Abstract
Aims
To examine the correlation between the fasting plasma glucose and HbA1c levels using regression equation and to assess the average fasting plasma glucose levels for the specific HbA1c (A1C) goals in the patients with diabetes using each A1C-and fasting plasma glucose-based diagnostic criteria.
Methods
This study included data from 4481 participants with A1C and fasting plasma glucose, but with no diabetic medications in the Korean National Health and Nutritional Examination Survey 2011. The correlation between fasting plasma glucose and A1C was examined using linear regression models.
Results
The A1C levels corresponding to the fasting plasma glucose of 5.5 and 7 mmol/L were 5.75 and 6.42 %. However, in the subjects with diabetes diagnosed by the A1C criteria only, 5.5 and 7 mmol/L in the fasting plasma glucose predicted A1C of 6.49 and 7.14 % respectively. The average fasting plasma glucose levels to achieve specified A1C levels of 5.0–5.9, 6.0–6.9, 7.0–7.9, 8.0–8.9, and 9.0–9.9 % were 5.1, 6.1, 7.7, 8.8 and 11.2 mmol/L, respectively.
Conclusions
The association between A1C and fasting plasma glucose levels is in concordance with the existing criteria for diagnosis of diabetes. However, the average fasting plasma glucose concentrations to achieve targeted A1C may be lower than those in western populations.
Keywords: Diabetes mellitus, Diagnosis, Glucose, Hemoglobin A, Glycated
Background
The incidence of type 2 diabetes is becoming an emergency worldwide, particularly in East Asia, potentially reaching a pandemic level in the very near future [1]. Although a significant progress has been made in reducing cardiovascular risk factors among Korean patients with diabetes [2], type 2 diabetes creates a considerable amount of economic and social burdens in Korea due to its long-term and wide-ranging multiple chronic complications [3, 4]. Therefore, it is important to prevent these complications through its early diagnosis and prevention.
Fasting plasma glucose (FPG) should be measured for the purpose of screening or diagnosis for impaired fasting glucose and diabetes (hyperglycemic states) [5]. However, there are certain limitations with it: patients must fast more than 8 h before getting screened for diabetes and there are numerous factors (e.g., stress, acute illness) that can alter glucose concentrations [6]. Hemoglobin A1c (A1C) is a standard monitoring test for long-term glycemic control and was recently recommended for the diagnosis of diabetes as well although the generalization of the A1C diagnostic level compared with a glucose-based diagnosis may still be debated across ethnic groups [7]. In 2010, The American Diabetes Association (ADA) has recommended the A1C as a diagnostic tool for diabetes [8, 9] and in 2011, Korean Diabetes Association (kDa) has recommend the A1C as a diagnostic criterion for diabetes in clinical practice guidelines for type 2 diabetes [10, 11]. Recent study and recommendation showed average fasting glucose concentrations associated with specific A1C levels [12, 13]. This result can help the clinicians explain the relationship between fasting glucose concentrations and specific A1C levels more easily to the patients, but no study on this relationship has been made in Korea. Although there have been several hospital-based studies that have evaluated appropriate FPG and A1C cutoff levels for identifying patients with hyperglycemic states [14–16], it is thought that larger epidemiological studies are required to confirm the correlation between A1C and FPG for the diagnosis of diabetes in Korean population. We conducted this study to examine the relationship between the FPG and A1C for diabetes diagnosis (using the regression equation) and to access the average FPG concentrations associated with specific A1C levels in diabetic patients (using each A1c-based and FPG-based diagnostic criteria) [4, 11, 17, 18] in the recent national representative sample of the Korean adult population.
Methods
The Korea National Health and Nutrition Examination Survey (KNHANES), a nationwide cross-sectional survey and representative of non-institutionalized civilians in Korea, is conducted periodically by the Korea Centers for Disease Control and Prevention (KCDC). KNHANES was initiated in 1998 and was designed to provide comprehensive information about Koreans’ health status, health behavior, and nutritional status [19]. Data from the second year (2011) of KNHANES V (including A1C and FPG), was used in this cross-sectional analysis. To produce an unbiased national estimate, a sample weight was assigned for the participating individuals to represent the Korean population. Sampling weights were designated by the Korean Centers for Disease Control and Prevention and were constructed to account for the complex survey design, survey non-response, and poststratification [19].
From an initial total of 8518 men and women, 6066 (2677 men and 3389 women) with A1C and FPG, but without previous pharmacological therapy, were evaluated. From these 6066 persons, 1585 were excluded for the following reasons: 82 lacked underlying disease information; 707 were below 18 years old; 262 had fasting time less than 10 h; 366 had anemia defined as a hemoglobin value <130 g/L in men (n = 67) and <120 g/L in women (n = 299); 8 were pregnant; 14 had the estimated glomerular filtration ratio (eGFR) defined by the modification of diet in renal disease (MDRD) study equation less than 60 mL/min/1.73 m2; 9 had liver cirrhosis; and 137 had cancer of different kinds. Therefore, the final number of participants used in this analysis was 4481 and the total number was estimated to be 29,186,176 after a sample weight was assigned for the participating individuals to represent the Korean population. All examination (or testing) protocols were approved by the Institutional Review Board of Korea Centers for Disease Control and Prevention with each subject providing written informed consent before participating and the study protocol using this date was reviewed and approved by the institutional review board of Hallym University Sacred Heart Hospital.
Blood samples of all subjects were collected and the specimens were immediately centrifuged, aliquoted, frozen at −70 °C, and moved to the central laboratory (Neo-DIN Medical Institute, Seoul, South Korea), where they were analyzed within 24 h. The FPG concentrations were measured using an automated analyzer with an enzymatic assay (Pureauto S GLU: Daiichi, Tokyo, Japan) and the A1C was measured using high performance liquid chromatography (HLC-723G7: Tosoh, Yamaguchi, Japan). The thresholds for the diagnosis of diabetes were 7.0 mmol/L or greater by the FPG criteria, and 6.5 % or higher by the A1C criteria.
The statistical analysis was performed using SPSS (Chicago, IL) and weighted to the Korean population to provide nationally representative estimates. The baseline characteristics are presented as the means for continuous variables, and proportions for categorical variables. The association between fasting blood glucose and A1C was examined using linear regression models.
Results
The characteristics of the study population are presented in Table 1. The study subjects included 1992 men (estimated to be 15,168,273) and 2489 women (estimated to be 14,017,903). The average FPG value was 5.2 ± 0.8 mmol/L (ranging from 2.8 to 16.2 mmol/L) and the average A1C value was 5.6 ± 0.5 % (ranging from 3.1 to 13.6 %). Using data from KNHANES, the results of the simple linear regression analysis between the A1C level and FPG are summarized in Fig. 1. The prediction model between the A1C and FPG in this study population, expressed as A1C = 3.146 + 0.468 × FPG (R2 = 0.519, p < 0.001), is shown in Fig. 1a. The A1C level increased by approximately 0.47 % per the increment of 1.0 mmol/L in FPG. In the cases with diabetes diagnosed only by the FPG criteria,, the Pearson correlation coefficient (r) was 0.726 and the prediction model was expressed as A1C = 2.033 + 0.612 × FPG (R2 = 0.686, p < 0.001) while the change in A1C per the increment of 1.0 mmol/L in FPG was 0.61 %. The correlation between the A1C level and the FPG in participants with diabetes diagnosed only by the A1C criteria, shown in Fig. 1b, is expressed as is A1C = 3.985 + 0.45 × FPG, (R2 = 0.695, p < 0.001). The change in A1C per the increment of 1.0 mmol/L in FPG was 0.45 % in these participants.
Table 1.
Clinical characteristics of the study population (n = 4481)
| Mean ± standard deviation | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 48.6 ± 16.3 | 18 | 90 |
| Sex (women %) | 55.5 | ||
| Waist circumflex (cm) | 81.2 ± 10.1 | 52 | 137 |
| Body mass index (kg/m2) | 23.6 ± 3.4 | 13 | 45 |
| Systolic blood pressure (mmHg) | 118 ± 17 | 79 | 205 |
| Diastolic blood pressure (mmHg) | 76 ± 10 | 40 | 119 |
| FPG (mmol/L) | 5.2 ± 0.8 | 2.8 | 16.2 |
| A1C (%) | 5.6 ± 0.5 | 3.1 | 13.6 |
| Total cholesterol (mmol/L) | 5.0 ± 0.9 | 2.4 | 14.3 |
| Triglyceride (mmol/L) | 3.4 ± 2.7 | 0.2 | 21.9 |
| LDL cholesterol (mmol/L) | 2.9 ± 0.8 | 0.6 | 8.1 |
A1C hemoglobin A1c, FPG fasting plasma glucose, LDL low density lipoprotein
Fig. 1.
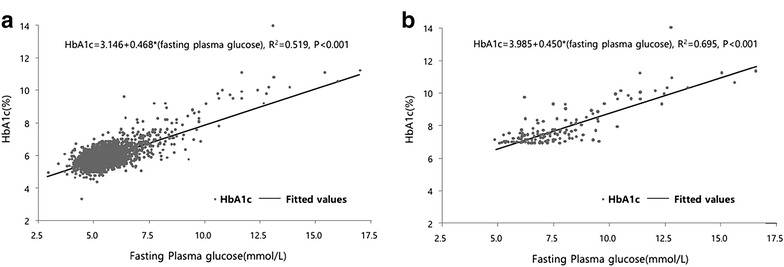
Linear regression analysis of fasting plasma glucose and HbA1c in this study population (a) (n = 4481) and in persons with diabetes by HbA1c criteria (b) (n = 199)
Based on these calculations, the correspondence of A1C to specific FPG were shown in Table 2. We found similar results of the linear regression between all study population and diabetics diagnosed only by the FPG criteria. However, A1C levels in the subjects diagnosed only by FPG criteria are slightly lower than those found in all study population. A FPG of 5.5 mmol/L predicted an A1C of 5.75 % in the study population and of 5.43 % in the subjects diagnosed only by FBS criteria. A FPG of 7 mmol/L also predicted an A1C of 6.42 and 6.32 % respectively. However, in the subjects with diabetes diagnosed only by the A1C criteria, 5.5 and 7 mmol/L predicted 6.49 and 7.14 % in the A1C respectively. From these regression equations, FPG levels for A1C of 6.5 % were calculated at approximately 7.3 mmol/L in diabetics diagnosed by the FPG criteria, and 5.5 mmol/L only by A1C criteria. Mean fasting plasma glucose concentrations for specific A1C groups are summarized in Table 3. The average FPG needed to achieve specified A1C levels of lower than 5, 5.0–5.9, 6.0–6.9, 7.0–7.9, 8.0–8.9, and 9.0–9.9 % and more than 10 % were 4.8 mmol/L with 95 % CI 2.8–6.8, 5.1 mmol/L (3.3–8.9), 6.1 mmol/L (4.0–8.7), 7.7 mmol/L (5.4–10.2), 8.8 mmol/L (7.4–12.1) 11.2 mmol/L (6.1–13.2) and 13.9 mmol/L (11.1–16.2) respectively. These results showed that mean FPG concentrations for specified A1C levels in this study were lower than those in previous western studies [12, 13] (Table 3).
Table 2.
A1C estimated from the regression analysis
| FPG (mmol/L) | Regression estimated A1C (%) | ||
|---|---|---|---|
| Study population | Diabetes by FPG criteria only | Diabetes by A1C criteria only | |
| 5.3 | 5.65 | 5.30 | 6.39 |
| 5.5 | 5.75 | 5.43 | 6.49 |
| 5.6 | 5.77 | 5.47 | 6.51 |
| 6.3 | 6.08 | 5.88 | 6.81 |
| 6.7 | 6.26 | 6.11 | 6.99 |
| 7 | 6.42 | 6.32 | 7.14 |
| 7.2 | 6.50 | 6.42 | 7.21 |
| 7.3 | 6.55 | 6.49 | 7.26 |
A1C hemoglobin A1c, FPG fasting plasma glucose
Table 3.
Mean fasting plasma glucose levels (95 % CI) for specified A1C levels in the ADAG study and KNHANES 2011
| A1C (%) | KNHANES 2011a | ADAG study [12]b | |
|---|---|---|---|
| Mean FPG | Mean fasting glucose | ||
| Mean (mmol/L) ± SD | Range | ||
| <5 | 4.8 ± 0.4 | 2.8–6.8 | |
| 5 | 5.1 ± 0.5 | 3.3–8.9 | |
| 6 | 6.1 ± 0.9 | 4.0–8.7 | |
| 6.0–6.4 | 5.9 ± 0.8 | 6.8a | |
| 6.5–6.9 | 6.8 ± 1.1 | 7.9 | |
| 7 | 7.7 ± 1.1 | 5.4–10.2 | |
| 7.0–7.4 | 7.5 ± 1.0 | 8.4 | |
| 7.5–7.9 | 8.0 ± 1.1 | 9.3 | |
| 8 | 8.8 ± 1.2 | 7.4–12.1 | 9.9c |
| 9 | 11.2 ± 1.3 | 6.1–13.2 | |
| ≥10 | 13.9 ± 1.8 | 11.1–16.2 | |
Data are expressed as mean (±SD)
ADAG A1c-derived average glucose, A1C hemoglobin A1c, FPG fasting plasma glucose, KNHANES Korea National Health and Nutrition Examination Survey
aThe range of specified A1C for this fasting glucose level (6.8 mmol/L) is from 5.5 to 6.49 %
bModified from Wei et al. Diabetes Care 2014;37:1048–1051 [12]. These estimates are based on the SMBG data from 470 of the ADAG study participants (237 with type 1 diabetes and 147 with type 2 diabetes) to determine the average fasting blood glucose for specified HbA1c groups
cThe range of specified A1C for this fasting glucose level (9.9 mmol/L) is from 8.0 to 8.5 %
Discussion
The results of this study supported a close association between A1C and FPG levels for the diagnosis of diabetes for the Korean population, which is in concordance with the recent recommendations [4, 11, 17, 18]. The A1C levels corresponding to a FPG of 5.5 mmol/L were 5.75 % in all study population and 5.43 % in participants with diabetes diagnosed by FPG-based diagnostic criteria only. The A1C levels corresponding to a FPG of 7 mmol/L were also 6.42 and 6.32 % respectively. This suggests that the established FPG criteria for the diagnosis of prediabetes and diabetes is relatively valid in Korean population. However, it is thought that there are several issues in order to adopt these diagnostic criteria for diabetes in Korea.
First, the relationship of A1C and blood glucose with the risk of diabetic retinopathy (DR) should be supported by epidemiological studies in Korean population. The association between chronic glucose concentrations and microvascular complications, particularly DR, has been the basis for the diagnosis of diabetes. The diagnostic A1C cutoff in diabetes (≥6.5 %) is associated with an optimal point for the prevalence of DR, as are the diagnostic values for FPG [8]. However, the KDA has adopted the diagnostic criteria for type 2 diabetes from the ADA recommendation so far because there has been no prospective epidemiological study in Korean population to demonstrate an association between blood glucose level and the development of DR [11]. A recent study provided important evidence that A1C threshold of 6.5 % is a suitable test for the diagnosis of diabetes mellitus in three major Asian ethnic groups, but this study also showed that specific A1C levels had different sensitivity and specificity in these populations [20]. Therefore, A1C should be used thoughtfully and in combination with traditional glucose criteria for screening and diagnosis of diabetes, although the reasons for these differences remain unknown [21]. One Korean community-based cross-sectional study showed that the A1C cutoff of 6.5 % allowed the proper detection of DM and supported the appropriate use of A1C for the diagnosis of diabetes [22]. However, the recent nationwide epidemiological study from the 2011 KNHANES demonstrated that both FPG and A1C levels were closely associated with DR [23]. This study showed that the optimal glycemic and A1C levels for detecting DR were 6.3 mmol/L for FPG and 6.2 % for A1C in Korean population suggesting that current diabetes diagnostic cutoffsshould be lower for Koreans [23]. The results of our study also show that a FPG of 6.3 mmol/L predicted an A1C of approximately 6.1 %, supporting the previous study in Korean population.
Second, the patients’ race/ethnicity may influence A1C [6, 24, 25]. A recent study showed that African Americans have higher A1C levels than non-Hispanic whites after adjustment for fasting glucose levels, but had lower levels of 1,5-anhydroglucitol suggesting that their postprandial glucose levels may be higher [26]. However, there was no report for correlation between FPG and specific A1C levels in Asian population after 2010 ADA indication that A1C should be performed according to the method certified by the National Glycohemo-globin Standardization Program (NGSP) and standardized to the diabetes control and complication trial (DCCT) assay [8, 9]. In the present study, the A1C levels corresponding to a FPG of 5.5 and 7 mmol/L were 6.49 and 7.14 % respectively in Korean population with newly diagnosed diabetes using A1C-based diagnostic criteria only. These findings are inconsistent with those in participants who are diagnosed by FPG-based diagnostic criteria only in this study and are also inconsistent with those in recent recommendations [4, 11, 17, 18]. In fact, it is difficult to directly compare mean FPG levels for specified A1C levels in the A1c-derived average glucose (ADAG) study and our study because the ADAG study was a multicenter observational study of serial FPG for specified A1C levels. However, it is reported that participants with an elevated A1C but “nondiabetic” FPG may have the likelihood of showing higher postprandial glucose levels or elevated glycation rates [4] and the results of our study rather supported these hypothesis in Korean population. Therefore, we need to pay more attention that the diabetic patients who are diagnosed by A1C-based criteria only have lower FPG compared to those who are diagnosed by FPG-based criteria only. It also needs to be noted that the patients diagnosed by A1C-based criteria only can have higher postprandial glucose levels than those who are diagnosed by FPG-based criteria only.
Third, it is important that FPG is accurately measured for the screening or diagnosis of prediabetes and diabetes, although it was reported that it would be inappropriate to use the fasting glucose criteria alone for screening diabetes and the 2-h glucose seems to be a particularly important diagnostic tool in Asian populations [27]. In fact, most Korean people take regular (biennial) medical examinations at the National Health Insurance Corp including fasting glucose measurement [28]. Any suspicious findings on the results can be sufficient to suggest that they need a doctor’s exam or inspections for an accurate diagnosis and any necessary treatments. However, many Korean people think that prediabetes and diabetes are screened as a result of these examinations even though health screening is not for diagnosis, and they are not worried about these hyperglycemic states if normal or slightly elevated serum glucose levels are measured. Furthermore, many laboratories including the National Health Examination measure serum glucose even though guidelines rather recommend measurement of plasma because large-scale medical checkups require a simple and cost-effective sample collection method [6]. Our previous hospital-based report using data from 2028 persons for the evaluation of metabolic abnormalities including impaired fasting glucose or dyslipidemia suspected through health examinations in the National Health Insurance Corp. or by personal medical checkup showed that 22.1 % had newly diagnosed diabetes according to FPG. Even, among participants with normoglycemia according to serum glucose, 14.2 % had newly diagnosed diabetes according to FPG diagnostic criteria [29]. It is thought that these findings may be one of the important reasons why the diagnosis of diabetes, which is the fifth-leading cause of death in Korea, could be underestimated.
Our study had several limitations. First, this study was based on a cross-sectional analysis. Second, participants self-reported whether they were taking any glucose-lowering medications and from this, recall bias may exist. Third, because we did not have the results of 75 g oral glucose tolerance test (OGTT), the diagnosis of diabetes among our participants may be underestimated and an association between A1C and 2 h postprandial glucose levels in Korean population with newly diagnosed diabetes by only A1C-based diagnostic criteria was not assessed. Fourth, we excluded participants with anemia but not hemoglobinopathies. This, however, does not seem to be an issue as the prevalence of hemoglobinopathy is very low in Korea [30, 31]. Finally, some clinical conditions that could influence the interpretation of A1C (such as inflammation or change of diet) were not included in this study. Despite these limitations, present study, to our knowledge, is the first epidemiological report showing the correlation between FPG concentrations and A1C levels after using A1C for the diagnosis of diabetes, and the association between the mean FPG concentrations and specific A1C levels. However, this study also shows a great difference in the correlation between FPG and A1C values in the two groups diagnosed only by FPG and only by A1C criteria respectively. These results require further study to compare the characteristics of individuals who are newly diagnosed by either only A1C- or FPG-based criteria. Large-scale longitudinal study should be made to investigate the association between levels of A1C and FPG with incident diabetic retinopathy and to determine the optimal values of A1C and FPG for detecting incident diabetic retinopathy in the Korean population.
Conclusions
Our study showed that a close association between A1C and FPG levels is in concordance with the previous criteria for diagnosis of diabetes. However, the average FPG concentrations to achieve targeted A1C in the subjects with diabetes diagnosed only by A1C-based criteria may be lower than those in western populations. Therefore, we need to pay attention that patients diagnosed by A1C-based criteria only can have higher postprandial glucose levels than those who are diagnosed by FPG-based criteria only, in real-life practice.
Authors' contributions
JGK, CBL, and SHI conceived and designed this study: SMH and JGK analyzed and interpreted the data: SMH and JGK wrote the manuscript: SMH, JGK, CSK, SJL, CBL and SHI contributed to the discussion and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- ADA
American Diabetes Association
- ADAG
A1c-derived average glucose
- DCCT
diabetes control and complication trial
- DR
diabetic retinopathy
- eGFR
estimated glomerular filtration ratio
- FPG
fasting plasma glucose
- A1C
hemoglobin A1c
- KCDC
Korean Centers for Disease Control and Prevention
- kDa
Korean Diabetes Association
- KNHANES
Korea National Health and Nutrition Examination Survey
- MDRD
modification of diet in renal disease
- NGSP
National Glycohemoglobin Standardization Program
- OGTT
oral glucose tolerance test
Contributor Information
Sangmo Hong, Email: lanugo35@gmail.com.
Jun Goo Kang, Phone: +82-31-380-3700, Email: kjg0804@empal.com.
Chul Sik Kim, Email: ironeat@gmail.com.
Seong Jin Lee, Email: leesj@hallym.ac.kr.
Chang Beom Lee, Email: lekang@hanyang.ac.kr.
Sung-Hee Ihm, Email: ihmsh@hallym.ac.kr.
References
- 1.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Yu SH, Kang JG, Hwang YC, Ahn KJ, Yoo HJ, Ahn HY, et al. Increasing achievement of the target goals for glycemic, blood pressure and lipid control for adults with diagnosed diabetes in Korea. J Diabetes Investig. 2013;4:460–465. doi: 10.1111/jdi.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IeB Park, Kim J, Kim DJ, Chung CH, Oh JY, Park SW, et al. Task Force Team for basic statistical study of Korean Diabetes Mellitus of Korean Diabetes Association, Diabetes epidemics in Korea: reappraise nationwide survey of diabetes “diabetes in Korea 2007”. Diabetes Metab J. 2013;37:233–239. doi: 10.4093/dmj.2013.37.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes A Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 5.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. National Academy of Clinical Biochemistry. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34:1419–1423. doi: 10.2337/dc11-9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34:518–523. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen DL, Witte DR, Kaduka L, Jørgensen ME, Borch-Johnsen K, et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care. 2010;33:580–582. doi: 10.2337/dc09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association Standards of medical care in diabetes-2010. Diabetes Care. 2010;33:S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J, Kwon KC, Kim JH, Kim JW, Min WK, Lee SY, et al. Annual report on external quality assessment in metabolic disorders in Korea (2008) J Lab Med Qual Assur. 2009;31:143–159. [Google Scholar]
- 11.Ko SH, Kim SR, Kim DJ, Oh SJ, Lee HJ, Shim KH, et al. Clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J. 2011;35:431–436. doi: 10.4093/dmj.2011.35.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei N, Zheng H, Nathan DM. Empirically establishing blood glucose targets to achieve HbA1c goals. Diabetes Care. 2014;37:1048–1051. doi: 10.2337/dc13-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AmericanDiabetes A. (6) Glycemic targets. Diabetes Care. 2015;38:S33–S40. doi: 10.2337/dc15-S009. [DOI] [PubMed] [Google Scholar]
- 14.Kim KS, Kim SK, Lee YK, Park SW, Cho YW. Diagnostic value of glycated haemoglobin HbA(1c) for the early detection of diabetes in high-risk subjects. Diabet Med. 2008;25:997–1000. doi: 10.1111/j.1464-5491.2008.02489.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim HK, Bae SJ, Choe J. Impact of HbA1c criterion on the detection of subjects with increased risk for diabetes among health check-up recipients in Korea. Diabetes Metab J. 2012;36:151–156. doi: 10.4093/dmj.2012.36.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, et al. Optimal hemoglobin A1C cutoff value for diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013;99:231–236. doi: 10.1016/j.diabres.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia Report of a WHO/IDF Consultation. https://www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf.
- 18.World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus abbreviated report of a WHO Consultation. http://www.who.int/diabetes/publications/report-hba1c_2011.pdf. [PubMed]
- 19.Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES) Int J Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabanayagam C, Khoo EY, Lye WK, Ikram MK, Lamoureux EL, Cheng CY, et al. Diagnosis of diabetes mellitus using HbA1c in Asians: relationship between HbA1c and retinopathy in a multiethnic Asian population. J Clin Endocrinol Metab. 2015;100:689–696. doi: 10.1210/jc.2014-2498. [DOI] [PubMed] [Google Scholar]
- 21.Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97:1067–1072. doi: 10.1210/jc.2011-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho NH, Kim TH, Woo SJ, Park KH, Lim S, Cho YM, et al. Optimal HbA1c cutoff for detecting diabetic retinopathy. Acta Diabetol. 2013;50:837–842. doi: 10.1007/s00592-013-0452-3. [DOI] [PubMed] [Google Scholar]
- 23.Park YM, Ko SH, Lee JM, Kim DJ, Kim DJ, Han K, et al. Committee of Clinical Practice Guideline, Korean Diabetes Association. Glycaemic and haemoglobin A1c thresholds for detecting diabetic retinopathy: the fifth Korea National Health and Nutrition Examination Survey (2011) Diabetes Res Clin Pract. 2014;104:435–442. doi: 10.1016/j.diabres.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152:770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur JS, et al. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: a community-based study. J Clin Endocrinol Metab. 2010;95:2832–2835. doi: 10.1210/jc.2009-2433. [DOI] [PubMed] [Google Scholar]
- 26.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med. 2011;154:303–309. doi: 10.7326/0003-4819-154-5-201103010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y. Tajima N; International Diabetes Epidemiology Group; DECODA Study Group. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia. 2000;43:1470–1475. doi: 10.1007/s001250051557. [DOI] [PubMed] [Google Scholar]
- 28.Jee SH, Suh I, Kim IS, Appel LJ. Smoking and atherosclerotic cardiovascular disease in men with low levels of serum cholesterol: the Korea Medical Insurance Corporation Study. JAMA. 1999;282:2149–2155. doi: 10.1001/jama.282.22.2149. [DOI] [PubMed] [Google Scholar]
- 29.Kang JG, Kim CS, Lee SJ, Ihm S-H, Choi MG, Yoo H-J, et al. Usual delay in sample processing can underestimate detection of prediabetes and diabetes in Korea. EASD 2010. http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=b715101f-a633-485d-a14d-b5f4bb1ce4f5&cKey=7009b655-6504-4dd7-98b1-6ba8dd3b4c8f&mKey=10a86782-07e4-4a2d-9100-f660e5d752a9.
- 30.Cho HS, Hah JO, Kang IJ, Kang HJ, Kwak JY, Koo HH, et al. Hereditary Hemolytic Anemia in Korea: a Retrospective Study from 1997 to 2006. Korean J Hematol. 2007;42:197–205. doi: 10.5045/kjh.2007.42.3.197. [DOI] [Google Scholar]
- 31.Shin JS, Lee MJ, Jung HJ, Park JE. A Clinical Study on Hereditary Hemoglobinopathy: a Single Institute Experience. Clin Pediatr Hematol Oncol. 2014;21:9–15. doi: 10.15264/cpho.2014.21.1.9. [DOI] [Google Scholar]


