and colleagues (2011) have begun to uncover the heterogeneity within TMD in the large study Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA), including variations in pain sensitivity, genetic haplotypes related to pain, immunologic factors, and psychosocial variables. Based on supervised cluster analysis, OPPERA has recently identified 3 clusters of TMD patients: the first characterized by low experimental pain sensitivity and low psychological distress, the second by higher pain sensitivity, and the third by higher pain sensitivity and psychological distress (Bair et al. 2016). The differences among these clusters point to mechanistic, etiologic factors that vary across patients in ways that are more meaningful than the distinctions often made in diagnosis (e.g., arthralgia vs. myalgia).
Although TMD treatments are not yet personalized in the clinic in many cases, there is good evidence that some treatments are more appropriate for some patients than others. This review examines an important dimension on which TMD and other types of chronic pain patients can differ, which we believe will be crucial to consider as the field moves toward the personalized treatment of TMD pain—the degree to which each individual’s pain has been centralized.
Centralized Pain
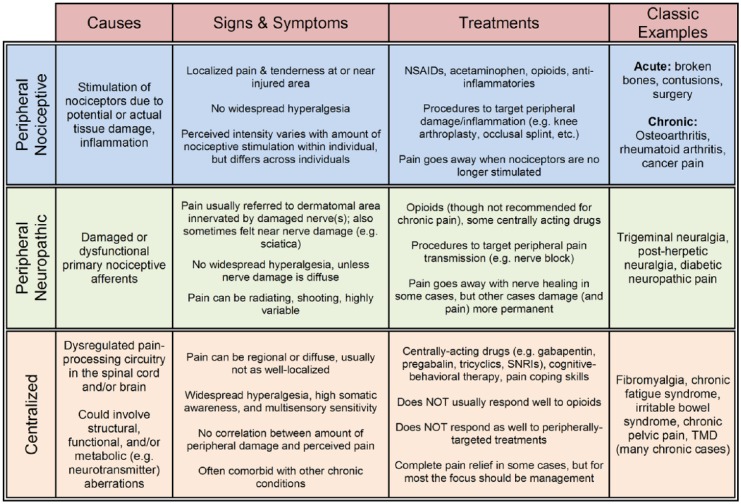
Classically, the term central pain was used to describe the condition of patients who experienced chronic pain as a result of a large-scale injury to the central nervous system, such as a stroke or a lesion, an example being thalamic pain syndrome. More recently, the term central sensitization has been used to describe the amplification of pain signals that can occur in chronic pain. However, its use in this context is debated since the term was coined to describe a specific mechanistic process in spinal cord neurons (Woolf and Thompson 1991), and this is but one of many processes that could be causing central pain amplification in patients. Because of the specificity of these terms and the myriad ways that one’s pain could be augmented via central means, our group uses the term centralized pain to more broadly refer to any condition in which pain is chronically perceived because of central nervous system adaptations that 1) abnormally amplify ascending peripheral input or 2) generate and maintain the perception of pain despite little or no peripheral nociceptive drive. The distinction between centralized and peripheral pain is important, since the mechanism of pain will ultimately affect its prognosis and treatment (see Fig.).
Figure.
Mechanistic characterization of pain. Pain mechanisms can be categorized as peripheral nociceptive, peripheral neuropathic, and centralized. While this classification scheme overly simplifies the vast array of possible mechanisms within each category, it does provide a framework through which clinicians can narrow down treatment options based on each patient’s most prevalent signs and symptoms. Although some chronic pain diagnoses are thought to be more centralized (e.g., fibromyalgia) and others more peripheral (e.g., osteoarthritis), on average, the reality is that no chronic pain state falls neatly into a single mechanistic category. NSAID, nonsteroidal anti-inflammatory drugs; SNRI, serotonin-norepinephrine reuptake inhibitor; TMD, temporomandibular disorder.
Centralized pain is often found in a set of idiopathic chronic pain conditions, including FM, chronic fatigue syndrome (CFS), irritable bowel syndrome, headache, TMD, and interstitial cystitis (Clauw 2014), which have moderate to high degrees of heritability and comorbidity (Diatchenko et al. 2006; Williams and Clauw 2009). The symptomatology of these conditions has been well characterized, with the most prevalent symptoms being both current and previous incidences of persistent multifocal pain, coupled with a host of additional somatic complaints, including sleep disturbance, memory problems, fatigue, and mood disorders (Williams and Clauw 2009). The striking similarity of these conditions, the exception being the region (or regions) where pain is experienced, strongly suggests that there are numerous commonalities in their pathophysiologic underpinnings. In fact, what is labeled a specific, new-onset chronic pain condition (e.g., TMD) by the clinician who specializes in the area of the body where the pain is experienced can, upon further examination, be identified as a more systemic chronic illness that began for the person much earlier in life, with the perceived pain temporally waxing and waning at various body locations.
Psychosocial Factors
The comorbidity of functional somatic syndromes has been illustrated by Kato and colleagues (2009). They have shown that FM, CFS, TMD, and other pain conditions share latent characteristics, such as widespread tenderness, sleep difficulties, and memory problems, which are largely distinct from “psychological” traits, such as anxiety and depression. For example, FM patients who are not depressed have increased pain-evoked activity in regions of the brain that process the sensory (i.e., intensive) aspects of pain (e.g., the primary and secondary somatosensory cortices and the posterior insula), while those who are also depressed show enhanced activation in areas that process the affective (e.g., unpleasant, distressing) component, including the amygdala and anterior insula (Giesecke et al. 2005). Genetic studies also support this idea that there are 2 overlapping sets of traits—those pertaining to pain and sensory amplification and others concerning mood and affect (Diatchenko et al. 2006). These findings suggest that while both sensory and affective amplifications of pain are features of centralized pain, psychological factors appear to more strongly influence the affective component. Within TMD cohorts, levels of depression and/or somatic complaints are associated with chronicity of the disorder (Reiter et al. 2015), palpation tenderness in the orofacial area (Sherman et al. 2004), nonsymptomatic pain tolerance (Koutris et al. 2013), and comorbid pain conditions (Dahan et al. 2015). These are important outcomes, as pain sensitivity, pain spread, chronicity, and complex presentation are hallmarks of centralized pain.
More generally, levels of depressive/anxious symptoms in TMD appear to be elevated as compared with healthy controls but comparable to other chronic pain conditions (Dworkin and Massoth 1994; Giannakopoulos et al. 2010). The same is true of nonspecific somatic complaints (i.e., abdominal pain, unrefreshing sleep; Aaron et al. 2000). There is some evidence that negative affective symptoms are a risk factor for developing TMD. The OPPERA study found a number of psychosocial factors that are associated cross sectionally with chronic TMD, including levels of depression, anxiety, and somatization (Fillingim et al. 2011). These results were corroborated by prospective analyses (n = 3,263), as levels of global psychological distress and somatic complaints were both robust predictors of incident TMD (Fillingim et al. 2013).
Of interest to clinicians treating TMD, axis II dimensions (i.e., psychosocial characteristics) have generally been found to be important predictors of treatment outcomes. For instance, levels of depression, catastrophizing, and somatic complaints are strong predictors of a worse response to standard treatment (Fricton and Olsen 1996; Velly et al. 2011; Litt and Porto 2013). Negative affect and psychosocial stress may contribute to the symptoms and incidence of TMD directly, by influencing neural substrates, and/or may indicate common etiologic factors that promote psychological vulnerabilities and chronic pain. For instance, experimental affective priming paradigms (e.g., visual cues designed to elicit anxious feelings or positive affect) modulate pain responses (Tang and Gibson 2005), and common neuroanatomic vulnerabilities to pain and negative affect have been identified (Robinson et al. 2009).
While centralized pain and psychological dysfunction appear to be distinct if overlapping constructs, for purposes of evaluation and treatment they must be considered in tandem. This may be due to common etiologic factors, such as altered neurotransmitter function (e.g., monoamines; discussed below) and psychological trauma in early life, which has been linked to the development of both psychopathology (MacMillan et al. 2001) and chronic pain (Paras et al. 2009). Prospective studies are needed to identify unique and common risk factors for each, and treatment studies should attempt, where possible, to measure both sensory and affective aspects of pain.
Genetics and Immunology
Currently, it is believed that genetic factors account for about half of the variability in sensitivity to experimental pain and that these same genes also increase one’s propensity to develop chronic pain. At least 5 of these sets of genes have been identified, including those that affect and/or regulate COMT (an estrogen-sensitive enzyme that could partially explain sex differences in chronic pain), sodium and potassium channel mutations, GTP cyclohydrolase, and adrenergic receptors (Diatchenko et al. 2005; Amaya et al. 2006; Tegeder et al. 2006; Costigan et al. 2010; McLean et al. 2011), but not all studies have confirmed these findings (Hocking et al. 2010; Nicholl et al. 2010).
These genetic predispositions might come into play only when activated through environmental triggers. For FM and CFS, potential stressors include early life trauma, physical trauma, certain infections, emotional stress, regional pain conditions, and autoimmune disorders (Clauw and Chrousos 1997; Ablin et al. 2009). However, only a small number of people who are exposed to any of the aforementioned conditions (~5% to 10%) go on to develop FM or CFS; the majority eventually return to a normal state of health. The complex interplay between genetic and environmental factors was recently illustrated by a study showing interactions among COMT haplotypes, sex, and psychological stress level, in terms of their effects on pain sensitivity (Meloto 2016). The current hypothesis is that the various factors that are known to be associated with centralized pain constitute a pain-prone phenotype, which causes people to develop a number of chronic pain conditions and which can predict who will transition from acute to chronic pain following an injury or environmental stressor.
The OPPERA study is the largest and most rigorous investigation of genetic risk factors for TMD and related conditions (Maixner et al. 2011). OPPERA contained a prospective analysis of >2,700 individuals designed to identify single-nucleotide polymorphisms (SNPs) of common genes associated with pain perception, affective processes, and inflammation that confer risk for TMD (Smith et al. 2013). While no SNP was associated with first-onset TMD, several emerged as predictors of intermediate phenotypes likely to be related to centralized pain. These included associations among:
nonspecific orofacial pain with SNPs of the SCN1A gene, which is implicated in the initiation and propagation of action potentials in afferent nerves and may impair gamma-aminobutyric acid (GABA)ergic interneuron function in the central nervous system, altering inhibitory tone (Martin et al. 2010);
general psychological symptoms and 1 SNP of the COX1 gene, a strong regulator of central neuroinflammation (Choi et al. 2009); and
temporal summation of heat pain sensation with an SNP of the MPDZ gene, which encodes proteins related to G protein–coupled receptors for neurotransmitters, including GABA (Balasubramanian et al. 2007).
Case-control genetic analyses were also conducted in OPPERA for chronic TMD, which is most likely maintained in part by centralized pain processes, and they revealed several SNPs associated with monoamine pathways as well as regulation of inflammation—namely, interleukin 10 (IL-10; an anti-inflammatory cytokine) and 1 type of glucocorticoid receptor where the anti-inflammatory endogenous hormone cortisol binds (Smith et al. 2011). Another recent study indicated monoamine involvement in TMD, as 1 SNP of the dopamine receptor 4 gene confers additional TMD risk (Aneiros-Guerrero et al. 2011).
Another large-scale study of TMD compared subjects without widespread palpation tenderness (i.e., less centralized pain) with subjects with widespread tenderness (i.e., more centralized pain). The latter group was found to be characterized by high levels of IL-8, a proinflammatory cytokine; this phenotypic relationship was confirmed by experimental pain testing, as higher levels of IL-8 were associated with reduced pressure pain thresholds in the whole TMD sample (Slade et al. 2011). An earlier study found a positive correlation between levels of circulating IL-6 and ischemic pain intensity (Costello et al. 2002). Higher plasma levels of acute-phase proinflammatory cytokines IL-6, IL-1β, and tumor necrosis factor α, as well as IL-10, were recently reported in TMD and were associated with more dysregulated sleep (Park and Chung 2016). These findings are important because circulating levels of proinflammatory cytokines have been linked to brain inflammation via positron emission tomography (Loggia et al. 2015).
Together these findings suggest that for some TMD patients, centralization of their pain might be due to a number of genetic and/or inflammatory alterations in neurotransmitter systems.
Pain Perception and Processing
Quantitative sensory testing (QST) and neuroimaging have helped us make significant advances in our understanding of chronic pain pathogenesis. There is a wide bell-shaped range of experimental pain sensitivity across the general population, with chronic pain patients more often found shifted to the right, hyperalgesic side of the curve (Diatchenko et al. 2005; Ablin and Clauw 2009). Centralized pain patients with regional pain (e.g., TMD) often exhibit hyperalgesia to pressure and thermal stimulation, even remote from where the ongoing pain is experienced, suggesting that the central gain control for pain is set higher for the entire body (Maixner et al. 1995; Kashima et al. 1999; Giesecke et al. 2004; Slade et al. 2014). There is also evidence that nonpainful sensory signals are perceptually amplified. For example, TMD patients have heightened sensitivity to innocuous pressure and auditory stimuli, though not to the extent of FM patients (Hollins et al. 2009).
QST is an excellent tool for determining the potential underlying mechanisms that may be leading to hyperalgesia and increased pain-evoked brain activity. The measurement of pressure thresholds at the site of an injury could indicate peripheral sensitization, but this mechanism cannot explain the widespread tenderness and hyperalgesia observed in centralized pain patients. Conditioned pain modulation, a QST paradigm in which 2 experimental pain stimuli are applied simultaneously, has also provided evidence for central pain–processing abnormalities in chronic pain. When most healthy individuals are tested for conditioned pain modulation, the stronger, more tonic (conditioning) pain will inhibit the weaker, phasic (test) pain. This form of endogenous analgesia is impaired in FM (Kosek and Hansson 1997), but in TMD the results have been mixed (King et al. 2009; Garrett 2013), possibly due to differences among the samples in the proportion of TMD patients with highly centralized pain. If TMD subjects are separated out according to their degree of centralized pain, we expect that differences from controls would be observed in the TMD patients with more centralized pain.
Functional magnetic resonance imaging studies have provided a neurologic basis for hyperalgesia by revealing increased pain-evoked brain activity in centralized pain (Gracely et al. 2002; Cook et al. 2004; Giesecke et al. 2004). The regions of activation by experimental pain vary slightly depending on the parameters of stimulation but, for the most part, are activated reliably in both pain patients and controls. The most common areas of activation are the thalamus, primary and secondary somatosensory cortices, and insular cortex, as well as the anterior, mid-, and posterior cingulate cortex—meaning that pain generates a complex network of activity in sensory, limbic, and associative brain regions. In general, for any given noxious stimulus intensity, individuals with centralized chronic pain will show greater amounts of pain-evoked activity in these brain regions.
Neuroimaging may hold one of the important keys for detecting, diagnosing, and treating centralized pain mechanisms. There is a growing literature on the structural, functional, and neurochemical alterations that are present in the brains of TMD patients. While the neuroimaging literature on other chronic pain conditions (e.g., FM) has begun to tell a consistent story regarding centralized pain, small sample sizes and inconsistent findings across studies currently make solid conclusions about TMD difficult to come by (Lin 2014; Walitt et al. 2016).
Central Neurotransmission: The Common Denominator
Given the high degree of comorbidity with other disorders and the frequency of certain symptoms, the most parsimonious pathologic theory for centralized pain is thus: imbalances in the neurotransmitters known to play a role in causing the pain of the disorder are contributing to the comorbid pain conditions and to disturbances with sleep, affect, memory, and other realms of function. The persistent pain could be due to 1) increased neurotransmission in pronociceptive systems, 2) decreased neurotransmission in antinociceptive pathways, or 3) some combination thereof. If there is increased glutamatergic tone, for example, this could lead to 1) an increase in the volume control for pain and other sensory stimuli because of its actions in ≥1 sensory brain regions and 2) a dysregulation of sleep due to its action in sleep-related brain areas. There is ample evidence that neurotransmitter levels are altered in FM and some evidence in TMD (Gerstner et al. 2012). Optimal treatment of centralized pain, including TMD, will likely entail a determination of which neurotransmitter systems are disrupted and the administration of the proper exogenous drug to rectify the imbalance. Still, nondrug therapies (e.g., cognitive behavioral therapy) will have a place in treatment, since they too are capable of altering neurotransmission. Also, because of the overlap in the brain’s use of neurotransmitter classes for different functions, drugs that might be good candidates for reducing pain will not be recommended due to side effects via interactions with other nonpain systems.
Mixed Pain States
There are both central and peripheral contributions to chronic pain in most patients—meaning that pain is mechanistically mixed, but central factors are more relevant in some cases and peripheral factors in others (possibly nociceptive and/or peripheral neuropathic). For further discussion of this topic, consult Appendix A.
Implications for the Treatment of TMD
Past lessons of failed TMJ implants, the often observed mismatch between peripheral pathology and pain level, and the fact that for many individuals TMD pain is self-limiting have brought the field to a point where conservative, reversible treatments are recommended. While this is an improvement from the most common standards of care decades ago, it is still the case that TMD treatment is not often personalized based on each patient’s underlying pain mechanism.
A recent review of 66 TMD treatment papers published between 1994 and 2014 concluded that conservative treatments—including counseling, occlusal splint therapy, physiotherapeutic techniques (e.g., massage), and drug therapy—are most often undertaken (Wieckiewicz 2015). When the results are broken down by disease entity, no clear pattern of specific treatments targeted to specific pathologies emerges. For example, occlusal splints were often utilized for treating myofascial TMD, disk displacements, and bruxism.
Although occlusal splints are often used to treat TMD, there is no definitive answer on whether they are more effective than placebo. This discrepancy could be easily explained if occlusal splints are an effective treatment option for particular TMD pathologies but not others; the failure of many studies to separate TMD patients out based on etiology has likely masked the observance of treatment effectiveness beyond placebo in some samples. For example, Raphael and Marbach (2001) conducted an oral splint treatment study in 63 women with myofascial TMD. Some women received an active splint and others a sham or placebo splint, and pain was measured at a 6-wk follow-up. Overall, there was no difference between the groups (i.e., no additional benefit of the active splint beyond placebo). However, when the researchers separated individuals out according to whether they had tenderness localized to the TMJ region or widespread tenderness, the active splint was shown to be significantly more effective than the sham in the people who had localized TMD pain (Raphael and Marbach 2001). It appears that this peripherally targeted treatment was effective in individuals whose pain was not centralized. In contrast, for centralized pain, this peripheral treatment did not target the source and was therefore ineffective.
The move from the sophisticated neuroimaging, genetic, and QST methods that have been used to uncover centralized pain features to the clinic may seem complicated, but much of the information about a patient’s degree of pain centralization can be obtained, albeit less precisely, through methods currently available to clinicians.
By taking a thorough history and conducting a physical examination, clinicians can gain insight into the amount of sensory amplification (and, thus, pain centralization) present in a patient. Centralized pain often entails a ramping up of the volume control for not only pain but also touch, heat, sounds, and light (Geisser et al. 2008). In addition, these patients have significantly higher somatic awareness, or hypervigilance—meaning that they are much more aware of sensations associated with their own bodies (e.g., indigestion, urinary urgency, eyelids twitching; Hollins et al. 2009). Discussing these factors with patients can be illuminating. Although experimental pain testing is not yet routine and standardized in clinical practice, one can gain insight into the degree of centralization in a patient by assessing pressure pain thresholds at asymptomatic locations, such as the arms or hands. By applying firm pressure over 1) several interphalangeal joints of each hand, 2) the adjacent phalanges, and 3) the forearm muscles (including the lateral epicondyle region), one can assess overall pain sensitivity and gain other potentially valuable information. If the patient is tender in most or all areas or just the forearm, they are likely a pain-sensitive individual, making the chances that their pain is centralized to some degree greater. If the patient is tender in only the fingers, this might indicate other pathologies (e.g., rheumatoid arthritis, lupus, metabolic bone disease).
The degree of pain centralization for each patient can be taken into account when determining the appropriate pharmacologic therapy. For patients with peripheral/nociceptive noninflammatory pain, acetaminophen and nonsteroidal anti-inflammatory drugs work well. The former is now thought to be safer but less effective than the latter. Although opioids can be effective in certain situations, they are known to be ineffective (and sometimes counterproductive) for chronic pain and are no longer recommended for it. Both inflammatory and noninflammatory peripheral pain syndromes, as well as peripheral neuropathic pain, can be treated with topical agents, anti-inflammatory drugs, or injections, depending on the mechanism.
Patients in whom centralized pain is suspected should generally respond better to drugs with centrally acting mechanisms. Tricyclics have been shown to be effective in some cases (Tversky et al. 1991), but they have significant toxicity. Newer drugs that target neurotransmitter systems (e.g., serotonin and norepinephrine) with better selectivity, such as tramadol or duloxetine, are more commonly prescribed. Alpha-2-delta calcium channel ligands, such as gabapentin and pregabalin, have also shown promise in treating centralized pain.
Finally, the potential for nondrug therapies to help individuals with centralized pain should not be overlooked. For example, TMD patients who score high on the biopsychosocial axis of the diagnostic criteria for TMD generally respond less well to peripherally targeted treatments, but for those who score high on this axis (i.e., those with more systemic symptoms and likely some degree of pain centralization), cognitive behavioral therapy has been shown to have some promise in helping them (Turner et al. 2006).
Summary and Conclusions
Advances in our understanding of pain mechanisms, especially those pertaining to the central nervous system, are bringing the possibility of providing personalized care for TMD in the clinical setting closer to the present. By identifying markers of pain centralization with a thorough history, physical examination, and questionnaires, clinicians can now identify patients who have a higher propensity to be helped by a peripherally targeted intervention and those who are more likely to respond to centrally acting treatments. In many patients, there are opportunities to treat both the peripheral and central components of their pain. However, we still have ample work to do to be able to differentiate among patients with the same degree of centralization but different underlying pathologies.
Author Contributions
D.E. Harper and A. Schrepf, contributed to conception and design, drafted and critically revised the manuscript; D.J. Clauw, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
Drs. Harper and Schrepf are supported by National Institutes of Health (K12-DE023574; D.J.C., principal investigator). Dr. Clauw has received research funding from Cerephex, Forest, Merck, and Pfizer and serves as a consultant for Tonix, Theravance, Cerephex, Pfizer, Abbott, Merck, Eli Lilly, UCB, Johnson & Johnson, Forest Laboratories, and Purdue Pharma.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Aaron LA, Burke MM, Buchwald D. 2000. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 160(2):221–227. [DOI] [PubMed] [Google Scholar]
- Ablin JN, Buskila D, Clauw DJ. 2009. Biomarkers in fibromyalgia. Curr Pain Headache Rep. 13(5):343–349. [DOI] [PubMed] [Google Scholar]
- Ablin K, Clauw DJ. 2009. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am. 35(2):233–251. [DOI] [PubMed] [Google Scholar]
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, et al. 2006. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 26(50):12852–12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneiros-Guerrero A, Lendinez AM, Palomares AR, Perez-Nevot B, Aguado L, Mayor-Olea A, Ruiz-Galdon M, Reyes-Engel A. 2011. Genetic polymorphisms in folate pathway enzymes, DRD4 and GSTM1 are related to temporomandibular disorder. BMC Med Genet. 12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair E, Gaynor S, Slade GD, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Smith SB, Diatchenko L, Maixner W. 2016. Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: the OPPERA study. Pain. 157(6):1266–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Fam SR, Hall RA. 2007. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J Biol Chem. 282(6):4162–4171. [DOI] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. 2009. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 30(4):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ. 2014. Fibromyalgia: a clinical review. JAMA. 311(15):1547–1555. [DOI] [PubMed] [Google Scholar]
- Clauw DJ, Chrousos GP. 1997. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. 4(3):134–153. [DOI] [PubMed] [Google Scholar]
- Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. 2004. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 31(2):364–378. [PubMed] [Google Scholar]
- Costello NL, Bragdon EE, Light KC, Sigurdsson A, Bunting S, Grewen K, Maixner W. 2002. Temporomandibular disorder and optimism: relationships to ischemic pain sensitivity and interleukin-6. Pain. 100(1–2):99–110. [DOI] [PubMed] [Google Scholar]
- Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu T, Kiselycznyk C, Poddar M, Lu Y, et al. 2010. Multiple chronic pain states are associated with a common amino acid-changing allele in KCNS1. Brain. 133(9):2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan H, Shir Y, Velly A, Allison P. 2015. Specific and number of comorbidities are associated with increased levels of temporomandibular pain intensity and duration. J Headache Pain. 16:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. 2006. Idiopathic pain disorders: pathways of vulnerability. Pain. 123(3):226–230. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, et al. 2005. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 14(1):135–143. [DOI] [PubMed] [Google Scholar]
- Dworkin SF, Massoth DL. 1994. Temporomandibular disorders and chronic pain: disease or illness? J Prosthet Dent. 72(1):29–38. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, et al. 2013. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 14(12 Suppl):T75–T90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Dubner R, Bair E, Baraian C, Slade GD, Maixner W. 2011. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. 12(11 Suppl):T46–T60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricton JR, Olsen T. 1996. Predictors of outcome for treatment of temporomandibular disorders. J Orofac Pain. 10(1):54–65. [PubMed] [Google Scholar]
- Garrett PH, Sarlani E, Grace EG, Greenspan JD. 2013. Chronic temporomandibular disorders are not necessarily associated with a compromised endogenous analgesic system. J Orofac Pain. 27(2):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisser ME, Strader DC, Petzke F, Gracely RH, Clauw DJ, Williams DA. 2008. Comorbid somatic symptoms and functional status in patients with fibromyalgia and chronic fatigue syndrome: sensory amplification as a common mechanism. Psychosomatics. 49(3):235–242. [DOI] [PubMed] [Google Scholar]
- Gerstner GE, Gracely RH, Deebajah A, Ichesco E, Quintero A, Clauw DJ, Sundgren PC. 2012. Posterior insular molecular changes in myofascial pain. J Dent Res. 91(5):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos NN, Keller L, Rammelsberg P, Kronmüller KT, Schmitter M. 2010. Anxiety and depression in patients with chronic temporomandibular pain and in controls. J Dent. 38(5):369–376. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. 2004. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 50(2):613–623. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Gracely RH, Williams DA, Geisser M, Petzke F, Clauw DJ. 2005. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 52(5):1577–1584. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ. 2002. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 46(5):1333–1343. [DOI] [PubMed] [Google Scholar]
- Hocking LJ, Smith BH, Jones GT, Reid DM, Strachan DP, Macfarlane GJ. 2010. Genetic variation in the beta2-adrenergic receptor but not catecholamine-o-methyltransferase predisposes to chronic pain: results from the 1958 british birth cohort study. Pain. 149(1):143–151. [DOI] [PubMed] [Google Scholar]
- Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W. 2009. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain. 141(3):215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima K, Rahman OI, Sakoda S, Shiba R. 1999. Increased pain sensitivity of the upper extremities of TMD patients with myalgia to experimentally-evoked noxious stimulation: possibility of worsened endogenous opioid systems. Cranio. 17(4):241–246. [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. 2009. A population-based twin study of functional somatic syndromes. Psychol Med. 39(3):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., 3rd 2009. Deficiency in endogenous modulation of prolonged heat pain in patients with irritable bowel syndrome and temporomandibular disorder. Pain. 143(3):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek E, Hansson P. 1997. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 70(1):41–51. [DOI] [PubMed] [Google Scholar]
- Koutris M, Visscher CM, Lobbezoo F, Naeije M. 2013. Comorbidity negatively influences the outcomes of diagnostic tests for musculoskeletal pain in the orofacial region. Pain. 154(6):927–932. [DOI] [PubMed] [Google Scholar]
- Lin CS. 2014. Brain signature of chronic orofacial pain: A systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PLoS ONE. 9(4):e94300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Porto FB. 2013. Determinants of pain treatment response and nonresponse: identification of TMD patient subgroups. J Pain. 14(11):1502–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji RR, et al. 2015. Evidence for brain glial activation in chronic pain patients. Brain. 138(Pt 3):604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH, Jamieson E, Duku EK, Walsh CA, Wong MY, Beardslee WR. 2001. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 158(11):1878–1883. [DOI] [PubMed] [Google Scholar]
- Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. 2011. Orofacial pain prospective evaluation and risk assessment study: the OPPERA study. J Pain. 12(11 Suppl):T4–T11.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Fillingim R, Booker D, Sigurdsson A. 1995. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 63(3):341–351. [DOI] [PubMed] [Google Scholar]
- Martin MS, Dutt K, Papale LA, Dube CM, Dutton SB, de Haan G, Shankar A, Tufik S, Meisler MH, Baram TZ, et al. 2010. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 285(13):9823–9834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max MB. 2000. Is mechanism-based pain treatment attainable? Clinical trial issues. J Pain. 1(3 Suppl):2–9. [DOI] [PubMed] [Google Scholar]
- McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, et al. 2011. Catechol o-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 12(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloto CB, Bortsov AV, Bair E, Helgeson E, Ostrom C, Smith SB, Dubner R, Slade GD, Fillingim RB, Greenspan JD, et al. 2016. Modification of comt-dependent pain sensitivity by psychological stress and sex. Pain (Amsterdam). 157(4):858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholl BI, Holliday KL, Macfarlane GJ, Thomson W, Davies KA, O’Neill TW, Bartfai G, Boonen S, Casanueva F, Finn JD, et al. 2010. No evidence for a role of the catechol-o-methyltransferase pain sensitivity haplotypes in chronic widespread pain. Ann Rheum Dis. 69(11):2009–2012. [DOI] [PubMed] [Google Scholar]
- Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, Elamin MB, Seime RJ, Prokop LJ, Zirakzadeh A. 2009. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 302(5):550–561. [DOI] [PubMed] [Google Scholar]
- Park JW, Chung JW. 2016. Inflammatory cytokines and sleep disturbance in patients with temporomandibular disorders. J Oral Facial Pain Headache. 30(1):27–33. [DOI] [PubMed] [Google Scholar]
- Plesh O, Adams SH, Gansky SA. 2011. Temporomandibular joint and muscle disorder-type pain and comorbid pains in a national US sample. J Orofac Pain. 25(3):190–198. [PMC free article] [PubMed] [Google Scholar]
- Raphael KG, Marbach JJ. 2001. Widespread pain and the effectiveness of oral splints in myofascial face pain. J Am Dent Assoc. 132(3):305–316. [DOI] [PubMed] [Google Scholar]
- Reiter S, Emodi-Perlman A, Goldsmith C, Friedman-Rubin P, Winocur E. 2015. Comorbidity between depression and anxiety in patients with temporomandibular disorders according to the research diagnostic criteria for temporomandibular disorders. J Oral Facial Pain Headache. 29(2):135–143. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Edwards SE, Iyengar S, Bymaster F, Clark M, Katon W. 2009. Depression and pain. Front Biosci (Landmark Ed). 14:5031–5051. [DOI] [PubMed] [Google Scholar]
- Sherman JJ, LeResche L, Huggins KH, Mancl LA, Sage JC, Dworkin SF. 2004. The relationship of somatization and depression to experimental pain response in women with temporomandibular disorders. Psychosom Med. 66(6):852–860. [DOI] [PubMed] [Google Scholar]
- Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, et al. 2011. Cytokine biomarkers and chronic pain: association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 152(12):2802–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. 2014. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain (Amsterdam). 155(10):2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, Knott C, Slade GD, Bair E, Gibson DG, et al. 2011. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 12(11 Suppl):T92–T101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Mir E, Bair E, Slade GD, Dubner R, Fillingim RB, Greenspan JD, Ohrbach R, Knott C, Weir B, et al. 2013. Genetic variants associated with development of TMD and its intermediate phenotypes: the genetic architecture of TMD in the OPPERA prospective cohort study. J Pain. 14(12 Suppl):T91–T101.e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Gibson SJ. 2005. A psychophysical evaluation of the relationship between trait anxiety, pain perception, and induced state anxiety. J Pain. 6(9):612–619. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, et al. 2006. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 12(11):1269–1277. [DOI] [PubMed] [Google Scholar]
- Turner JA, Mancl L, Aaron LA. 2006. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain. 121(3):181–194. [DOI] [PubMed] [Google Scholar]
- Tversky J, Reade PC, Gerschman JA, Holwill BJ, Wright J. 1991. Role of depressive illness in the outcome of treatment of temporomandibular joint pain-dysfunction syndrome. Oral Surg Oral Med Oral Pathol. 71(6):696–699. [DOI] [PubMed] [Google Scholar]
- Velly AM, Look JO, Carlson C, Lenton PA, Kang W, Holcroft CA, Fricton JR. 2011. The effect of catastrophizing and depression on chronic pain: a prospective cohort study of temporomandibular muscle and joint pain disorders. Pain. 152(10):2377–2383. [DOI] [PubMed] [Google Scholar]
- Walitt B, Ceko M, Gracely JL, Gracely RH. 2016. Neuroimaging of central sensitivity syndromes: Key insights from the scientific literature. Curr Rheumatol Rev. 12(1):55–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckiewicz MM. 2015. Reported concepts for the treatment modalities and pain management of temporomandibular disorders. J Headache Pain. 16:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Clauw DJ. 2009. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 10(8):777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. 1991. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 44(3):293–299. [DOI] [PubMed] [Google Scholar]
- Yunus MB. 2012. The prevalence of fibromyalgia in other chronic pain conditions. Pain Res Treat. 2012:584573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.