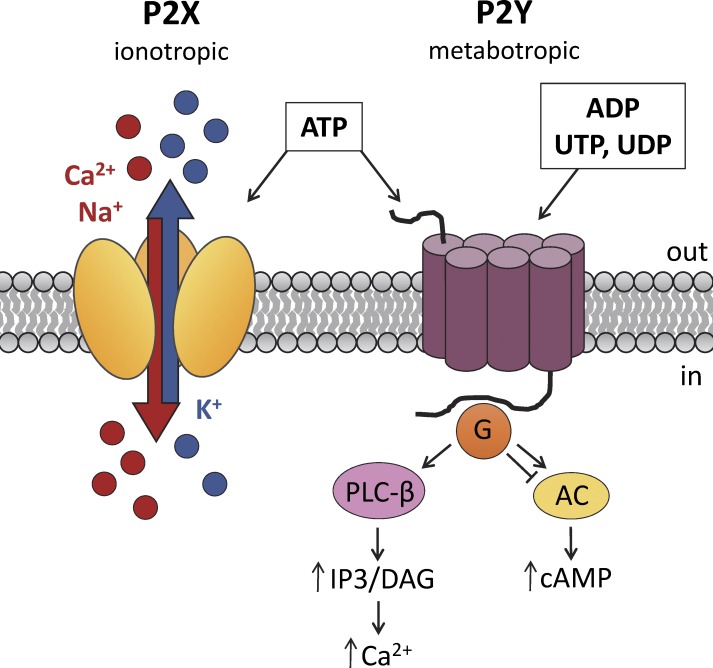
Key physiological functions of organisms, such as sensory transduction, regulation of heart rate, smooth muscle contraction, bile secretion, endocrine regulation, immune responses, and various pathophysiological conditions, including neuropathic pain, diabetes, kidney failure, and cancer, are regulated by purinergic signaling (Burnstock, 2013). The pathway begins with extracellular purine nucleotides, such as ATP and UTP, or nucleosides, such as adenosine, binding to their cognate purinergic receptors in the plasma membrane (Burnstock, 1972). Purinergic receptors are divided into two types: ligand-gated ion channels (P2X) and G protein–coupled receptors (P2Y and P1). Whereas P1 is activated specifically by adenosine, P2Y receptors are activated by a wide range of nucleotides, including ATP, ADP, and UTP, among others. P2X receptors are specifically activated by ATP and are further divided into seven different subtypes: P2X1 to P2X7. P2X receptors form homomeric or heteromeric trimers (Jiang et al., 2003; Kawate et al., 2009), thus resulting in nonselective ATP-gated ion channels that are permeable to Ca2+, Na+, and K+ (Fig. 1). An important role for P2X receptors in male fertility has been proposed after the detection of several members of the P2X family in mammalian spermatozoa (Banks et al., 2010). In the well-executed study reported in this issue, Fleck et al. perform electrophysiological profiling of murine spermatogonia and describe functional expression of two types of purinergic receptors, P2X4 and P2X7, as well as large-conductance Ca2+-activated K+ (BK, or “Big Potassium”) channels, in immature male germ cells.
Figure 1.
Purinergic signaling. P2X receptors are trimeric ion channels in which each subunit consists of two membrane-spanning domains. Binding of ATP to P2X channels causes a conformational change that opens the pore and allows Ca2+ and/or Na+ to enter and K+ to leave the cell (Hattori and Gouaux, 2012). P2Y receptors consist of seven transmembrane domains and are G protein–coupled receptors. Binding of ATP (or ADP, UTP, or UDP) to P2Y receptors activates different G protein signaling pathways, depending on the specific G protein associated with the receptor (P2Y1,2,4,6,11 with Gq/G11, P2Y11 with Gs, and P2Y12,13,14 with Gi/G0; Burnstock, 2012).
ATP is known to mediate an increase in intracellular calcium concentration in sperm and also to stimulate the acrosome reaction, in which enzymes stored in the anterior cap-like structure of the sperm head are released, allowing sperm to penetrate the egg (Luria et al., 2002). The P2X2 receptor was recently characterized in mouse sperm (Navarro et al., 2011). P2rx2-deficient mice are fertile and have normal sperm morphology; however, their fertility declines over days with frequent mating, suggesting that the P2X2 receptor can provide a selection advantage under certain conditions. P2X receptors are also involved in sperm transport through vas deferens: double deletion of P2X1 and α1A-adrenergic G protein–coupled receptors in male mice led to a complete infertility caused by inhibition of sperm transport (White et al., 2013). P2X receptors have also been reported to be expressed in mammalian testes (Glass et al., 2001); however, their physiological importance for spermatogenesis and sperm maturation attracted attention only recently.
In this issue, Fleck et al. (2016) perform a comprehensive evaluation of the physiological function of P2X4 and P2X7 receptors, as well as BK channels, in mammalian spermatogonial cells, the undifferentiated germ cells that produce spermatozoa. This work reveals new insights into autocrine/paracrine regulation of mammalian spermatogenesis. The authors suggest that spermatogonial purinergic signaling might be required for synchronized sperm development and release into the epididymis (Fleck et al., 2016). The important determinant of male fertility is sperm count; therefore, testes are required to produce a steady number of spermatozoa to provide continuous sperm transport through the male reproductive tract. The testis is an immune-privileged organ in which Sertoli cells prevent spermatogonia and developing sperm cells from direct contact with the blood. Thus, spermatogonia lack the ability to coordinate their division through direct communication via the blood stream. Paracrine signaling, using ATP, could therefore be a useful alternative mechanism to solve this communication problem and ensure coordinated and synchronized sperm development and release. ATP could be released by either spermatogonia or adjacent Sertoli cells, resulting in effective intercellular communication. The current work by Fleck et al. (2016) raises an interesting question about whether synchronized sperm maturation is under the control of purinergic paracrine/autocrine signaling in adult testes.
Testicular purinergic signaling
Male germ cell differentiation, i.e., spermatogenesis, takes place in the seminiferous tubules of the testis. Before puberty, the seminiferous tubules consist of a homogenous group of undifferentiated germ cells, type A spermatogonia. With the onset of puberty, and in response to surging levels of pituitary and male steroid hormones, mitotic division of spermatogonia commences and gives rise to round-shaped primary spermatocytes that undergo meiosis to form haploid round spermatids. The spermatids differentiate further to develop the typical shape of a spermatozoon with a long flagellum and a head containing densely packed DNA (Paniagua and Nistal, 1984; de Kretser et al., 1998; Neto et al., 2016).
The interstitial compartment surrounding the seminiferous tubules contains Leydig cells, which are responsible for the production of testosterone and other androgens involved in spermatogenesis. Steroidogenesis in Leydig cells is initiated through binding of luteinizing hormone (LH) to receptors in the cell membrane, but the process is further regulated by several factors, including gonadotropin-releasing hormone (GnRH), growth factors, cytokines, and ATP (Saez, 1994; Foresta et al., 1996). Purinergic signaling causes an increased influx of Ca2+ from the extracellular environment as well as a release of Ca2+ from intracellular stores (Pérez-Armendariz et al., 1996), which in turn leads to increased secretion of testosterone (Foresta et al., 1996). Several subunits of P2X receptors have been detected in Leydig cells, including P2X2, P2X4, P2X6, and P2X7 (Poletto Chaves et al., 2006; Antonio et al., 2009). Further electrophysiological experiments have suggested that the observed Ca2+ influx after ATP application to Leydig cells stems from opening of heteromeric P2X2/4/6 channels, whose properties are most similar to P2X2 (Antonio et al., 2009).
Androgens exert their effect on spermatogenesis by binding to receptors in Sertoli cells. These somatic cells lie in close proximity to the germ cells and provide them with the nutrients and growth factors needed for proper development (Wang et al., 2009). Furthermore, Sertoli cells secrete a fluid that makes up the tubular environment and transports the differentiated spermatozoa from the seminiferous tubules to the epididymis (Rato et al., 2010). Purinergic signaling regulates Sertoli cell fluid secretion as well as enzyme activity, secretion of estradiol, and the cell’s response to follicle-stimulating hormone (FSH; Rudge et al., 1995; Ko et al., 1998; Meroni et al., 1998; Rossato et al., 2001; Gelain et al., 2005). This effect is elicited by both metabotropic P2Y and ionotropic P2X receptors (Filippini et al., 1994; Foresta et al., 1995; Rudge et al., 1995). Extracellular ATP triggers an influx of Na+, followed by depolarization and increased intracellular Ca2+ levels in cultured Sertoli cells (Foresta et al., 1995; Rossato et al., 2001). However, further characterization of the receptors responsible for ATP signaling is needed because different studies have shown diverse expression patterns and activity of P2X receptors. These disparate results were suggested to be caused by altered expression of P2X subunits in Sertoli cells during the different developmental stages of sperm cells (Ko et al., 2003). For example, P2X2 and P2X3 receptors were only found at certain developmental stages, whereas P2X7 was expressed in all Sertoli cells of the seminiferous tubules of rats (Glass et al., 2001). This could also explain the observed differences in studies of cultured Sertoli cells.
The role of ionotropic P2X receptors in mammalian sperm development
Spermatogenesis is a tightly regulated process subject to ongoing influence by a myriad of paracrine and endocrine stimuli. As reported in this issue (Fleck et al., 2016), functional expression of P2X4 and P2X7 receptors in murine spermatogonia indicates that sperm development can be influenced by ATP signaling via these P2X receptors. The recently published human sperm transcriptome (Miller et al., 2016) also reveals the high levels of residual mRNA for P2X4 and P2X7 (as well as P2X3 and P2X5) receptors found in human spermatozoa, indicating that ATP-regulating pathways in murine and human spermatogenesis might be similar. The fact that spermatogonia have high expression of several types of P2X receptors, particularly given their genetic redundancy, indicates that their function could be critical for sperm development. Although male infertility has not been reported for P2X4- or P2X7-deficient mice, despite their immunological, cardiovascular, and neurological phenotypes, it is possible that conditional deletion of both P2X receptors in the testes will eventually yield an infertility phenotype. Another interesting finding, reported by Fleck et al. (2016), is the functional expression in spermatogonia of the large-conductance calcium-activated K+ channel (BK). The BK can provide the necessary feedback mechanism for restoring membrane potential after P2X receptor–triggered depolarization. Because P2X receptors conduct Na+ and Ca2+, depolarization and elevation of cytoplasmic Ca2+ will lead to activation of the BK, which in in turn triggers K+ efflux, subsequent membrane hyperpolarization, and eventually restoration of resting membrane potential.
Future directions
Purinergic signaling has broad effects on endocrine pathways, from secretion of GnRH from the hypothalamus to the release of testosterone and estradiol from testicular cells (Foresta et al., 1996; Rossato et al., 2001; Burnstock, 2014). As these pathways are vital for male fertility, ablation of one of the P2X channels may be compensated for by increased activity of other subunits, either in the same cell or in signaling pathways upstream or downstream from the affected tissue. This could also be true for P2X4 and P2X7, which in this issue are shown to be active in differentiating spermatogonia (Fleck et al., 2016). Although mice deficient in either of these two channels do not display a reduction in fertility (Solle et al., 2001; Ke et al., 2003; Brône et al., 2007), ablation of one channel could give rise to increased activity of other purinergic signaling pathways, either as a direct response in spermatogonia or indirectly, for example, through the release of hormones from adjacent cells. In support of this hypothesis, studies of P2X4−/− mouse kidneys and an alveolar cell line have shown overexpression of either P2X4 or P2X7 when the other subtype was down-regulated (Weinhold et al., 2010; Craigie et al., 2013). However, others have found a direct interaction between homotrimeric P2X4 and P2X7 channels. For example, murine macrophages do not display a change in expression of P2X7 after P2X4 ablation; however, protein–protein interaction between the two channels was instead suggested to be involved in P2X7-mediated cell death and release of inflammatory signals (Kawano et al., 2012a,b; Pérez-Flores et al., 2015). Although Fleck et al. (2016) show a two-step dose–response curve that corresponds to independent activation of P2X4 and P2X7 channels, additional studies are needed to rule out any change in gene expression or channel interaction after protein ablation in vivo.
Several nonsynonymous single nucleotide polymorphisms (NS-SNPs) have also been detected for both P2X4 and P2X7 receptors in humans. Both of these receptors are highly expressed in immune, epithelial, and endothelial cells (Soto et al., 1996; Nicke, 2008). NS-SNPs of P2X7 cause increased risk of, among other conditions, osteoporosis in fracture patients and postmenopausal women, tuberculosis, and multiple sclerosis (Jiang et al., 2013). Similarly, P2X4 NS-SNPs cause increased risk of osteoporosis (Wesselius et al., 2013) as well as increased pulse pressure, the latter likely caused by defects in the compliance of large arteries (Stokes et al., 2011). However, none of the NS-SNPs show a direct effect on male fertility. To reveal the importance of purinergic signaling in the human testis, it would be necessary to study the expression pattern of P2X subtypes in the different stages of human spermatogenesis and compare the results with those of rodents. Ion channels and transporters that are functionally expressed in sperm cells could differ among even closely related species, and this could also be true for sperm precursors. In fact, rat spermatogonia do not express P2X4 (Glass et al., 2001), whereas Fleck et al. (2016) show this receptor to be an important modulator of purinergic signaling in mouse spermatogonia. Thus, the question of which P2X subtypes conduct purinergic signaling in human sperm cells remains open.
Purinergic signaling often leads to increased release of ATP into the extracellular environment. Although ATP functions at a very short range (Fitz, 2007), it permits positive feedback through autocrine signaling as well as communication with adjacent cells (Corriden and Insel, 2010). Fleck et al. (2016) show an increased release of ATP after activation of P2X channels, most likely P2X7, in spermatogonia. A previous study also showed release of ATP after FSH stimulation of Sertoli cells (Lalevée et al., 1999; Gelain et al., 2005). The close proximity between Sertoli and germ cells in the testis could thus allow for changes in cell function, whether ATP is released by Sertoli cells or the developing germ cells themselves. Future studies should therefore take into account the role of purinergic signaling in Sertoli–germ cell interactions, which could give rise to activation of P2X channels and increased uptake of Ca2+ by spermatogonia. Fleck et al. (2016) also suggest the possibility that the increased Ca2+ levels observed after purinergic signaling could regulate spermatogonial gene expression. Therefore, even a brief release of ATP into the extracellular environment could lead to long-term effects on sperm differentiation. It must be noted that the current study by Fleck et al. (2016) investigates purinergic signaling in spermatogonia of 7-d-old mice. Although electrophysiological measurements of cultured cells correspond to results from intact seminiferous cord preparations of similarly aged mice (Fleck et al., 2016), it would be important to test whether ATP gives rise to a similar response in spermatogonia of adult mice. If cell–cell communication takes part in purinergic signaling in the testis, increased hormonal levels at puberty could alter signaling events. For example, the newborn male mouse experiences low serum levels of FSH until 10 d postnatal, after which a surge in FSH stimulates the differentiated Sertoli cells to support the developing sperm cells (Barakat et al., 2008). As previously mentioned, increased FSH levels would also lead to ATP release from Sertoli cells, which could cause influx of Ca2+ into spermatogonia (Lalevée et al., 1999; Gelain et al., 2005). Thus, the expression levels or function of P2X channels in the adult testis could be different to those observed in younger animals. However, measurement of ion channel activity in the testis remains a challenge. Since the adult testis contains a heterogeneous population of differentiating germ cells, a method to distinguish the different cell types with specific live cell markers is vital for correct interpretation of electrophysiological data from this tissue. The development of such techniques could also further our understanding of the role of other ion channels present during spermatogenesis.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01GM111802 and R21HD081403, the Pew Biomedical Scholars Pew Charitable Trust, an Alfred P. Sloan Award, and the Packer Wentz Endowment Will to P.V. Lishko.
The authors declare no competing financial interests.
Sharona E. Gordon served as editor.
References
- Antonio L.S., Costa R.R., Gomes M.D., and Varanda W.A.. 2009. Mouse Leydig cells express multiple P2X receptor subunits. Purinergic Signal. 5:277–287. 10.1007/s11302-008-9128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks F.C., Calvert R.C., and Burnstock G.. 2010. Changing P2X receptor localization on maturing sperm in the epididymides of mice, hamsters, rats, and humans: a preliminary study. Fertil. Steril. 93:1415–1420. 10.1016/j.fertnstert.2009.02.061 [DOI] [PubMed] [Google Scholar]
- Barakat B., O’Connor A.E., Gold E., de Kretser D.M., and Loveland K.L.. 2008. Inhibin, activin, follistatin and FSH serum levels and testicular production are highly modulated during the first spermatogenic wave in mice. Reproduction. 136:345–359. 10.1530/REP-08-0140 [DOI] [PubMed] [Google Scholar]
- Brône B., Moechars D., Marrannes R., Mercken M., and Meert T.. 2007. P2X currents in peritoneal macrophages of wild type and P2X4−/− mice. Immunol. Lett. 113:83–89. 10.1016/j.imlet.2007.07.015 [DOI] [PubMed] [Google Scholar]
- Burnstock G. 1972. Purinergic nerves. Pharmacol. Rev. 24:509–581. [PubMed] [Google Scholar]
- Burnstock G. 2012. Purinergic signalling: Its unpopular beginning, its acceptance and its exciting future. BioEssays. 34:218–225. 10.1002/bies.201100130 [DOI] [PubMed] [Google Scholar]
- Burnstock G. 2013. Introduction and perspective, historical note. Front. Cell. Neurosci. 7:227 10.3389/fncel.2013.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. 2014. Purinergic signalling in endocrine organs. Purinergic Signal. 10:189–231. 10.1007/s11302-013-9396-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriden R., and Insel P.A.. 2010. Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci. Signal. 3:re1 10.1126/scisignal.3104re1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie E., Birch R.E., Unwin R.J., and Wildman S.S.. 2013. The relationship between P2X4 and P2X7: a physiologically important interaction? Front. Physiol. 4:216 10.3389/fphys.2013.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kretser D.M., Loveland K.L., Meinhardt A., Simorangkir D., and Wreford N.. 1998. Spermatogenesis. Hum. Reprod. 13:1–8. 10.1093/humrep/13.suppl_1.1 [DOI] [PubMed] [Google Scholar]
- Filippini A., Riccioli A., De Cesaris P., Paniccia R., Teti A., Stefanini M., Conti M., and Ziparo E.. 1994. Activation of inositol phospholipid turnover and calcium signaling in rat Sertoli cells by P2-purinergic receptors: modulation of follicle-stimulating hormone responses. Endocrinology. 134:1537–1545. [DOI] [PubMed] [Google Scholar]
- Fitz J.G. 2007. Regulation of cellular ATP release. Trans. Am. Clin. Climatol. Assoc. 118:199–208. [PMC free article] [PubMed] [Google Scholar]
- Fleck D., Mundt N., Bruentgens F., Geilenkirchen P., Machado P.A., Veitinger T., Veitinger S., Lipartowski S.M., Engelhardt C.H., Oldiges M., et al. 2016. Distinct purinergic signaling pathways in prepubescent mouse spermatogonia. J. Gen. Physiol. 148 10.1085/jgp.201611636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C., Rossato M., Bordon P., and Di Virgilio F.. 1995. Extracellular ATP activates different signalling pathways in rat Sertoli cells. Biochem. J. 311:269–274. 10.1042/bj3110269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C., Rossato M., Nogara A., Gottardello F., Bordon P., and Di Virgilio F.. 1996. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem. J. 320:499–504. 10.1042/bj3200499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelain D.P., Casali E.A., de Oliveira R.B., de Souza L.F., Barreto F., Dal-Pizzol F., and Moreira J.C.. 2005. Effects of follicle-stimulating hormone and vitamin A upon purinergic secretion by rat Sertoli cells. Mol. Cell. Biochem. 278:185–194. 10.1007/s11010-005-7500-4 [DOI] [PubMed] [Google Scholar]
- Glass R., Bardini M., Robson T., and Burnstock G.. 2001. Expression of nucleotide P2X receptor subtypes during spermatogenesis in the adult rat testis. Cells Tissues Organs (Print). 169:377–387. 10.1159/000047905 [DOI] [PubMed] [Google Scholar]
- Hattori M., and Gouaux E.. 2012. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 485:207–212. 10.1038/nature11010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.H., Kim M., Spelta V., Bo X., Surprenant A., and North R.A.. 2003. Subunit arrangement in P2X receptors. J. Neurosci. 23:8903–8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.H., Baldwin J.M., Roger S., and Baldwin S.A.. 2013. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front. Pharmacol. 4:55 10.3389/fphar.2013.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano A., Tsukimoto M., Mori D., Noguchi T., Harada H., Takenouchi T., Kitani H., and Kojima S.. 2012a Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem. Biophys. Res. Commun. 420:102–107. 10.1016/j.bbrc.2012.02.122 [DOI] [PubMed] [Google Scholar]
- Kawano A., Tsukimoto M., Noguchi T., Hotta N., Harada H., Takenouchi T., Kitani H., and Kojima S.. 2012b Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem. Biophys. Res. Commun. 419:374–380. 10.1016/j.bbrc.2012.01.156 [DOI] [PubMed] [Google Scholar]
- Kawate T., Michel J.C., Birdsong W.T., and Gouaux E.. 2009. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 460:592–598. 10.1038/nature08198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H.Z., Qi H., Weidema A.F., Zhang Q., Panupinthu N., Crawford D.T., Grasser W.A., Paralkar V.M., Li M., Audoly L.P., et al. 2003. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 17:1356–1367. 10.1210/me.2003-0021 [DOI] [PubMed] [Google Scholar]
- Ko W.H., Chan H.C., Chew S.B., and Wong P.Y.. 1998. Regulated anion secretion in cultured epithelia from Sertoli cells of immature rats. J. Physiol. 512:471–480. 10.1111/j.1469-7793.1998.471be.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko W.H., Au C.L., and Yip C.Y.. 2003. Multiple purinergic receptors lead to intracellular calcium increases in cultured rat Sertoli cells. Life Sci. 72:1519–1535. 10.1016/S0024-3205(02)02410-4 [DOI] [PubMed] [Google Scholar]
- Lalevée N., Rogier C., Becq F., and Joffre M.. 1999. Acute effects of adenosine triphosphates, cyclic 3′,5′-adenosine monophosphates, and follicle-stimulating hormone on cytosolic calcium level in cultured immature rat Sertoli cells. Biol. Reprod. 61:343–352. 10.1095/biolreprod61.2.343 [DOI] [PubMed] [Google Scholar]
- Luria A., Rubinstein S., Lax Y., and Breitbart H.. 2002. Extracellular adenosine triphosphate stimulates acrosomal exocytosis in bovine spermatozoa via P2 purinoceptor. Biol. Reprod. 66:429–437. 10.1095/biolreprod66.2.429 [DOI] [PubMed] [Google Scholar]
- Meroni S.B., Cánepa D.F., Pellizzari E.H., Schteingart H.F., and Cigorraga S.B.. 1998. Effects of purinergic agonists on aromatase and gamma-glutamyl transpeptidase activities and on transferrin secretion in cultured Sertoli cells. J. Endocrinol. 157:275–283. 10.1677/joe.0.1570275 [DOI] [PubMed] [Google Scholar]
- Miller M.R., Mannowetz N., Iavarone A.T., Safavi R., Gracheva E.O., Smith J.F., Hill R.Z., Bautista D.M., Kirichok Y., and Lishko P.V.. 2016. Unconventional endocannabinoid signaling governs sperm activation via the sex hormone progesterone. Science. 352:555–559. 10.1126/science.aad6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B., Miki K., and Clapham D.E.. 2011. ATP-activated P2X2 current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA. 108:14342–14347. 10.1073/pnas.1111695108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto F.T., Bach P.V., Najari B.B., Li P.S., and Goldstein M.. 2016. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 10.1016/j.semcdb.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Nicke A. 2008. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem. Biophys. Res. Commun. 377:803–808. 10.1016/j.bbrc.2008.10.042 [DOI] [PubMed] [Google Scholar]
- Paniagua R., and Nistal M.. 1984. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J. Anat. 139:535–552. [PMC free article] [PubMed] [Google Scholar]
- Pérez-Armendariz E.M., Nadal A., Fuentes E., and Spray D.C.. 1996. Adenosine 5′-triphosphate (ATP) receptors induce intracellular calcium changes in mouse leydig cells. Endocrine. 4:239–247. 10.1007/BF02738690 [DOI] [PubMed] [Google Scholar]
- Pérez-Flores G., Lévesque S.A., Pacheco J., Vaca L., Lacroix S., Pérez-Cornejo P., and Arreola J.. 2015. The P2X7/P2X4 interaction shapes the purinergic response in murine macrophages. Biochem. Biophys. Res. Commun. 467:484–490. 10.1016/j.bbrc.2015.10.025 [DOI] [PubMed] [Google Scholar]
- Poletto Chaves L.A., Pontelli E.P., and Varanda W.A.. 2006. P2X receptors in mouse Leydig cells. Am. J. Physiol. Cell Physiol. 290:C1009–C1017. 10.1152/ajpcell.00506.2005 [DOI] [PubMed] [Google Scholar]
- Rato L., Socorro S., Cavaco J.E., and Oliveira P.F.. 2010. Tubular fluid secretion in the seminiferous epithelium: ion transporters and aquaporins in Sertoli cells. J. Membr. Biol. 236:215–224. 10.1007/s00232-010-9294-x [DOI] [PubMed] [Google Scholar]
- Rossato M., Merico M., Bettella A., Bordon P., and Foresta C.. 2001. Extracellular ATP stimulates estradiol secretion in rat Sertoli cells in vitro: modulation by external sodium. Mol. Cell. Endocrinol. 178:181–187. 10.1016/S0303-7207(01)00426-9 [DOI] [PubMed] [Google Scholar]
- Rudge S.A., Hughes P.J., Brown G.R., Michell R.H., and Kirk C.J.. 1995. Inositol lipid-mediated signalling in response to endothelin and ATP in the mammalian testis. Mol. Cell. Biochem. 149-150:161–174. 10.1007/BF01076574 [DOI] [PubMed] [Google Scholar]
- Saez J.M. 1994. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr. Rev. 15:574–626. 10.1210/edrv-15-5-574 [DOI] [PubMed] [Google Scholar]
- Solle M., Labasi J., Perregaux D.G., Stam E., Petrushova N., Koller B.H., Griffiths R.J., and Gabel C.A.. 2001. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276:125–132. 10.1074/jbc.M006781200 [DOI] [PubMed] [Google Scholar]
- Soto F., Garcia-Guzman M., Gomez-Hernandez J.M., Hollmann M., Karschin C., and Stühmer W.. 1996. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc. Natl. Acad. Sci. USA. 93:3684–3688. 10.1073/pnas.93.8.3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes L., Scurrah K., Ellis J.A., Cromer B.A., Skarratt K.K., Gu B.J., Harrap S.B., and Wiley J.S.. 2011. A loss-of-function polymorphism in the human P2X4 receptor is associated with increased pulse pressure. Hypertension. 58:1086–1092. 10.1161/HYPERTENSIONAHA.111.176180 [DOI] [PubMed] [Google Scholar]
- Wang R.S., Yeh S., Tzeng C.R., and Chang C.. 2009. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr. Rev. 30:119–132. 10.1210/er.2008-0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhold K., Krause-Buchholz U., Rödel G., Kasper M., and Barth K.. 2010. Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell. Mol. Life Sci. 67:2631–2642. 10.1007/s00018-010-0355-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselius A., Bours M.J., Jørgensen N.R., Wiley J., Gu B., van Helden S., van Rhijn L., and Dagnelie P.C.. 2013. Non-synonymous polymorphisms in the P2RX4 are related to bone mineral density and osteoporosis risk in a cohort of Dutch fracture patients. Purinergic Signal. 9:123–130. 10.1007/s11302-012-9337-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C.W., Choong Y.T., Short J.L., Exintaris B., Malone D.T., Allen A.M., Evans R.J., and Ventura S.. 2013. Male contraception via simultaneous knockout of α1A-adrenoceptors and P2X1-purinoceptors in mice. Proc. Natl. Acad. Sci. USA. 110:20825–20830. 10.1073/pnas.1318624110 [DOI] [PMC free article] [PubMed] [Google Scholar]