Abstract
Hypertension (HTN) induced by bevacizumab is a side effect that is often thought to resolve after treatment. However, there are currently no reports on rates of resolution. We assess the incidence and timing of the resolution of bevacizumab induced HTN.
We evaluated all patients treated with bevacizumab for gynecologic malignancies at a single institution from 2012 through 2014. HTN was retrospectively diagnosed and staged by CTCAE v4.0 criteria. Resolution of HTN was defined as ≥ 2 values return to baseline blood pressure and/or discontinuation/decrease of blood pressure medications.
We identified 104 patients; 35 were excluded due to receiving bevacizumab at time of analysis. Grade 2 or higher induced HTN was identified in 34/69 (49.3%) patients, of which 26/69 (37.7%) had grade 2 HTN and 8/69 (11.6%) had grade 3/4 HTN. Onset of HTN occurred at a median of 67 (14–791) days. Resolution of HTN occurred in 28/34 (82.4%) patients with a median time to resolution of 87 (3–236) days. BMI, history of HTN, blood pressure medications, prior bevacizumab treatment, number of bevacizumab cycles, CA-125 and albumin at initiation of treatment were not independent risk factors associated with developing HTN in multivariate analysis. Median PFS for those with HTN was 12.5 (1.9–45.8) months vs 11.0 (2.1–44.7) for those without (p = 0.17).
Hypertension induced by bevacizumab resolved in 82% of patients in a median of 87 days. There were no identifiable risk factors associated with induced HTN and HTN was not a biomarker for improved prognosis in our cohort.
Keywords: Bevacizumab, Hypertension, Chemotherapy
Highlights
-
•
Bevacizumab induced HTN in gynecologic cancers has a resolution rate of 82% with a median of 87 days.
-
•
We found no specific risk factors for development of bevacizumab induced HTN in gynecologic cancers.
-
•
Development of bevacizumab induced hypertension does not correlate with improved PFS in our cohort.
1. Introduction
Bevacizumab is a monoclonal antibody that targets vascular endothelial growth factor (VEGF) and is approved by the US FDA for colorectal cancer, renal cell cancer, glioblastoma, non-small cell lung cancer, ovarian cancer and cervical cancer. Although FDA approval for gynecologic malignancies was only obtained in 2014, Bevacizumab has been utilized as an adjuvant or single agent chemotherapy for well over a decade.
Hypertension (HTN) associated with the use of bevacizumab is one of the most common side effects reported in the literature across oncologic fields. Meta-analyses have demonstrated overall incidence of hypertension ranging from 2.7 to 32% and incidence of grade 3 or 4 HTN ranging from 1.8 to 22% (Ranpura et al., 2010). Incidence of grade 3 or 4 HTN when treating gynecologic malignancies ranges from 17–25% (Burger et al., 2011, Perren et al., 2011, Aghajanian et al., 2012, Tewari et al., 2014). If uncontrolled, hypertension can lead to many significant medical comorbidities such as heart disease, stroke, and kidney disease. When hypertension is iatrogenically induced from cancer related therapy, it is imperative for providers to understand, and report to patients, the expected duration and risks.
The half-life of bevacizumab is estimated to be approximately 20 days (range 10–50 days) with doses of 1–20 mg/kg either weekly or every three weeks (Syrigos et al., 2011). This infers that time to drug clearance would be an estimated 100 days (5 half-lives). Time analysis of onset and resolution of bevacizumab induced hypertension is limited within the current literature. It is our hypothesis that hypertension will resolve with drug clearance of 100 days. The primary objective of this study was to identify the duration and rate of resolution for bevacizumab induced hypertension. Secondary analysis were performed to evaluate incidence of hypertension, identification of risk factors for induced hypertension, and evaluation of prognosis in those with induced hypertension compared to those without.
2. Methods and materials
All patients treated with bevacizumab for gynecologic malignancies at a single institution from 2012 through 2014 were reviewed. Approval was obtained from the Institutional Review Board (IRB). Blood pressure values were obtained and reviewed from all clinic visits prior to, during, and post bevacizumab therapy. HTN was diagnosed based on ≥ 2 recorded elevated blood pressure values in the medical record and staged by Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 published by the National Institutes of Health and the National Cancer Institute (Table 1). Resolution of HTN was defined as ≥ 2 values of a return to baseline blood pressure and/or discontinuation/decrease of blood pressure medications. Patients with preexisting HTN were graded with the same criteria and noted as preexisting controlled HTN if ≤ grade 1 HTN at time of presentation. Bevacizumab induced HTN was diagnosed in patients with preexisting HTN if there was an elevation of grade by CTCAE criteria from their presenting pretreatment baseline blood pressure. All demographic and clinical information was abstracted from the medical chart. This information included age, BMI, malignancy, history of HTN and/or blood pressure medication use, bevacizumab treatment history, and presenting lab values of CA-125 and albumin. Baseline medical comorbidities were quantified using the Charlson Comorbidity Index (CCI). The CCI is a validated instrument used to estimate risk of death. It is a weighted index that assigns a score based on medical comorbidities such as coronary artery disease, diabetes, kidney or liver disease, or metastatic solid tumors. All patient deaths related to disease at time of analysis were noted and PFS was calculated as length of time from initiation of bevacizumab treatment to documented date of disease progression or recurrence by radiography and/or provider.
Table 1.
CTCAE v4.0 (U.S. Department of Health and Human Services, NIH, 2009.)
| Grade 1 | Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated. | Prehypertension (systolic BP 120–139 mm Hg or diastolic BP 80–89 mm Hg) |
| Grade 2 | Moderate; minimal, local or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living (ADL). | Stage 1 hypertension (systolic BP 140–159 mm Hg or diastolic BP 90–99 mm Hg); medical intervention indicated; recurrent or persistent (≥ 24 h); symptomatic increase by > 20 mm Hg (diastolic) or to > 140/90 mm Hg if previously WNL; monotherapy indicated Pediatric: recurrent or persistent (≥ 24 h) BP > ULN; monotherapy indicated |
| Grade 3 | Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self care ADL. | Stage 2 hypertension (systolic BP ≥ 160 mm Hg or diastolic BP ≥ 100 mm Hg); medical intervention indicated; more than one drug or more intensive therapy than previously used indicated |
| Grade 4 | Life-threatening consequences; urgent intervention indicated. | Life-threatening consequences (e.g., malignant hypertension, transient or permanent neurologic deficit, hypertensive crisis); urgent intervention indicated |
| Grade 5 | Death related to adverse event. | Death |
Study data was collected and managed using the Research Electronic Data Capture (REDCap). Descriptive statistics and tests of normality were computed. Kolmogorov-Smirnov tests were used to assess normality of continuous variables. Non-normally distributed data were compared using Mann-Whitney U tests. Continuous variables were compared with Student’s t-tests. Dichotomous were compared using chi-square or Fisher’s exact test for comparisons with cells < 5. Kaplan-Meier was used to compute median time to resolution of HTN and a survival curve. Statistical analysis was performed using IBM SPSS version 23.
3. Results
Of the 104 patients identified, 35 were excluded as they were still receiving bevacizumab at the time of analysis. Patient characteristics are presented in Table 2. Median follow-up time was 17.5 (2–50) months. Overall incidence of ≥ grade 2 bevacizumab induced hypertension was 49.3% (34/69). The majority of HTN requiring treatment was grade 2 with 26/69 (37.7%) and 8/69 (11.6%) patients had grade 3 or 4 (Table 3).
Table 2.
Characteristics of those with and without bevacizumab induced HTN.
| HTN n = 34 | No HTN n = 35 | p-value | |
|---|---|---|---|
| Age (years; mean ± SD) | 58.6 ± 11.6 | 57.3 ± 7.4 | 0.56 |
| BMI (kg/m2; median (range)) | 25.3 (19.7–46.4) | 24.6 (17.5–125.0) | 0.59 |
| Malignancy | 0.11 | ||
| Ovary | 32 (94.1%) | 28 (80.0%) | |
| Uterine | 0 | 4 (11.4%) | |
| Cervix | 2 (5.9%) | 3 (8.6%) | |
| Hx of HTN | 16 (47.1%) | 14 (40.0%) | 0.55 |
| Prior BP medication use | 13 (81.3%) | 10 (71.4%) | 0.53 |
| Prior bevacizumab exposure | 4 (11.8%) | 5 (14.3%) | 1.0 |
| Number of bevacizumab cycles (median (range)) | 11 ( 3–57) | 8 (1–49) | 0.09 |
| CA-125a (median (range)) | 358 (24–8107) | 300 (12–6980) | 0.48 |
| Albuminb (g/dL; median (range)) | 3.8 (2.9–4.5) | 3.7 (2.8–4.5) | 0.35 |
| Charlson Comorbidity Index score (median (range)) | 7.5 (0 − 12) | 7 (2 − 10) | 0.61 |
| PFS (months; median (range)) | 12.5 (1.9–45.8) | 11.0 (2.1–44.7) | 0.17 |
CA-125 at initiation of bevacizumab treatment.
Albumin at initiation of bevacizumab treatment.
Table 3.
Induced hypertension as categorized by CTCAE v4.0 criteria.
| n = 69 (%) | |
|---|---|
| None | 22 (31.9) |
| Grade 1 | 13 (18.8) |
| Grade 2 | 26 (37.7) |
| Grade 3 | 7 (10.1) |
| Grade 4 | 1 (1.5) |
| Grade 5 | 0 |
Median number of bevacizumab cycles in those with HTN was 11 (3–57) compared to 8 (1–49) in those without HTN (p = 0.09). Ninety four percent (65/69) of patients received dosing of 15 mg/kg every three weeks, six patients received 10 mg/kg every two weeks. Of these six patients, 2 received dosing of 15 mg/kg every three weeks during primary treatment and were reduced to 10 mg/kg every two weeks for maintenance therapy, three patients received single agent treatment of 10 mg/kg every two weeks for recurrent disease, and one patient received 10 mg/kg every two weeks on trial.
Onset of HTN occurred at a median of 67 (14–791) days. Resolution of HTN after the last dose of bevacizumab occurred in 28/34 (82.4%) patients with a median of 87 (3–236) days. Analysis of all patients who developed HTN, including those who did not resolve in the study period, demonstrates a median time to resolution of 104 days (Fig. 1). Of the 34 patients with bevacizumab induced HTN, 16/34 (46%) had an elevation of BP to ≥ grade 2 HTN who had a pre-existing diagnosis of ≥ grade 2 HTN that was controlled on medications prior to initiation of bevacizumab, compared to 14/35 (40%) patients who had an elevation of BP to ≥ grade 2 HTN who previously had no HTN or grade 1 HTN. In patients requiring initiation of at least one new blood pressure medication, there was no consistency in type of medication started. Nine patients received diuretics, four β-blockers, seven angiotensin-converting-enzyme inhibitors (ACE-I), two angiotensin II receptor blockers (ARB), three calcium channel blockers (CCB), and one α-blockers. Ten patients were either on a β-blocker prior to initiation of bevacizumab or started on one during treatment. Median progression free survival (PFS) for those with HTN was 12.5 (1.9–45.8) months compared to 11.0 (2.1–44.7) for those without HTN (p = 0.17). Median PFS in patients with HTN on a β-blocker was 11.5 (4.0–21.9) months.
Fig. 1.
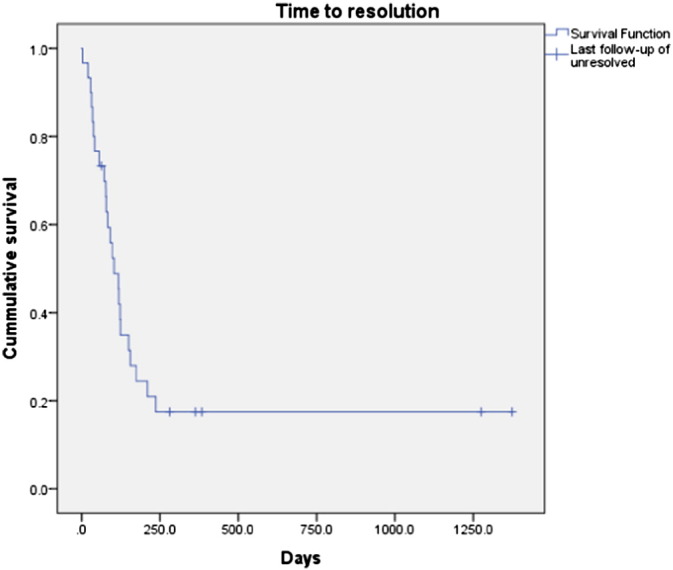
Hypertension time to resolution curve
Survival curve for time to resolution of all patients who developed HTN on bevacizumab, including those who did not have resolution of HTN within the time period studied.
BMI, history of HTN, prior use of blood pressure medications, prior bevacizumab treatment, number of bevacizumab cycles, CA-125 and albumin at initiation of treatment, and the Charlson Comorbidity Index were similar for the two groups in bivariate analyses (Table 2). The number of bevacizumab cycles was slightly but not statistically significantly higher for those who develop HTN (median 11 vs. 8; p = 0.09). No identifiable risk factor, of those evaluated, was identified for development of induced HTN.
4. Discussion
This study demonstrates an incidence of ≥ grade 2 bevacizumab induced HTN of 49%, which is higher than the previously reported literature for gynecologic malignancies. In the AURELIA trial for platinum-resistant recurrent ovarian cancer the incidence of grade 2 or higher hypertension was 20% with the addition of bevacizumab to chemotherapy, compared to 7% in the control arm (Pujade-Lauraine et al., 2014). Tewari et al demonstrated an incidence of 25% grade 2 or higher hypertension compared to 2% with the addition of bevacizumab in the treatment of cervical cancer (Tewari et al., 2014). The OCEANS, ICON7 and GOG218 trials incidence of grade 3 or 4 hypertension was 17%, 18%, and 22.9% with the addition of bevacizumab, compared to 1%, 2%, and 7.2% in the control arms respectively (Burger et al., 2011, Perren et al., 2011, Aghajanian et al., 2012). We demonstrate a rate of 11.6% of grade 3 or 4 hypertension. We suspect that the increased incidence of grade 2 HTN in this study is due to our retrospective analysis of HTN being the primary outcome. We diagnosed HTN using CTCAE guidelines (Table 1), analyzing all blood pressures in the medical chart whether diagnosis was documented or not. While our criteria for HTN diagnosis is identical to the Joint National Committee of prevention, detection, evaluation, and treatment of High blood pressure (JNC 7) (Moser, 2004), we speculate that a retrospective analysis looking specifically at blood pressure values over multiple visits would increase the diagnosis rate compared to a prospective analysis looking at individual blood pressure values, or ICD-9 codes for HTN.
Despite a high incidence of HTN, this study was unable to demonstrate any associated risk factors associated with development of bevacizumab induced HTN. We specifically analyzed age, BMI, history of HTN, prior BP medication use, prior exposure to bevacizumab, CA-125 values, albumin, and the Charlson comorbidity score (Table 2). Although we did not find any risk factors associated with bevacizumab induced HTN, the sample size was inadequate to detect a difference in these secondary outcomes. Of note, number of cycles and PFS may be slightly higher but did not reach statistical significance. This contradicts existing literature by Hamnvik et al. that demonstrated age, BMI, and history of HTN as risk factors for anti-VEGF therapy induced HTN in renal cell, hepatocellular, gastrointestinal, and sarcoma tumors (Hamnvik et al., 2015). However, Hamnvik’s study looked at other anti-VEGF therapies that did not include bevacizumab which may account for the variation in results. A separate established risk factor is the dose of bevacizumab. Our study did not demonstrate dose to be an associated risk factor, however 94% of our patients received 15 mg/kg every three weeks and therefore this was not properly evaluated in this study. This may be less relevant in treating gynecologic malignancies as there is often little variation in dosing, as demonstrated in this study.
The median time to onset of HTN in this study was 67 days. In relation to bevacizumab induced HTN in renal cell carcinoma, Yang et al. describes median time to onset from first dose as 131 days and states that hypertension uniformly decreased after cessation of therapy for metastatic renal cancer (Yang et al., 2003). Escalante et al. describe the median time to onset of hypertension in lung, breast and colorectal cancers from first dose as 7 days and post treatment decrease of hypertensive medications as 30.5 days (11–162) (Escalante et al., 2013).
These trials, including ours, were relatively small cohorts that lack consistency in malignancy treated as well as regimens used. These differences may account for the discrepancy in time of onset.
In this study we demonstrate that 82% of bevacizumab induced HTN resolved with a median of 87 days. This is consistent with the pharmacokinetics of the drugs clearance, which would predict resolution within 100 days. To understand resolution we must also understand the mechanism of action for its onset. The pathogenesis of VEGF inhibitor induced hypertension has several potential mechanisms and is likely multifactorial. Syrigos, et al. discusses the decreased nitric oxide synthesis leading to vasoconstriction, increased vascular tone, and decreased sodium ion renal excretion. A decrease in capillaries and arterioles due to VEGF inhibition will also increase peripheral resistance. Furthermore, there may be an effect on renal impairment by decreasing functionality between the podocytes and endothelial cells of the kidney as demonstrated by proteinuria associated with bevacizumab use (Ranpura et al., 2010, Syrigos et al., 2011). It is unclear which of these mechanisms plays a majority roll in this drug induced HTN, however a multifactorial etiology may explain why there is not complete resolution of HTN.
Grade 2 HTN is indication for intervention with medication. Of the 34 patients with ≥ grade 2 HTN, 15 (44%) patients in our cohort were started on a new medication or medication added to their existing regimen. There are currently no guidelines as to the most appropriate blood pressure management for bevacizumab induced hypertension. This is likely due to a multifactorial pathogenesis as well as a void in the existing study of this subject. Our data indicates that the majority of bevacizumab induced HTN is temporary. However, the JNC guidelines do recommend treatment for grade 2 HTN and we suggest appropriate adherence to these guidelines. Future studies should evaluate if strict adherence to guidelines and tight blood pressure control during chemotherapy treatment has an impact on overall hypertension related morbidity such as end organ damage, ischemia, or stroke.
Recently Watkins et al. demonstrated that the use of non-selective β-blocker therapy in patients with ovarian cancer is associated with longer overall survival (OS) (Watkins et al., 2015). While our study did not have significant power to demonstrate benefit in those with induced HTN already on, or started on, a β-blocker it is an interesting inference to consider β-blockade in those who develop bevacizumab induced HTN. Currently there is no recommendation on which antihypertensive therapy is the most appropriate for anti-VEGF induced HTN.
Given the high incidence of HTN with anti-VEGF therapy and its potential mechanisms of action, it has been hypothesized that HTN may be a marker for drug efficacy. Several studies have indicated that treatment related hypertension can predict improved outcomes in renal cell, colorectal and pancreatic cancers (Kindler et al., 2005, Osterlund et al., 2011). Bono et al. found a PFS improvement of 4.1 months in patients with renal cell cancer who had bevacizumab induced HTN compared to those who did not (Bono et al., 2009). Our study did not demonstrate a statistically significant improvement in PFS in those who developed bevacizumab induced HTN compared to those who did not. This remains a relevant and interesting question that should be further evaluated in larger trials.
Limitations of this study include its retrospective evaluation of a small cohort at a single institution, analysis of heterogeneous malignancies and variation in dosing of bevacizumab. Poor compliance with initiation of treatment for grade 2 hypertension may also alter the time to resolution, however we would not expect it to alter the incidence. Further limitations are related to a lack of data in other potential confounding factors such as treatment of recurrent or primary disease, combination of other chemotherapy regimens, race, urine protein/creatinine and preexisting or induced renal pathology. Further studies are warranted to evaluate risk factors for induced HTN, relation to associated hypertension related comorbidities, as well as effect on prognosis within gynecologic malignancy. HTN is the most prevalent side effect of bevacizumab in gynecologic malignancies. This paper is the first to demonstrate that the majority of bevacizumab induced HTN is transient and resolves within the expected drug clearance timeframe.
Footnotes
This project was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. Its contents are the authors' sole responsibility and do not necessarily represent official NIH views.
References
- Aghajanian C., Blank S.V., Goff B.A., Judson P.L., Teneriello M.G., Husain A. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30(17):2039–2045. doi: 10.1200/JCO.2012.42.0505. (Jun 10, PubMed PMID: 22529265. Pubmed Central PMCID: 3646321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono P., Elfving H., Utriainen T., Osterlund P., Saarto T., Alanko T. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann. Oncol. 2009;20(2):393–394. doi: 10.1093/annonc/mdn729. (Feb, PubMed PMID: 19211503) [DOI] [PubMed] [Google Scholar]
- Burger R.A., Brady M.F., Bookman M.A., Fleming G.F., Monk B.J., Huang H. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. (Dec 29, PubMed PMID: 22204724) [DOI] [PubMed] [Google Scholar]
- Escalante C.P., AL, Palla S.L., Lu M., Overman M.J., George M., Baker R.A., Shakar S.P., Durand J.-B. Practice patterns of bevacizumab-induced hypertension in cancer patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013 (2013 ASCO Annual Meeting) [Google Scholar]
- Hamnvik O.P., Choueiri T.K., Turchin A., McKay R.R., Goyal L., Davis M. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121(2):311–319. doi: 10.1002/cncr.28972. (Jan 15, PubMed PMID: 25236375. Pubmed Central PMCID: 4293233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler H.L., Friberg G., Singh D.A., Locker G., Nattam S., Kozloff M. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005;23(31):8033–8040. doi: 10.1200/JCO.2005.01.9661. (Nov 1, PubMed PMID: 16258101) [DOI] [PubMed] [Google Scholar]
- Moser M. Update on the management of hypertension: recent clinical trials and the JNC 7. J. Clin. Hypertens. 2004;6(10 Suppl 2):4–13. doi: 10.1111/j.1524-6175.2004.03830.x. (Oct, PubMed PMID: 15470293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund P., Soveri L.M., Isoniemi H., Poussa T., Alanko T., Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br. J. Cancer. 2011;104(4):599–604. doi: 10.1038/bjc.2011.2. (Feb 15, PubMed PMID: 21304526. Pubmed Central PMCID: 3049598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365(26):2484–2496. doi: 10.1056/NEJMoa1103799. (Dec 29, PubMed PMID: 22204725) [DOI] [PubMed] [Google Scholar]
- Pujade-Lauraine E., Hilpert F., Weber B., Reuss A., Poveda A., Kristensen G. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. (May 1, PubMed PMID: 24637997) [DOI] [PubMed] [Google Scholar]
- Ranpura V., Pulipati B., Chu D., Zhu X., Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am. J. Hypertens. 2010;23(5):460–468. doi: 10.1038/ajh.2010.25. (May, PubMed PMID: 20186127) [DOI] [PubMed] [Google Scholar]
- Syrigos K.N., Karapanagiotou E., Boura P., Manegold C., Harrington K. Bevacizumab-induced hypertension: pathogenesis and management. BioDrugs. 2011;25(3):159–169. doi: 10.2165/11590180-000000000-00000. (Jun 1, PubMed PMID: 21627340) [DOI] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Long H.J., 3rd, Penson R.T., Huang H., Ramondetta L.M. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. (Feb 20, PubMed PMID: 24552320. Pubmed Central PMCID: 4010094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J.L., Thaker P.H., Nick A.M., Ramondetta L.M., Kumar S., Urbauer D.L. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121(19):3444–3451. doi: 10.1002/cncr.29392. (Oct 1, PubMed PMID: 26301456. Pubmed Central PMCID: 4575637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.C., Haworth L., Sherry R.M., Hwu P., Schwartzentruber D.J., Topalian S.L. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. (Jul 31, PubMed PMID: 12890841. Pubmed Central PMCID: 2275324) [DOI] [PMC free article] [PubMed] [Google Scholar]


