Abstract
To explore the impact of interactions between smoking and symptoms of posttraumatic stress disorder (PTSD) on pain intensity, psychological distress, and pain-related functioning in patients with orofacial pain, a retrospective review was conducted of data obtained during evaluations of 610 new patients with a temporomandibular disorder who also reported a history of a traumatic event. Pain-related outcomes included measures of pain intensity, psychological distress, and pain-related functioning. Main effects of smoking status and PTSD symptom severity on pain-related outcomes were evaluated with linear regression analyses. Further analyses tested interactions between smoking status and PTSD symptom severity on pain-related outcomes. PTSD symptom severity and smoking predicted worse pain-related outcomes. Interaction analyses between PTSD symptom severity and smoking status revealed that smoking attenuated the impact of PTSD symptom severity on affective distress, although this effect was not found at high levels of PTSD symptom severity. No other significant interactions were found, but the present results identifying smoking as an ineffective coping mechanism and the likely role of inaccurate outcome expectancies support the importance of smoking cessation efforts in patients with orofacial pain. Smoking is a maladaptive mechanism for coping with pain that carries significant health- and pain-related risks while failing to fulfill smokers’ expectations of affect regulation, particularly among persons with orofacial pain who also have high levels of PTSD symptom severity. Addressing smoking cessation is a critical component of comprehensive treatment. Further research is needed to develop more effective ways to help patients with pain and/or PTSD to replace smoking with more effective coping strategies.
Keywords: temporomandibular disorders, nicotine, tobacco, psychology, behavioral science, dental public health
Introduction
Posttraumatic stress disorder (PTSD) comprises “characteristic symptoms following exposure to one or more traumatic events” (American Psychiatric Association 2013). Symptoms of PTSD include intrusive thoughts about the trauma, avoidance of stimuli associated with the trauma, negative alterations in cognitions and mood, and alterations in arousal and reactivity (American Psychiatric Association 2013). Sharp and Harvey (2001) suggested that there is a relationship of mutual maintenance between PTSD and pain. Evidence suggests a higher prevalence of PTSD among chronic pain patients as compared with the general population (McWilliams et al. 2003). Elevated PTSD symptoms are associated with increased pain, disability, and psychological dysfunction in temporomandibular disorder (TMD) patients (de Leeuw et al. 2005; Bertoli et al. 2007; Cyders et al. 2011).
Cyders et al. (2011) investigated the ability of different conceptual models of PTSD to predict pain severity and pain-related disability. The authors applied structural equation modeling to data from a sample of 411 female orofacial pain patients with a history of trauma. Two models, each containing 4 symptom clusters (factors), provided the best fit to the data. Both of these models included the symptom clusters “reexperiencing,” “avoidance,” and “hyperarousal,” and each model contained an additional unique factor: “numbing” in one model and “dysphoria” in the other.
Smoking is a possible moderating factor of the relationship between pain and PTSD. Zvolensky et al. (2008) noted 3 lines of evidence for associations between PTSD and smoking: 1) rates of lifetime and current smoking are higher in individuals with PTSD as compared with those without PTSD; 2) smokers with PTSD smoke more cigarettes per day and display higher levels of nicotine dependence than do smokers without PTSD; and 3) among individuals who experience a traumatic event, those who develop PTSD report increased smoking behavior versus those who do not develop the disorder. Data suggest higher rates of smoking among chronic pain patients (Zvolensky et al. 2009) when compared with estimates in the general population (Agaku et al. 2014). Among individuals with chronic pain, smokers have been found to report higher pain intensity and an increased number of painful sites when compared with nonsmokers. Smokers display a greater impact of pain on occupational and social functioning and worse treatment outcomes than do nonsmokers (see review by Shi et al. 2010). Despite evidence for acute analgesic effects of nicotine (Shi et al. 2010; Ditre et al. 2011), smokers in pain use greater amounts of analgesic medication than do nonsmokers in both postoperative and general population settings (see review by Ditre et al. 2011). Smokers with pain experience increased severity of psychiatric comorbidities when compared with nonsmokers with pain, including higher levels of depression, suicidal ideation, affective distress, and pain-related anxiety (see review by Ditre et al. 2011).
Adverse effects of smoking have been specifically demonstrated in orofacial pain populations. Studies of TMD patients have found more severe pain intensity, greater pain-related interference, elevated anxiety, elevated global psychological distress, and reduced sleep quality among smokers as compared with nonsmokers (Weingarten et al. 2009; de Leeuw et al. 2013; Custodio et al. 2015) with some evidence of dose-dependent effects (Burris et al. 2013). These findings suggest that smoking is associated with worse pain outcomes and worse psychological functioning in TMD patients.
Purpose of This Study
The purpose of the current study was to explore the impact of interactions between PTSD symptomatology and smoking on pain intensity, psychological distress, and pain-related functioning in the setting of orofacial pain. A further purpose was to evaluate the impact of smoking on different PTSD symptom clusters.
It was hypothesized that PTSD symptomatology would interact with smoking status to predict worse outcomes than either PTSD or smoking status alone. A second exploratory hypothesis was that smokers with orofacial pain would report levels of hyperarousal, dysphoria, and numbing higher than those of nonsmokers with orofacial pain.
Materials and Methods
Participants
A retrospective review was conducted of data obtained during initial evaluations of individuals seen at a university-based orofacial pain center from 1997 through 2013. Participants were eligible for inclusion if they were 18 to 80 y old and reported a history of a traumatic experience. Inclusion also required a primary diagnosis of a TMD. Diagnoses were made at the initial evaluation by residents and supervising faculty trained in the diagnosis and management of orofacial pain disorders in accordance with the guidelines of the American Academy of Orofacial Pain (de Leeuw 2008). Primary diagnoses were reviewed and grouped according to the appropriate corresponding categories of the research diagnostic criteria for TMDs (RDC/TMD; Dworkin and LeResche 1992) as either muscle (group 1: muscle diagnoses) or joint (group 2/3: disc displacements/arthralgia, arthritis, arthrosis).
Participants were excluded if their primary diagnosis indicated a condition other than a TMD (e.g., neuropathic pain or headache). Patients with secondary diagnoses incompatible with RDC/TMD were likewise excluded with the exceptions of temporomandibular joint subluxation, bruxism, and occlusal instability. Further exclusion criteria included incomplete, incorrect, or missing data for any variables of interest or selected potential covariates. Participants who reported a rating of “0” for average pain on the visual analog scale (VAS) of pain intensity or who reported an invalid pain rating (i.e., VAS average pain rated higher than VAS maximum pain level or lower than VAS minimum pain level) were likewise excluded. The study was approved by the Institutional Review Board of the university where the study was conducted (14-0473-P6H). This study was in concordance with STROBE guidelines for observational studies.
Measures
Smoking Status
Participants were characterized as smokers or nonsmokers based on their response of “yes” or “no” to the question “Do you smoke?” Given the large proportion of smokers who reported smoking at least a pack per day, as well as the doubts about the reliability of self-reported packs per day, smoking status was examined as a dichotomous variable.
Pain Intensity
Participants reported their maximum, average, and minimum pain intensities over the past month on a 100-mm VAS. The present study used only the average pain intensity rating. A second measure of pain intensity was obtained from the pain intensity subscale of the Multidimensional Pain Inventory (MPI; described in the Pain-Related Functioning section).
PTSD Symptom Severity
Measures of PTSD symptom severity were derived from the PTSD Checklist–Civilian Version (PCL-C) (Blanchard et al. 1996). The PCL-C asks patients to report the number and nature of their lifetime traumatic experiences, and it measures the severity of symptoms associated with the “most significant” traumatic experience. Patients rate how much they have been bothered over the past month by symptoms described in 17 items, using a response scale ranging from 1 (“not at all”) to 5 (“very much”). Scores from these 17 items are added to obtain an overall estimate of the severity of PTSD symptoms. In addition to this total score, the items can be grouped according to PTSD symptom clusters. The current study used the total PCL-C score as well as scores for the 5 symptom clusters included in models of PTSD by Cyders et al. (2011). Reliability analyses yielded the following Cronbach’s alpha values for PCL-C scores from the present sample: .93 for total PCL-C score, .88 for reexperiencing, .74 for avoidance, .81 for numbing, .84 for hyperarousal, and .86 for dysphoria. Although it was not possible to ascertain whether any participant would have met diagnostic criteria for PTSD, previous research has established a PCL-C score of 41 as an optimal cutoff for a probable diagnosis of PTSD in a population of orofacial pain patients (Sherman et al. 2005). Therefore, the participants were not diagnosed formally with PTSD but represented a group of individuals with a range of trauma-related symptom severity, some of whom would likely have received a PTSD diagnosis.
Psychological Distress
Measures of psychological distress were obtained from the Global Severity Index (GSI) of the Symptom Checklist-90-Revised (SCL-90R; Derogatis 1979). The SCL-90R assesses the severity of symptoms described in 90 self-report items as experienced by the patient over the preceding week. Responses are calculated to generate 9 primary symptom dimensions and 3 global indices of functioning. Previous research has demonstrated considerable overlap among the 9 symptom dimensions (Hardt et al. 2000); therefore, only the GSI was used in the present study. Calculations of internal consistency for the GSI subscale in previous studies have yielded a Cronbach’s alpha of .97 (Hardt et al. 2000; Arrindell et al. 2006).
Pain-Related Functioning
Measures of life interference, life control, affective distress, and general activity were obtained from the MPI: a 52-item self-report questionnaire that assesses the impact of pain, the extent of patients’ participation in daily activities, and levels of social support. Calculations of internal consistencies for MPI subscales in previous research have found Cronbach’s alpha values ranging from .70 to .90 (Kerns et al. 1985).
Statistical Analyses
Analyses were performed with SPSS 22 (IBM). First, chi-square tests for independence (for categorical variables) and independent samples t tests (for continuous variables) were used to compare selected demographic characteristics between smokers and nonsmokers. Independent samples t tests were used to compare total PCL-C scores and mean symptom cluster scores with respect to smoking status. Next, main effects of smoking and PTSD symptom severity on pain, psychological distress, and pain-related functioning were analyzed independently with simple linear regression analyses. After that, linear regression analyses were performed to test whether smoking status moderated the effects of PTSD symptom severity on these outcomes. Separate analyses were run for each outcome variable. Three participants were missing MPI general activity data only. These cases were handled with listwise deletion for analyses pertaining to MPI general activity. All models were tested with and without a quadratic PTSD symptom severity variable to test for potential nonlinear effects of PTSD symptom severity. Quadratic terms were not significant; as such, only linear models are shown below. Moderation analyses were conducted with the PROCESS macro by Andrew F. Hayes (2012). Main effects were added in the first step, and the interaction term was added in the next so that the additional variance explained by the interaction (ΔR2) could be computed. Coefficients for these models are provided as unstandardized betas (B), representing the number of points that the outcome variable changes for each unit change in the predictor variable. For all regression models, a total adjusted R2 was computed to represent the total variance in the outcome explained by the predictors.
Results
Demographics
A total of 5,029 potential participants seen within the specified time frame were reviewed for eligibility: 2,487 were excluded due to a lack of reported trauma-related stressors; 840 were excluded for missing or invalid data on variables/covariates of interest; and 1,091 were excluded due to ineligible primary or secondary diagnoses. One was a duplicate case and was therefore excluded.
The final sample consisted of 610 participants. The mean age of the sample was 39.6 y (SD = 14, range: 18 to 77), and the sex composition of the sample was 85% female. Patients in the sample had a mean pain duration of 44.8 mo (SD = 76) and a median pain duration of 12 mo (range: 1 to 480). Mean pain intensity on the VAS was 47.2 mm (SD = 23).
Smoking Status
Twenty-five percent of the sample reported smoking. Among smokers, the self-reported average number of cigarettes smoked per day was 18 (assuming 20 cigarettes per pack; this calculation excluded 9 smokers for whom these data were unavailable or incorrectly filled out). Approximately 67% of smokers reported smoking ≥1 pack per day. Smokers and nonsmokers did not differ in primary diagnostic category per RDC/TMD or in sex proportions. Smokers were more likely than nonsmokers to be unemployed and unmarried. Smokers were younger on average than nonsmokers. The proportion of participants likely to meet diagnostic criteria for PTSD (based on a PCL-C score ≥41) was significantly larger in smokers versus nonsmokers. These findings are presented in Table 1.
Table 1.
Comparison of Demographic Characteristics between Smokers and Nonsmokers.
| Total (N = 610, 100%) | Smokers (n = 155, 25.4%) | Nonsmokers (n = 455, 74.6%) | χ2/t | P Value | |
|---|---|---|---|---|---|
| Sex | 0.76 | .383 | |||
| Male | 93 (15.2) | 27 (17.4) | 66 (14.5) | ||
| Female | 517 (84.8) | 128 (82.6) | 389 (85.5) | ||
| RDC/TMD primary diagnosis | 1.67 | .196 | |||
| Muscle (group 1) | 319 (52.3) | 88 (56.8) | 231 (50.8) | ||
| Joint (group 2/3) | 291 (47.7) | 67 (43.2) | 224 (49.2) | ||
| Employment status | 22.14 | <.001 | |||
| Unemployed | 198 (32.5) | 74 (47.7) | 124 (27.3) | ||
| Employed | 412 (67.5) | 81 (52.3) | 331 (72.7) | ||
| Marital status | 13.48 | <.001 | |||
| Single | 239 (39.2) | 80 (51.6) | 159 (34.9) | ||
| Married | 371 (60.8) | 75 (48.4) | 296 (65.1) | ||
| PTSD categorya | 26.58 | <.001 | |||
| Positive | 134 (22.0) | 57 (36.8) | 77 (16.9) | ||
| Negative | 476 (78.0) | 98 (63.2) | 378 (83.1) | ||
| Age, y | 39.6 ± 14.0 | 35.4 ± 11.5 | 41.1 ± 14.5 | 4.97b | <.001 |
| Pain intensity (VAS), mm | 47.2 ± 23.0 | 59.9 ± 21.8 | 42.9 ± 21.7 | −8.4c | <.001 |
| Pain duration, mo | 44.8 ± 76.0 | 48.9 ± 71.7 | 43.4 ± 77.5 | −0.78c | .436 |
Values are presented as n (%) or mean ± SD.
PCL-C, PTSD Checklist–Civilian Version; PTSD, posttraumatic stress disorder; RDC/TMD, research diagnostic criteria for temporomandibular disorders; VAS, visual analog scale.
Positive: PCL-C score ≥41. Negative: PCL-C score <41.
df = 332.11 (equal variances not assumed).
df = 608.
Main Effects of PTSD and Smoking Status on Pain Outcomes
Main effects of smoking and PTSD symptom severity on pain and psychological variables were evaluated with simple linear regression analyses (hypothesis 1; Table 2). Both smoking and PTSD symptom severity significantly predicted increases in pain, psychological distress, and pain-related dysfunction, with the exception of general activity—in which case, a decrease was significantly predicted by PTSD symptom severity but not by smoking. The impact of smoking appeared to be most pronounced on average pain intensity (adjusted R2 = .103) with relatively small effect sizes on SCL-90R and MPI measures. PTSD symptom severity exhibited a robust effect size on global psychological functioning and affective distress.
Table 2.
Results of Linear Regression Analyses.
| Smoking: Main Effectsa |
PCL-C Total Score: Main Effects |
|||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variable | R2 (Adj) | β | t | P Value | R2 (Adj) | β | t | P Value |
| VAS average pain intensity | .103 | .323 | 8.403 | <.001 | .090 | .302 | 7.806 | <.001 |
| MPI subscales | ||||||||
| Pain severity | .063 | .255 | 6.491 | <.001 | .098 | .316 | 8.208 | <.001 |
| Life interference | .042 | .208 | 5.243 | <.001 | .155 | .395 | 10.597 | <.001 |
| Life control | .038 | −.198 | −4.992 | <.001 | .176 | −.421 | −11.450 | <.001 |
| Affective distress | .042 | .209 | 5.272 | <.001 | .287 | .537 | 15.690 | <.001 |
| General activityb | .003 | −.070 | −1.736 | .083 | .049 | −.224 | −5.659 | <.001 |
| Global severity indexc | .056 | .239 | 6.072 | <.001 | .500 | .708 | 24.714 | <.001 |
All models were tested separately.
Adj, adjusted; MPI, Multidimensional Pain Inventory; PCL-C, PTSD Checklist–Civilian Version; VAS, visual analog scale.
Nonsmoking = 0, smoking = 1.
n = 607.
Symptom Checklist-90-Revised.
Interaction of Smoking Status and PTSD Symptom Severity
Linear regression models were used to test whether smoking status moderated the effects of PTSD symptom severity on pain, psychological distress, and pain-related functioning (Table 3). Models were run with and without the following covariates: primary diagnostic category (RDC/TMD), age, sex, marital status, employment status, and duration of pain. Inclusion of the covariates did not substantively change the results, and as such, only the full model (i.e., with all covariates) is reported for ease of interpretation. Smoking significantly attenuated the impact of PTSD symptom severity on affective distress. A graphic analysis of the interaction revealed that this moderating effect of smoking on affective distress was most pronounced at low levels of PTSD symptom severity (i.e., 1 SD below the average PCL-C score of the sample) but was attenuated at a higher level of PTSD symptom severity (i.e., 1 SD above the average PCL-C score; see Fig.). Although this interaction was statistically significant, the impact on the overall effect size of the model was relatively small, with smoking as a moderator introducing a change in R2 of .014. Inclusion of MPI pain intensity as an additional covariate did not substantially alter the model (data not shown). No other significant interactions were found.
Table 3.
Linear Regression Models: Measures of Pain, Psychological Distress, and Pain-Related Functioning Predicted by PTSD Symptom Severity (Total PCL-C Score) with Smoking as Moderator.
| Outcome Variable (Total R2): Predictive Variable | B | SE | P Value | ΔR2 |
|---|---|---|---|---|
| VAS average pain intensity (.179) | ||||
| PCL-C score | 0.42 | 0.078 | .005 | |
| Smoking | 14.71 | 5.191 | <.001 | |
| PCL-C × smoking | −0.05 | 0.142 | .731 | <.001 |
| Pain severitya (.164) | ||||
| PCL-C score | 0.34 | 0.053 | <.001 | |
| Smoking | 10.44 | 3.525 | .003 | |
| PCL-C × smoking | −0.13 | 0.096 | .175 | .003 |
| Interferencea (.200) | ||||
| PCL-C score | 0.44 | 0.053 | <.001 | |
| Smoking | 6.82 | 3.535 | .054 | |
| PCL-C × smoking | −0.07 | 0.097 | .489 | .001 |
| Life controla (.217) | ||||
| PCL-C score | −0.32 | 0.035 | <.001 | |
| Smoking | −4.59 | 2.307 | .047 | |
| PCL-C × smoking | 0.067 | 0.063 | .275 | .002 |
| Affective distressa (.348) | ||||
| PCL-C score | 0.50 | 0.034 | <.001 | |
| Smoking | 10.07 | 2.259 | <.001 | |
| PCL-C × smoking | −0.22 | 0.062 | <.001 | .014 |
| General activitya,b (.112) | ||||
| PCL-C score | −0.14 | 0.033 | <.001 | |
| Smoking | 0.55 | 2.181 | .80 | |
| PCL-C × smoking | −0.01 | 0.060 | .89 | <.001 |
| Global severity indexc (.533) | ||||
| PCL-C score | 0.56 | 0.028 | <.001 | |
| Smoking | 4.62 | 1.831 | .012 | |
| PCL-C × smoking | −0.06 | 0.050 | .207 | .001 |
Controlled for potential covariates: RDC/TMD primary diagnostic category, sex, age, marital status, employment status, duration of pain; Linear regression models: df1 = 9, df2 = 600).
PCL-C, PTSD Checklist–Civilian Version; PTSD, posttraumatic stress disorder; RDC/TMD, research diagnostic criteria for temporomandibular disorders; VAS, visual analog scale.
Multidimensional Pain Inventory subscale.
n = 607; df1 = 9, df2 = 597.
Symptom Checklist-90-Revised subscale.
Figure.
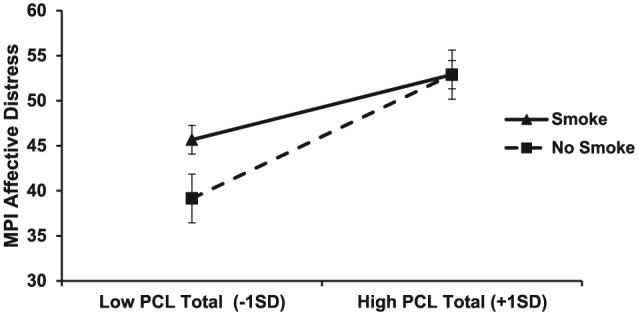
Interaction of posttraumatic stress disorder symptoms and smoking status on affective distress. MPI, Multidimensional Pain Inventory; PCL, PTSD Checklist–Civilian Version.
Smoking and PTSD Symptom Clusters
Independent samples t tests compared total PCL-C scores and PTSD symptom cluster scores in smokers and nonsmokers (hypothesis 2; Table 4). Smokers displayed significantly higher total scores. Differences in mean scores of all PCL-C symptom clusters were significant, with smokers displaying more severe symptoms of reexperiencing, avoidance, hyperarousal, numbing, and dysphoria.
Table 4.
Results of Independent Samples t Tests Comparing Smokers and Nonsmokers Regarding Total PCL-C Score and Mean Scores Loading on PCL-C Symptom Clusters.
| PCL-C | Smokers | Nonsmokers | t | df | P Value |
|---|---|---|---|---|---|
| Total score | 35.7 ± 14.4 | 29.7 ± 12.7 | −4.64a | 240.67 | <.001a |
| Symptom clustersb | |||||
| Reexperiencing | 2.13 ± 1.0 | 1.86 ± .92 | −3.05 | 608 | .002 |
| Avoidance | 2.06 ± 1.15 | 1.78 ± 1.04 | −2.84 | 608 | .005 |
| Numbing | 1.82 ± .90 | 1.53 ± .76 | −3.56a | 234.03 | <.001a |
| Hyperarousal | 2.37 ± 1.07 | 1.83 ± .88 | −5.63a | 229.22 | <.001a |
| Dysphoria | 2.09 ± .88 | 1.70 ± .79 | −4.94a | 243.01 | <.001a |
Values are presented as mean ± SD.
PCL-C, PTSD Checklist–Civilian Version.
Levene’s test for equality of variances, P < .05; equal variances not assumed.
Means calculated according to mean scores of PCL-C items that load on individual symptom clusters.
Discussion
The results revealed significant main effects of smoking and PTSD symptom severity in predicting pain severity, psychological distress, and pain-related dysfunction. These results are consistent with empirical evidence from previous studies (de Leeuw et al. 2005; Bertoli et al. 2007; Weingarten et al. 2009; Burris et al. 2013; de Leeuw et al. 2013; Custodio et al. 2015). The consistency and strength of these findings support the importance of smoking cessation efforts for patients with orofacial pain.
Despite the main effects of smoking and PTSD, our hypothesis of an interaction between PTSD symptom severity and smoking was not confirmed. No significant interactions between smoking status and PTSD symptom severity were found for any outcomes, with the exception of affective distress. The lack of a synergistic effect of smoking status and PTSD symptom severity on most outcomes is surprising given the high levels of PTSD symptom severity among smokers, the high prevalence of smokers among patients with probable PTSD, and the prediction of worse pain-related outcomes associated with both pain and smoking when considered separately as predictive variables. The question arises whether the combined effects of smoking and PTSD symptom severity on pain-related outcomes might be characterized as additive rather than synergistic, in which case smoking would be expected to increase levels of pain and dysfunction uniformly across all levels of PTSD symptom severity.
Affective distress as measured by the MPI was the sole exception to the lack of interactions between smoking and PTSD symptom severity. Smoking attenuated the increase in affective distress that occurred with increasing PCL-C scores.
Previous research suggests that smoking may uniquely moderate affect among subjects with PTSD. Affect diary data from male Vietnam combat veterans (61 with PTSD, 56 without PTSD) showed higher anger/hostility and lower positive affect among smokers with PTSD as compared with smokers without PTSD (Beckham et al. 2004). However, among participants with PTSD, smokers reported higher positive affect than did nonsmokers, a phenomenon not observed within the non-PTSD group. These findings and those of the present study may reflect use of smoking as a mechanism for affect regulation by individuals with PTSD and pain.
A reduction in affective distress could be a significant incentive for continued smoking, especially in pain patients whose coping mechanisms may be overwhelmed by the superimposition of a persistent pain condition on a background of PTSD. However, in the present study the change in the variance of the PTSD symptom severity-affective distress relationship with smoking included as a moderator was only .014. Such a small change is of dubious clinical significance. Furthermore, our data suggest that smoking does not regulate affective distress at high levels of PTSD symptom severity. Therefore, unrealistic outcome expectancies that exceed actual benefits may be at play in any effort by these patients to regulate affect by smoking. The present study’s data do not reveal whether the participants in the smoking group consciously chose smoking as such a coping mechanism, but they do suggest that the smokers gained minimal to no reduction in affective distress in exchange for an increased burden of overall psychological distress, pain, and pain-related dysfunction.
The increase in affective distress as a function of PCL-C score may be steeper among nonsmokers than among smokers in part because smokers display a relatively high level of affective distress even at lower levels of PTSD symptomatology (see Fig.). These data have intriguing clinical implications, suggesting that smoking cessation may reduce affective distress in TMD patients with a history of trauma but that achieving this effect in persons with high levels of PTSD symptomatology may require effective management of their trauma-related symptoms. The anticipated and actual changes in affect that result from smoking and smoking cessation in patients with orofacial pain and PTSD should be tested in future research.
Exploration of our hypothesis regarding smoking within PTSD symptom clusters revealed that smoking was indeed associated with increased mean symptom cluster scores, but the effect was generalized across all clusters rather than limited to select symptoms. These results do not suggest that smoking cessation may target any particular set of PTSD-related symptoms; rather, they reinforce the importance of smoking cessation for pain patients with PTSD and suggest that cessation may be an important component in achieving global reduction of PTSD symptoms.
The association of both PTSD and smoking with worse pain-related outcomes highlights the complexity of pain management in patients with these conditions. Smoking seems to replace and may hinder the implementation of more adaptive coping strategies (Ditre et al. 2011). Despite the frustration and challenge involved in trying to help these patients, there is reason for hope: probably all clinicians know patients who have successfully quit smoking. Furthermore, experimental evidence suggests that interventions such as coping enhancement and expectancy modification may influence smokers to change their behavior (Ditre et al. 2010). Further research is needed to develop optimal treatments to help patients in pain rid themselves of smoking in favor of truly helpful coping strategies. Patients who suffer from PTSD may present special challenges and may particularly benefit from such efforts. For example, integrated treatment of smoking and PTSD has demonstrated more success than conventional smoking cessation treatment in achieving 12-mo smoking abstinence (McFall et al. 2010).
Several limitations of this study should be noted. The retrospective design of this study precludes drawing conclusions about causality among any of the variables examined. Dichotomizing current smoking status limited our ability to investigate certain lines of inquiry; future studies of smoking and PTSD in orofacial pain populations would do well to use a valid measure of smoking or a nicotine dependence scale as a continuous variable. Our discussion of possible links among motivation to regulate affect, outcome expectancies, and the moderating impact of smoking on the relationship between PTSD symptom severity and affective distress appears to be consistent with our data and with the characteristics of smoking in PTSD as noted in previous studies (Beckham et al. 2004; Beckham et al. 2005; Beckham et al. 2007; Cook et al 2007; Feldner et al. 2007; Marshall et al. 2008). However, no direct measures of smoking motives or expectancies were included in this study. Our suggestions therefore require verification by future research using valid and reliable measures of smoking motives and expectancies.
Conclusion
Smoking and PTSD symptom severity each predict worse levels of pain, psychological distress, and pain-related dysfunction in TMD patients. Smoking slightly reduces the impact of PTSD symptom severity on affective distress, though this effect dissipates at high levels of PTSD symptomatology. Otherwise, smoking does not modify relationships between PTSD symptom severity and pain, global psychological distress, or pain-related functioning. For TMD patients with a history of trauma, smoking is an ineffective mechanism for coping with affective distress. Addressing smoking and PTSD symptoms are critical components of the comprehensive care of orofacial pain patients. Providers who treat patients with orofacial pain should screen for both smoking and PTSD symptoms and should be prepared to refer patients for management of these conditions.
Author Contributions
T. Weber, I.A. Boggero, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; C.R. Carlson, contributed to conception, design, data acquisition, analysis, and interpretation, and critically revised the manuscript; E. Bertoli, contributed to conception, design, data analysis, and interpretation, and critically revised the manuscript; J.P. Okeson, contributed to conception and design, and critically revised the manuscript; R. de Leeuw, contributed to conception, design, data analysis, and interpretation, and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors express gratitude to Ms. Eilene Foster and Dr. Pam Driggers for editorial and technical support. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor the official policy or position of the U.S. government, the Department of Defense, or the Department of the Air Force.
Footnotes
I.A. Boggero’s research activities, including his participation as a coauthor of this study, are supported by the National Institute on Aging of the National Institutes of Health (F31AG048692).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Agaku IT, King BA, Dube SR. 2014. Current cigarette smoking among adults: United States, 2005–2012. Morbidity and Mortality Weekly Report. 63(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Trauma- and stressor-related disorders. In: Diagnostic and statistical manual of mental disorders. 5th ed Washington (DC): American Psychiatric Publishing; p. 271–290. [Google Scholar]
- Arrindell WA, Barelds DP, Janssen IC, Buwalda FM, van der Ende J. 2006. Invariance of SCL-90R dimensions of symptom distress in patients with peri partum pelvic pain (PPPP) syndrome. Br J Clin Psychol. 45(Pt 3):377–391. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Dennis MF, McClernon FJ, Mozley SL, Collie CF, Vrana SR. 2007. The effects of cigarette smoking on script-driven imagery in smokers with and without posttraumatic stress disorder. Addict Behav. 32(12):2900–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JC, Feldman ME, Vrana SR, Mozley SL, Erkanli A. 2005. Immediate antecedents of cigarette smoking in smokers with and without posttraumatic stress disorder: a preliminary study. Exp Clin Psychopharm. 13(3):219–228. [DOI] [PubMed] [Google Scholar]
- Beckham JC, Gehrman PR, McClernon FJ, Collie CF, Feldman ME. 2004. Cigarette smoking, ambulatory cardiovascular monitoring, and mood in Vietnam veterans with and without chronic posttraumatic stress disorder. Addict Behav. 29(8):1579–1593. [DOI] [PubMed] [Google Scholar]
- Bertoli E, de Leeuw R, Schmidt JE, Okeson JP, Carlson CR. 2007. Prevalence and impact of post-traumatic stress disorder symptoms in patients with masticatory muscle or temporomandibular joint pain: differences and similarities. J Orofac Pain. 21(2):107–119. [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. 1996. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 34(8):669–673. [DOI] [PubMed] [Google Scholar]
- Burris JL, Perez C, Evans DR, Carlson CR. 2013. A preliminary study of cigarette smoking in female orofacial pain patients. Behav Med. 39(3):73–79. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. 2007. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 192(1):87–95. [DOI] [PubMed] [Google Scholar]
- Custodio L, Carlson CR, Upton B, Okeson JP, Harrison AL, de Leeuw R. 2015. The impact of cigarette smoking on sleep quality of patients with masticatory myofascial pain. J Oral Facial Pain Headache. 29(1):15–23. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Burris JL, Carlson CR. 2011. Disaggregating the relationship between posttraumatic stress disorder symptom clusters and chronic orofacial pain: implications for the prediction of health outcomes with PTSD symptom clusters. Ann Behav Med. 41(1):1–12. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, editor. 2008. Orofacial pain: guidelines for assessment, diagnosis, and management. 4th ed Chicago (IL): Quintessence. [Google Scholar]
- de Leeuw R, Bertoli E, Schmidt JE, Carlson CR. 2005. Prevalence of post-traumatic stress disorder symptoms in orofacial pain patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 99(5):558–568. [DOI] [PubMed] [Google Scholar]
- de Leeuw R, Eisenlohr-Moul T, Bertrand P. 2013. The association of smoking status with sleep disturbance, psychological functioning, and pain severity in patients with temporomandibular disorders. J Orofac Pain. 27(1):32–41. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. 1979. Symptom Checklist-90-R. Minneapolis (MN): National Computer Systems. [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. 2011. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 137(6):1065–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. 2010. Effects of expectancies and coping on pain-induced motivation to smoke. J Abnorm Psychol. 119(3):524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. 1992. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 6(4):301–355. [PubMed] [Google Scholar]
- Feldner MT, Babson KA, Zvolensky MJ, Vujanovic AA, Lewis SF, Gibson LE, Monson CM, Bernstein A. 2007. Posttraumatic stress symptoms and smoking to reduce negative affect: an investigation of trauma-exposed daily smokers. Addict Behav. 32(2):214–227. [DOI] [PubMed] [Google Scholar]
- Hardt J, Gerbershagen HU, Franke P. 2000. The symptom check-list, SCL-90-R: its use and characteristics in chronic pain patients. Eur J Pain. 4(2):137–148. [DOI] [PubMed] [Google Scholar]
- Hayes AF. 2012. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. http://www.afhayes.com/public/process2012.pdf.
- Kerns RD, Turk DC, Rudy TE. 1985. The West Haven–Yale Multidimensional Pain Inventory (WHYMPI). Pain. 23(4):345–356. [DOI] [PubMed] [Google Scholar]
- Marshall EC, Zvolensky MJ, Vujanovic AA, Gibson LE, Gregor K, Bernstein A. 2008. Evaluation of smoking characteristics among community-recruited daily smokers with and without posttraumatic stress disorder and panic psychopathology. J Anxiety Disord. 22(7):1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall M, Saxon AJ, Malte C, Chow B, Bailey S, Baker DG, Beckham JC, Boardman KD, Carmody TP, Joseph AM, et al. 2010. Integrating tobacco cessation into mental health care for posttraumatic stress disorder: a randomized controlled trial. JAMA. 304(22):2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. 2003. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 106(1–2):127–133. [DOI] [PubMed] [Google Scholar]
- Sharp TJ, Harvey AG. 2001. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. 21(6):857–877. [DOI] [PubMed] [Google Scholar]
- Sherman JJ, Carlson CR, Wilson JF, Okeson JP, McCubbin JA. 2005. Post-traumatic stress disorder among patients with orofacial pain. J Orofac Pain. 19(4):309–317. [PubMed] [Google Scholar]
- Shi Y, Weingarten TN, Mantilla CB, Hooten WM, Warner DO. 2010. Smoking and pain: pathophysiology and clinical implications. Anesthesiology. 113(4):977–992. [DOI] [PubMed] [Google Scholar]
- Weingarten TN, Iverson BC, Shi Y, Schroeder DR, Warner DO, Reid KI. 2009. Impact of tobacco use on the symptoms of painful temporomandibular joint disorders. Pain. 147(1–3):67–71. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Legues CW, Brown RA, Feldner MT. 2008. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine Tob Res. 10(8):1415–1427. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. 2009. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine Tob Res. 11(12):1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]


