The ClC-K2 chloride channel has long been known to robustly express in the basolateral plasma membranes of epithelial cells lining the thick ascending limb, distal convoluted tubule, and distal nephron (including the collecting duct system; Stauber et al., 2012; Jentsch, 2015). Our understanding of the function of this channel in the thick ascending limb and distal convoluted tubule is known to a degree of certainty; however, the function of this channel in the distal nephron has been more obscure. In this issue, Pinelli et al. provide compelling evidence that ClC-K2 Cl− channels in the basolateral membrane of β intercalated cells function as key regulators of NaCl reabsorption and Cl−/HCO3− exchange in the distal nephron. This is important because the distal nephron is the final segment of the nephron to have an effect on urine, and thus, it fine-tunes the electrolyte, pH, and water balance of urine excreted from the body.
Pinelli et al. (2016) perform detailed biophysical analysis of the effects of voltage, extra- and intracellular pH, and intracellular Cl− and extracellular Ca2+ concentrations on native ClC-K2 channels expressed in murine connecting tubules. The results of this study show that extra- and intracellular pH synergistically modulate the voltage dependence of ClC-K2. By increasing basolateral Cl− conductance and, as a consequence, transcellular pendrin (Slc26a4)-dependent absorptive Cl− flux, activation of ClC-K2 by alkaline pH is proposed to decrease Na+ reabsorption by β intercalated cells, thus maximizing Cl−/HCO3− exchange during metabolic alkalosis. Such regulation of ClC-K2 provides a mechanism whereby β intercalated cells can switch from primarily reabsorbing urinary Na+ with Cl− (during times of low urinary Na+ and little pressure for excretion of base) to exchanging luminal Cl− with HCO3− in the absence of Na+ reabsorption (during a need for base excretion). Such “switching” from NaCl reabsorbing to HCO3− secreting enables β intercalated cells to contribute to the kidneys’ ability to appropriately modulate acid–base balance.
The ClC proteins are members of an ancient family of Cl− transport proteins that are widely expressed in every animal and most, if not all, bacteria (Stauber et al., 2012; Jentsch, 2015). These proteins serve diverse functions in both prokaryotes and eukaryotes. The ClC family is large, containing both electrogenic transporters, antiporters moving 2Cl− and one H+ in opposite directions in a secondary active manner, and bona fide ion channels that selectively conduct anions (Cl−, given the composition of physiological solutions) through a gated pore. The family is commonly divided into two branches, transporters and channels, with antiporters expressed primarily in the older bacteria branch. The junior branch contains proteins that have lost their need for H+ to move Cl− in a coupled manner such that anions move through the ClC channel pore via restrictive diffusion.
ClC channels are unique for ion channel proteins in the sense that they contain two distinct protopores, conduction pathways, for Cl−. This has led to them being referred to as “double-barreled” channels. Permeation through these two parallel protopores is independently controlled by protopore gates, with the holochannel also containing a common gate that modulates permeation through both protopores uniformly (Miller, 2015). Supporting the theory that the ligand binding sites and route used by ClC antiporters to translocate Cl− across the plasma membrane evolved into the conduction pathway of ClC ion channels is the observation that the line separating rudimentary Cl− conductance from translocation in ClC transporters is thin, involving replacement of only two amino acids (Jayaram et al., 2008).
The hallmark pH dependence of ClC ion channel gating in the junior branch also seems likely to be a vestigial remnant of antiporters that moved 2Cl− with one H+. As highlighted in the current study by Pinelli et al. (2016), while possibly vestigial, this sensitivity to protons has important physiological consequences when considering modulation of ClC channels by pH, particularly those expressed in the distal nephron. The fact that alkaline pH in the physiological range activates ClC-K2 channels enables β intercalated cells to switch from reabsorbing NaCl to secreting HCO3−.
Thomas Jentsch’s laboratory in 1990 was the first to clone the double-barreled ClC channel (Jentsch et al., 1990). They expression cloned ClC-0 from the electric organ of the Torpedo ray. The same laboratory cloned the first mammalian homologue, ClC-1, a year later from murine skeletal muscle (Steinmeyer et al., 1991). We now know that mammals express nine CLCN genes encoding ClC channels (Stauber et al., 2012; Jentsch, 2015). Some of these are ubiquitously expressed, whereas others are expressed in a highly tissue-specific manner. For instance, ClC-1 encoded by CLCN1 is expressed primarily in muscle, and mutation of this gene thus causes muscle-specific diseases such as myotonia.
Mammalian ClC-K channels, which encompass the distinct, but very similar, ClC-K1 and ClC-K2 channels encoded by ClCNKA and ClCNKB, respectively, are primarily expressed in epithelial cells, in particular kidney epithelial cells that are involved in ion transport (reviewed in Stockand [2013] and Zaika et al. [2016]). ClC-K1 and ClC-K2 were originally cloned from both human and rat kidney (Uchida et al., 1993; Kieferle et al., 1994). The “K” in ClC-K represents the fact that these channels are expressed predominantly in the kidneys. However, both channels also can be found in neuroepithelial cells in the inner ear where they function as key components of the basolateral Cl− conductance that participates in K+ secretion into the endolymph. Because of their renal expression, mutation of ClC-K1 and ClC-K2 causes kidney disease associated with electrolyte and acid–base imbalance. For clarity, it is noted that the ClC-K2 orthologue in humans is referred to as ClC-Kb.
ClC-K1 is expressed in the kidney, primarily in the apical and basolateral membranes of epithelial cells lining the ascending thin limb and in the basolateral membrane of epithelial cells lining the thick ascending limb of the loop of Henle. In the ascending thin limb, ClC-K1 activity contributes to the generation and maintenance of the hypertonic medulla and countercurrent mechanism (Matsumura et al., 1999). In the thick ascending limb, ClC-K1, in compliment with ClC-K2, serves as a basolateral exit pathway for transcellular Cl− flux (Krämer et al., 2008). This allows NaCl reabsorption across the thick ascending limb and ultimately the concentration of urine.
Despite close structural and functional similarity with ClC-K1, ClC-K2 has a distinct expression profile in the kidneys, with only modest overlap with that of ClC-K1. ClC-K2 is expressed in the basolateral membranes of all epithelial cells in the thick ascending limb and distal convoluted tubule and in the basolateral membranes of intercalated cells in the connecting tubule and collecting duct system (Matsumura et al., 1999; Vitzthum et al., 2002). The function of ClC-K2 in these cells as a basolateral exit pathway for Cl− flux is conserved (reviewed in Zaika et al. [2016]).
Barttin is an obligatory accessory subunit, encoded by BSND, which is required for activity by both ClC-K1 and ClC-K2 and which is coexpressed with these pore-forming subunits (Estévez et al., 2001). Because of their overlapping, but differential, expression profiles, mutation of ClC-K1 and ClC-K2 results in different tubulopathies. For instance, inactivation of ClC-K2 results in classic (type III) Bartter’s syndrome, caused by disruption of NaCl reabsorption in the thick ascending limb (Hebert, 2003; Krämer et al., 2008). In contrast, inactivation of ClC-K1 results in a phenotype more resembling diabetes insipidus (Matsumura et al., 1999). Loss of function of Barttin also results in a severe form of Bartter’s syndrome (type IV), but this disease presents with accompanying sensorineural deafness caused by complete loss of all ClC-K channel activity (Estévez et al., 2001; Krämer et al., 2008). Because the expression of ClC-K1 and ClC-K2 completely overlaps in epithelia of the inner ear, loss of function of either pore-forming protein can be compensated, but inactivation of the obligatory accessory subunit cannot and results in deafness.
In their study of ClC-K2 in the murine connecting tubule, Pinelli et al. (2016) describe a 10-pS Cl− channel located in the basolateral membrane that is activated by alkaline pH, increases in external Ca2+ concentration, and membrane depolarization. These are hallmark properties of ClC-K2 and are consistent with a basolateral channel identified previously in the distal nephron by the Teulon and Pochynyuk laboratories (Nissant et al., 2006; Zaika et al., 2015). Hennings et al. (2016) very recently used ClC-K2 knockout mice to demonstrate definitively that this basolateral 10-pS Cl− channel is indeed ClC-K2.
The primary importance of the current study by Pinelli et al. (2016) is their finding that alkaline pH shifts the voltage dependence of activation of the native connecting tubule ClC-K2 channel toward more hyperpolarizing potentials. These results can be found in Figs. 4 and 5 of their paper. Because of this leftward shift in voltage dependence, ClC-K2 activity is elevated in the presence of alkaline pH at physiological resting membrane potentials (approximately −15 mV; Muto et al., 1990) across the basolateral membrane of intercalated cells. Thus, an increase in basolateral Cl− permeability sets ideal conditions for transcellular Cl− flux from the lumen to the interstitial fluid in exchange for the movement of HCO3− in the reverse direction. As depicted in their Fig. 8, the results from the study by Pinelli et al. (2016) are consistent with ClC-K2 serving this function when serum pH is in the alkaline range and necessitates base secretion from β cells.
The likelihood that activation of ClC-K2 in the distal nephron by alkaline pH facilitates base secretion by β intercalated cells, as argued by Pinelli et al. (2016), raises an interesting possibility. Bartter’s syndrome is a tubulopathy that is characterized by urinary salt wasting, hypokalemia, metabolic alkalosis, low blood pressure, resistance to loop diuretics, and secondary compensatory hyperaldosteronism (Kleta and Bockenhauer, 2006; Seyberth, 2008). Loss of function mutations in NKCC2 (SLC12A2; type I), ROMK (KCNJ1; type II), ClC-K2 (type III), and Barttin (type IV) cause autosomal recessive Bartter’s syndrome, whereas gain of function mutations of the Ca2+ receptor (type V; CASR) cause autosomal-dominant Bartter’s syndrome. The pathology of Bartter’s syndrome is understood as follows: The primary insult is disruption of NaCl reabsorption at the thick ascending limb. Inactivating mutations in ClC-K2 and Barttin result in Bartter’s syndrome because they disrupt the basolateral exit pathway for Cl− in thick ascending limb epithelial cells, resulting in compromised NaCl reabsorption. Compromised NaCl reabsorption in the thick ascending limb results in loss of the ability to concentrate urine because the hypertonic medulla is lost. This manifests as renal salt wasting, lower blood pressure, secondary hyperaldosteronism, and resistance to loop diuretics. Also, the resulting increase in NaCl-rich luminal fluid delivery to the distal nephron in the face of compromised reabsorption in the thick ascending limb favors an attempt by the distal nephron to compensate via increases in Na+ reabsorption by principal cells. The distal nephron, though, is incapable of adequately compensating because of its more limited capacity for absorption. Across principal cells, Na+ is reabsorbed in exchange for K+ secretion. Thus, the futile attempt of the distal nephron to compensate for disrupted absorption at the thick ascending limb leads to avid K+ secretion. This results in the hypokalemia of Bartter’s syndrome and is further exacerbated by the hyperaldosteronism of the condition. Avid K+ secretion from principal cells is thought to promote K+ reabsorption and complementary acid secretion from α intercalated cells via H+/K+ exchange in addition to an increase in acid secretion from these cells via the V-ATPase activated by secondary hyperaldosteronism. As shown in Fig. 1, it is this acid secretion that is often used to explain the metabolic alkalosis observed in Bartter’s syndrome.
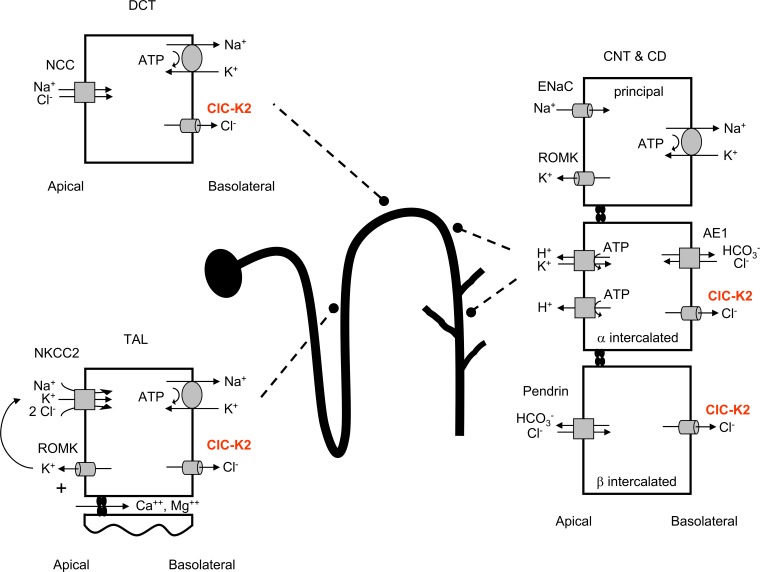
Figure 1.
The metabolic alkalosis of Bartter’s syndrome can arise from an increase in acid secretion from α intercalated cells and a decrease in base secretion from β intercalated cells. Depiction of the renal nephron showing the cells and transporters involved in the renal salt wasting and alkalosis of Bartter’s syndrome. CD, collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; NCC, sodium chloride cotransporter; ROMK, renal outer medullary potassium channel; TAL, thick ascending limb.
The current work of Pinelli et al. (2016), as shown in Fig. 1, offers a complementary mechanism to explain the metabolic alkalosis observed in Bartter’s syndrome caused by dysfunction of ClC-K2 and Barttin. Alkalinization of serum during Bartter’s syndrome associated with ClC-K2 and Barttin dysfunction may also result from a compromised ability of β intercalated cells in the distal nephron to secrete base. Although it is possible that both an increase in acid secretion from α intercalated cells and a decrease in base secretion from β intercalated cells synergistically contribute to the alkalosis apparent in Bartter’s syndrome caused by ClC-K2 and Barttin dysfunction, it is likely that loss of the ability to secrete base is the primary cause.
The rationale for this is that the ability of α intercalated cells to secrete acid is also dependent on the function of ClC-K2 channels in the basolateral membrane (see Fig. 1). Loss of ClC-K2 activity in the basolateral membrane of α intercalated cells disrupts a required Cl− recycling mechanism necessary for HCO3−/Cl− exchange across this membrane. Loss of basolateral HCO3−/Cl− exchange diminishes acid secretion across the apical membrane of α intercalated cells because an increase in cellular HCO3− slows the dissociation of cellular carbonic acid into bicarbonate and hydrogen. Thus, in the absence of basolateral ClC-K2 channel function, there is a diminished gradient for hydrogen secretion across the apical membrane of α intercalated cells, making it unlikely that hyperactive acid secretion is causative for the metabolic alkalosis apparent in Bartter’s syndrome resulting from ClC-K2 and Barttin dysfunction.
This study by Pinelli et al. (2016) thus extends our understanding of acid–base regulation by the kidney and, importantly, provides a cellular mechanism explaining the alkalosis apparent in Bartter’s syndrome type III and IV. The etiology of alkalosis in these forms (type III and IV) of Bartter’s syndrome—decreased base secretion from β intercalated cells—thus is likely to be fundamentally different to that in other forms (types I, II, and V) of Bartter’s syndrome, which result from an increase in acid secretion from α intercalated cells.
ACKNOWLEDGMENTS
The authors declare no competing financial interests.
Merritt Maduke served as editor.
References
- Estévez R., Boettger T., Stein V., Birkenhäger R., Otto E., Hildebrandt F., and Jentsch T.J.. 2001. Barttin is a Cl− channel β-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature. 414:558–561. 10.1038/35107099 [DOI] [PubMed] [Google Scholar]
- Hebert S.C. 2003. Bartter syndrome. Curr. Opin. Nephrol. Hypertens. 12:527–532. 10.1097/00041552-200309000-00008 [DOI] [PubMed] [Google Scholar]
- Hennings J.C., Andrini O., Picard N., Paulais M., Huebner A.K., Cayuqueo I.K., Bignon Y., Keck M., Cornière N., Böhm D., et al. . 2016. The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J. Am. Soc. Nephrol. 10.1681/ASN.2016010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H., Accardi A., Wu F., Williams C., and Miller C.. 2008. Ion permeation through a Cl−-selective channel designed from a CLC Cl−/H+ exchanger. Proc. Natl. Acad. Sci. USA. 105:11194–11199. 10.1073/pnas.0804503105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J. 2015. Discovery of CLC transport proteins: cloning, structure, function and pathophysiology. J. Physiol. 593:4091–4109. 10.1113/JP270043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch T.J., Steinmeyer K., and Schwarz G.. 1990. Primary structure of Torpedo marmorata chloride channel isolated by expression cloning in Xenopus oocytes. Nature. 348:510–514. 10.1038/348510a0 [DOI] [PubMed] [Google Scholar]
- Kieferle S., Fong P., Bens M., Vandewalle A., and Jentsch T.J.. 1994. Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc. Natl. Acad. Sci. USA. 91:6943–6947. 10.1073/pnas.91.15.6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleta R., and Bockenhauer D.. 2006. Bartter syndromes and other salt-losing tubulopathies. Nephron, Physiol. 104:p73–p80. 10.1159/000094001 [DOI] [PubMed] [Google Scholar]
- Krämer B.K., Bergler T., Stoelcker B., and Waldegger S.. 2008. Mechanisms of disease: the kidney-specific chloride channels ClCKA and ClCKB, the Barttin subunit, and their clinical relevance. Nat. Clin. Pract. Nephrol. 4:38–46. 10.1038/ncpneph0689 [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Uchida S., Kondo Y., Miyazaki H., Ko S.B., Hayama A., Morimoto T., Liu W., Arisawa M., Sasaki S., and Marumo F.. 1999. Overt nephrogenic diabetes insipidus in mice lacking the CLC-K1 chloride channel. Nat. Genet. 21:95–98. 10.1038/5036 [DOI] [PubMed] [Google Scholar]
- Miller C. 2015. In the beginning: a personal reminiscence on the origin and legacy of ClC-0, the ‘Torpedo Cl− channel’. J. Physiol. 593:4085–4090. 10.1113/jphysiol.2014.286260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto S., Yasoshima K., Yoshitomi K., Imai M., and Asano Y.. 1990. Electrophysiological identification of alpha- and beta-intercalated cells and their distribution along the rabbit distal nephron segments. J. Clin. Invest. 86:1829–1839. 10.1172/JCI114913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissant A., Paulais M., Lachheb S., Lourdel S., and Teulon J.. 2006. Similar chloride channels in the connecting tubule and cortical collecting duct of the mouse kidney. Am. J. Physiol. Renal Physiol. 290:F1421–F1429. 10.1152/ajprenal.00274.2005 [DOI] [PubMed] [Google Scholar]
- Pinelli L., Nissant A., Edwards A., Lourdel S., Teulon J., and Paulais M.. 2016. Dual regulation of the native ClC-K2 chloride channel in the distal nephron by voltage and pH. J. Gen. Physiol. 148 10.1085/jgp.201611623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyberth H.W. 2008. An improved terminology and classification of Bartter-like syndromes. Nat. Clin. Pract. Nephrol. 4:560–567. 10.1038/ncpneph0912 [DOI] [PubMed] [Google Scholar]
- Stauber T., Weinert S., and Jentsch T.J.. 2012. Cell biology and physiology of CLC chloride channels and transporters. Compr. Physiol. 2:1701–1744. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Ortland C., and Jentsch T.J.. 1991. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature. 354:301–304. 10.1038/354301a0 [DOI] [PubMed] [Google Scholar]
- Stockand J.D. 2013. Renal ion channels, electrophysiology of transport, and channelopathies. In Seldin and Giebisch’s The Kidney Physiology and Pathophysiology. Fifth edition Alpern R.J., Caplan M.J., and Moe O.W., editors. Elsevier Inc., Waltham, MA: 217–262. 10.1016/B978-0-12-381462-3.00008-2 [DOI] [Google Scholar]
- Uchida S., Sasaki S., Furukawa T., Hiraoka M., Imai T., Hirata Y., and Marumo F.. 1993. Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in kidney medulla. J. Biol. Chem. 268:3821–3824. [PubMed] [Google Scholar]
- Vitzthum H., Castrop H., Meier-Meitinger M., Riegger G.A., Kurtz A., Krämer B.K., and Wolf K.. 2002. Nephron specific regulation of chloride channel CLC-K2 mRNA in the rat. Kidney Int. 61:547–554. 10.1046/j.1523-1755.2002.00165.x [DOI] [PubMed] [Google Scholar]
- Zaika O., Mamenko M., Boukelmoune N., and Pochynyuk O.. 2015. IGF-1 and insulin exert opposite actions on ClC-K2 activity in the cortical collecting ducts. Am. J. Physiol. Renal Physiol. 308:F39–F48. 10.1152/ajprenal.00545.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O., Tomilin V., Mamenko M., Bhalla V., and Pochynyuk O.. 2016. New perspective of ClC-Kb/2 Cl− channel physiology in the distal renal tubule. Am. J. Physiol. Renal Physiol. 310:F923–F930. 10.1152/ajprenal.00577.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]