Introduction
Post-mastectomy radiotherapy has become increasingly common for patients with advanced breast cancer since the initial indications were first described by Ragaz and Overgaard in 1997.1-3 Breast reconstruction has also become more frequent with the most common technique being two stage-prosthetic reconstruction.4 The optimal timing and sequence of mastectomy, reconstruction, adjuvant chemotherapy and radiotherapy remains unresolved. If there is insufficient time to undergo the exchange procedure before radiation starts, then patients must receive radiation to the tissue expander (TE). Alternatively, radiation may be delivered to the permanent implant following the exchange procedure and completion of chemotherapy.5, 6 Results associated with these two algorithms demonstrate variable outcomes and have focused principally on surgical outcomes such as loss of the TE or permanent implant, and overall reconstructive failure. A systematic review demonstrated that studies in the literature are limited by a small case number, retrospective nature, absence of a control group, and omission of aesthetic outcomes and patient satisfaction.7
The senior author (P.G.C) has maintained a prospective database of two-stage prosthetic reconstruction that includes short-term complications, long-term outcomes, aesthetic results including capsular contracture, and more recently patient-reported outcomes. The objective of this study is to assess this prospectively collected data to evaluate and compare both clinician and patient-reported outcomes using two different algorithms: radiation of the TE followed by the exchange procedure versus radiation to the permanent implant after the exchange procedure. A group of non-irradiated patients was included as a control group.
Methods
Following institutional IRB approval, all patients who underwent prosthetic based reconstruction without radiation (non-XRT), radiation to the tissue expander (TE-XRT) or to the permanent implant (implant-XRT) from 2003 to 2012 by the senior author (P.G.C) were included in this study. This decade of experience was selected in order to maximize the overlap of all three cohorts being evaluated. Patients with radiation prior to the mastectomy (breast conserving therapy + radiation), combined techniques (implant + flap), and delayed reconstructions were excluded. The mastectomies were performed by a variety of oncologic surgeons at memorial Sloan Kettering Cancer Center (New York, NY). The surgical technique for TE implantation and exchange to permanent implant was uniform for the three groups of patients as described previously.8, 9
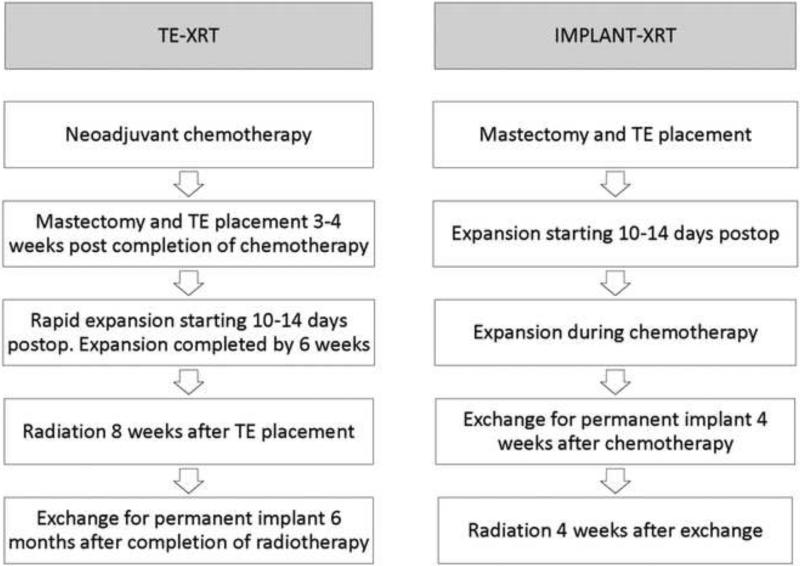
Timing of radiotherapy was determined by whether patients needed neoadjuvant chemotherapy or not. For those requiring neoadjuvant chemotherapy, radiation was delivered before the exchange of TE for permanent devices; if adjuvant chemotherapy was required, radiation was delivered after the exchange. Timing of radiotherapy for each group is illustrated in Figure 1. For TE-XRT patients, 3-4 weeks after completion of chemotherapy, a total mastectomy with immediate placement of a submuscular TE utilizing complete musculofascial coverage was performed. Axillary lymph node dissection/sentinel lymph node biopsy was performed as necessary. Approximately fifty percent of tissue expansion was performed intraoperatively with weekly expansions starting two weeks postoperatively. Patients were expanded rapidly to final volume by six weeks postoperatively with radiotherapy begun by eight weeks. Radiotherapy was then administered to the chest wall and regional lymph nodes with the TE fully expanded. Exchange to the permanent device and an extensive capsulotomy was performed 6 months after completing radiation therapy. For implant-XRT ppatients, the exchange procedure to permanent implant was performed four weeks after completion of chemotherapy followed by radiotherapy to the permanent implant 4 weeks therafter.5 The radiation field always included the para-clavicular nodal region, but the internal mammary chain and axillary nodes were irradiated based on pre-operative imaging or pathology. A single-isocenter technique was used throughout the study period and intensity-modulated tangent beams were used to achieve dose homogeneity. Unless the patient was very large-breasted, the prescribed energy was 6 MV photons for the implant-XRT patients. Daily bolus of 0.5 cm was placed over the chest wall fields only to insure adequate dose to the skin surface and mastectomy scar. For the TE-XRT group, 15 MV photons were used for the reconstructed chest wall to minimize “scatter” dose off the magnetic TE valve, and the bolus increased to 1 cm.
Figure 1.
Timing of radiation and exchange to permanent implant in TE-XRT and implant-XRT patients.
Patients were evaluated yearly. Demographics, treatment, surgical and aesthetic outcomes were prospectively recorded in the institutional database. Outcomes included reconstructive failure, capsular contracture, aesthetic results and patient satisfaction. Reconstructive failure was defined as explantation of the TE or the permanent implant. Capsular contracture was evaluated using the modified Baker classification for reconstructed breasts with the most recent evaluation used in the data analysis.10 For reconstructive failure and capsular contracture, each implant breast reconstruction was analyzed individually. Aesthetic results were evaluated by the surgeon using a categorical scale (poor, fair, good, very good, excellent). Patient reported outcomes (satisfaction and health-related quality of life) were evaluated with the BREAST-Q© Reconstructive module.
Outcomes for TE-XRT and implant-XRT were compared to the control group (non-XRT), in addition to comparisons between TE-XRT and implant-XRT. Chi-square test, ANOVA, and student t-test were used as indicated. The association between radiotherapy timing and reconstructive failure was evaluated using logistic regression after adjusting by variable clinical factors. Variables with a p<0.1 in the univariable analysis were included in the multivariable model. Since duration of follow-up time differed between the groups, predicted rates of reconstructive failure were assessed by Kaplan-Meier analysis and log rank test. Time to event was defined as the time from TE implantation to TE or permanent implant removal. The advantage of this type of analysis is that it allows for prediction of progressive events (e.g reconstructive failure) at different times, including data from patients who are currently in follow-up and those who were lost before the occurrence of the event. The BREAST-Q© Reconstructive module is a condition-specific, validated questionnaire designed to measure quality of life and satisfaction in patients with breast reconstruction. 11 Scales used were Satisfaction with Breasts, Satisfaction with Outcome, Psychosocial Well-being, Sexual Well-being, and Physical Well-being (chest and upper body). For each scale, items are summed and transformed on a 0 to 100 scale, with greater values indicating higher levels of satisfaction and health-related quality of life. Linear regression was used to adjust BREAST-Q© scores by factors shown to be associated with satisfaction and quality of life outcomes (body mass index [BMI], laterality, implant type, and time to follow-up).12, 13 A minimal important difference was defined as a 5 point difference between groups.13 All statistical analyses were performed using Stata 11.0™ (Stata Corporation, College Station, Texas).
Results
A total of 1,143 patients with 1,486 non-XRT, 94 TE-XRT, and 210 implant-XRT implant reconstructions were included in the study (Table 1). The three groups were similar in age and comorbidities (p=NS). Non-XRT patients had a significantly higher proportion of silicone implant use (p=0.01), bilateral reconstructions (p<0.01), and longer follow-up than the TE-XRT and implant-XRT patients (p<0.01). Compared to the implant-XRT group, TE-XRT patients had a greater proportion of silicone implants (p<0.01), greater number of bilateral reconstructions (p=0.02), and shorter follow-up time (p<0.01).
Table 1.
Characteristics of the groups
| Non-XRT n=1,486 | TE-XRT n=94 | Implant-XRT n=210 | p | p* | |
|---|---|---|---|---|---|
| Age | 47.8 (±9.6) | 46.1 (±10.6) | 46.3 (±8.9) | NS | NS |
| Body mass index | 24.6 (±4.8) | 26.3 (±5.6) | 25.3 (±5.0) | <0.01 | NS |
| Implant type (%) | 0.01 | <0.01 | |||
| Saline | 531 (37.2) | 31 (38.8) | 98 (47.1) | ||
| Silicone | 896 (62.8) | 49 (61.2) | 110 (52.8) | ||
| Comorbidities | 187 (12.5) | 11 (12.9) | 26 (12.6) | NS | NS |
| Laterality (%) | <0.01 | 0.02 | |||
| Unilateral | 39.0 | 57.0 | 71.6 | ||
| Bilateral | 61.0 | 43.0 | 28.4 | ||
| Mean follow up time (months) | 45.6 | 30.1 | 40.3 | <0.01 | <0.01 |
| Range | 0.3-133 | 0.5-118 | 1.6-113 | ||
| Mean interval between TE insertion and exchange to implant (months) | 6.1 | 13.2 | 6.2 | <0.01 | <0.01 |
between TE and implant radiation
Table 2 shows reconstructive failure rates by group. Reconstructive failure was lower for non-XRT patients compared to TE-XRT and implant-XRT patients (4.6% compared to 18.1% and 12.4% respectively, p<0.01). TE-XRT patients had reconstructive failure rates greater than implant-XRT patients, 18.1% vs. 12.4%, although the difference did not reach statistical significance (p=NS). Since characteristics such as implant type, laterality and follow-up time were different between the groups, a multivariable analysis of the odds to have a reconstructive failure adjusting by these factors was performed (Table 3). Radiation to either the implant or TE was independently associated with a greater likelihood of reconstructive failure when compared to non-XRT; however, the odds were greater for TEXRT (OR=5.75, p<0.01) than implant-XRT patients (OR= 5.19, p<0.01).
Table 2.
Failure rates
| Non-XRT (n=1,486) | TE-XRT (n=94) | Implant-XRT (n=210) | p | p* | |
|---|---|---|---|---|---|
| TE removal | 54 (3.6) | 8 (8.5) | 2 (1.0) | <0.01 | <0.01 |
| Implant removal | 14 (1.0) | 9 (9.6) | 24 (11.4) | <0.01 | NS |
| Reconstructive failure | 68 (4.6) | 17 (18.1) | 26 (12.4) | <0.01 | NS |
between TE and implant radiation
Table 3.
Multivariable analysis of reconstructive failure. Failure includes tissue expander and implant loss
| Odds Ratio Reconstructive failure | CI 95% | p | |
|---|---|---|---|
| Group | |||
| Non-XRT | 1.00 | ||
| TE-XRT | 5.75 | 2.58-12.03 | <0.01 |
| Implant-XRT | 5.19 | 2.71-9.99 | <0.01 |
| Implant type | |||
| Saline implants | 1.00 | ||
| Silicone implants | 0.61 | 0.36-1.07 | NS |
| Laterality | |||
| Unilateral | 1.00 | ||
| Bilateral | 1.16 | 0.50-1.92 | NS |
| Time to follow up | 0.98 | 0.95-0.99 | <0.01 |
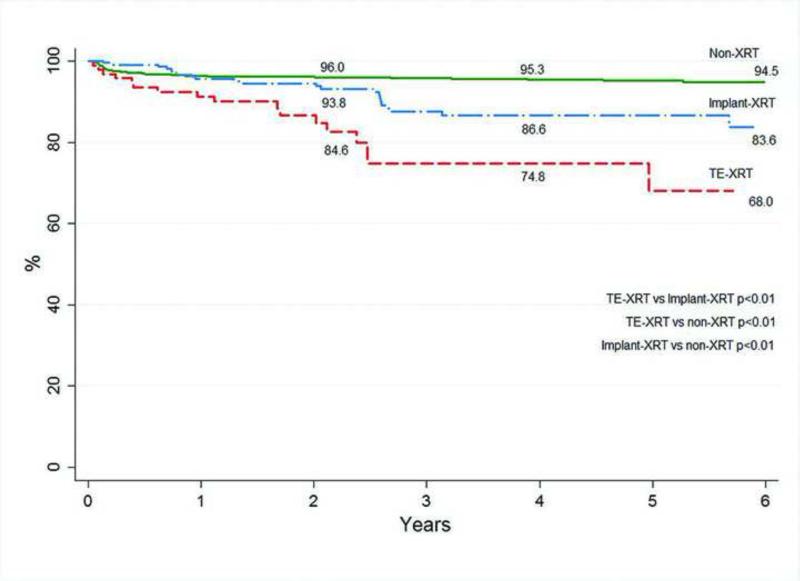
Figure 2 demonstrates a Kaplan-Meier analysis of the predicted reconstructive failure rates. Predicted failure rates at 6 years for TE-XRT are greater than for patients with implant-XRT, 32.0% compared to 16.4% (p<0.01). Reconstructive failure in non-irradiated patients is significantly lower (5.5%) than TE-XRT or implant-XRT groups (p<0.01).
Figure 2.
Long-term two-stage implant reconstruction survival. Failure includes tissue expander and implant loss
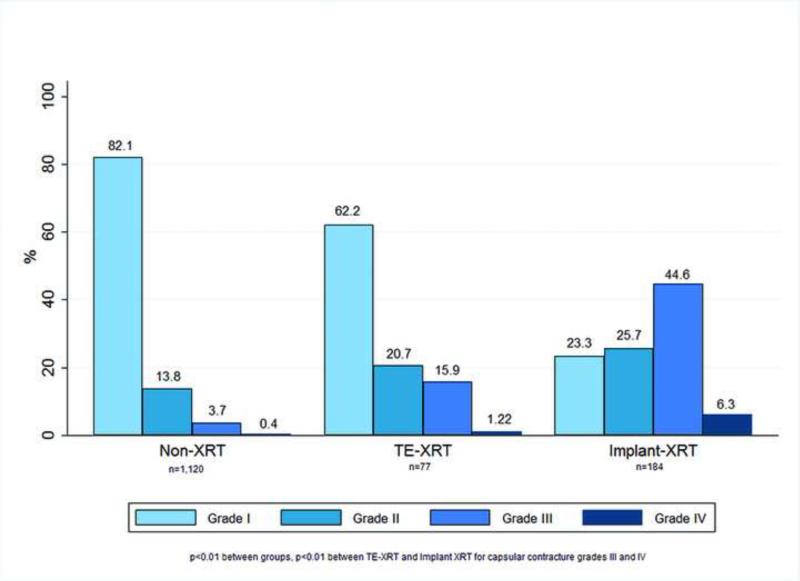
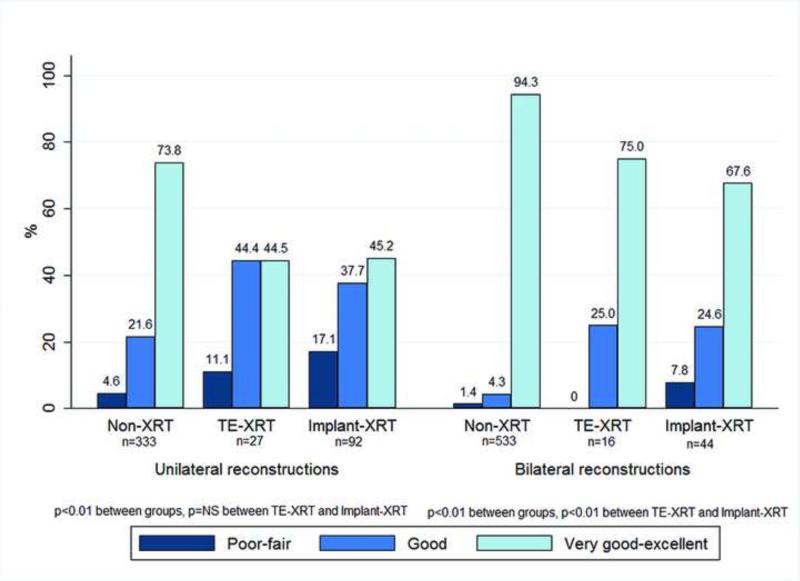
Aesthetic outcomes of patients who did not have a reconstructive failure are as follows. Capsular contracture grade III was present in 3.7% of implants without radiation, 15.9% of TE-XRT patients, and 44.6% of implant-XRT implants (p<0.01). Capsular contracture grade IV was present in 0.4%, 1.22%, and 6.3% of each group respectively (p<0.01) (Figure 3). The proportion of implants with capsular contracture grade IV was significantly higher in the implant-XRT compared to TE-XRT group (6.3% compared to 1.22, p<0.01). A comparison of aesthetic results is shown in Figure 4. For patients with unilateral reconstructions a very good to excellent result was present in 73.8% of non-XRT, compared to 44.5% of TE-XRT and 45.2% of the implant-XRT group (p<0.01); the proportion of patients with very good to excellent aesthetic results was not different for TE-XRT compared to implant-XRT patients (p=NS). For patients with bilateral reconstructions a very good to excellent aesthetic result was present in 94.3% of non-XRT patients, compared to 75.0% of TE-XRT and 67.6% of implant-XRT patients (p<0.01); patients with TE-XRT had a marginally higher proportion of very good to excellent results than implant-XRT patients (p<0.01).
Figure 3.
Capsular contracture rates by group
Figure 4.
Surgeon evaluated aesthetic results by radiation group
Health-related quality of life and satisfaction from the patients’ perspective were evaluated comparing BREAST-Q© scores adjusted by BMI, laterality, implant type, and follow-up time (Table 4). Non-XRT patients had significantly higher levels of satisfaction with breasts, satisfaction with outcome, psychosocial well-being, physical well-being and sexual well-being than TE-XRT or implant-XRT patients (p<0.01). Although some of the scale scores were significantly higher for TE-XRT compared to implant-XRT, none of them reached a minimal important difference.
Table 4.
Adjusted median BREAST-Q© scores (by BMI, laterality, implant type, follow-up)
| BREAST-Q© scale | Non-XRT (n=520) | TE-XRT (n=22) | Implant-XRT (n=84) | p | p* |
|---|---|---|---|---|---|
| Satisfaction with breasts (SD) | 64.1 (3.2) | 57.2 (3.1) | 56.2 (3.3) | <0.01 | NS |
| Satisfaction with outcome (SD) | 73.5 (3.9) | 70.2 (3.0) | 68.4 (3.8) | <0.01 | 0.02 |
| Psychosocial well-being (SD) | 76.4 (1.4) | 72.3 (1.2) | 71.1 (1.4) | <0.01 | <0.01 |
| Sexual well-being (SD) | 55.7 (0.9) | 55.4 (0.7) | 54.0 (0.9) | <0.01 | <0.01 |
| Physical well-being (SD) | 78.5 (2.4) | 73.4 (1.9) | 72.5 (2.6) | <0.01 | NS |
between TE and implant radiation
Discussion
The current data demonstrate that patients who receive radiation to the TE are significantly more likely to lose the TE than those with radiation to the final implant (8.5% vs. 1.0%). This finding may simply reflect that the TE remains in place for a lengthier period of time in the TE-XRT than the implant-XRT group (13.2 versus 6.2 months). It is our approach to wait for 6 months prior to doing the exchange procedure in order to minimize chances of having a problem during the acute phase of radiation. It is likely that if the TE was replaced earlier during the inflammatory period of radiation, the actual TE failure rate would be even higher as has been demonstrated by other surgeons who do use this approach.14 When evaluating the overall reconstructive failure rate, univariate analysis demonstrated that the TEXRT group had a higher failure rate than the implant-XRT group (18.1 vs. 12.4%), although this difference was not statistically significant. Since the outcome of reconstructive failure can be influenced by other important variables, such as duration of follow, laterality, or implant type, a multivariable analysis was completed. This demonstrated that the odds of reconstructive failure adjusted by these other variables, are higher with radiation to either the TE or the implant compared to non-XRT patients. In addition, those who receive radiation to the TE are 10% more likely to lose the overall reconstruction than the implant-XRT group. These results are consistent with other publications, although the overall loss rate of both the TE and implant is somewhat lower than other authors. Kronowitz et al. reported a 32% loss of the TE at 40 months when following his described algorithm for radiation to the TE.14 Nava, in a prospectively designed study, which also included a control group, demonstrated significantly higher loss of the reconstruction in the TE-XRT group, 40% vs. 6.7% in implant-XRT at 50 months of follow-up.15 Neither of these authors evaluated implant outcomes using Kaplan-Meier analysis; Kaplan-Meier analysis most closely approximates what occurs in reality, since the predicted long-term failure rates incorporate information from patients with variable follow up times and those who are lost to follow up that is not considered in univariate analysis. When applying this type of analysis, the potential for reconstructive failure is significantly higher for TE-XRT as compared to implant-XRT (32.0% at 6 years vs. 16.4%). The current data therefore supports the algorithm that one should radiate the final implant if one wishes to minimize reconstructive failure
To date, literature evaluating long-term aesthetic outcomes in radiated two-stage prosthetic based breast reconstructions is scant. Nava's data suggest that radiating the final implant provides slightly better aesthetic outcomes from both the surgeon and patient standpoint.15 We have recently published our comprehensive long-term results evaluating patients who received radiation using the implant-XRT algorithm demonstrating that greater than 62% of patients have at least a good to very good result with 29% having an excellent result.16 This is certainly not as good as non-radiated patients, but affords many high-risk surgical and oncologic patients the opportunity to reconstruct the breast mound. In the current publication, it appears that radiation to the TE may result in slightly better outcomes than radiation to the final implant both with regard to aesthetic results as evaluated by the surgeon, but also capsular contracture. Severe capsular contracture (grade III and IV) in patients receiving radiation to the TE is lower than that seen in patients with radiation to the implant (15.9% and 1.22% vs. 44.6% and 6.3%, respectively). Lower rates of severe capsular contracture in the TE-XRT group are likely due to the fact that aggressive capsulotomy is performed at the time of the exchange procedure. TE-XRT patients may benefit from the fact that the capsulotomy is performed after radiation which may result in a better aesthetic result, allowing the skin envelope to re-drape over the implant. For implant-XRT patients, a circumferential capsulotomy is performed during the exchange prior to radiation
Potential weaknesses of the current study are that the overall follow-up in TE-XRT patients is slightly shorter than the implant-XRT group, and longer follow-up might increase severe capsular contracture rates for that cohort. Patients who received TE-XRT also have a greater proportion of silicone implants and bilateral reconstructions which potentially skews the data towards better aesthetic results in this group of patients. In order to minimize these differences, aesthetic results were stratified by laterality of reconstruction, and although the difference in aesthetic outcomes with TE-XRT and implant-XRT is less evident for unilateral reconstructions, there remains a small difference between groups with good to excellent outcomes for TE-XRT patients in both unilateral and bilateral reconstructions (88.9% vs. 82.9% in unilateral, and 100% vs. 92.2% in bilateral, respectively). Other factors as breast size/shape, and quality of the tissues post mastectomy were not specifically evaluated in the current study. The evaluation of aesthetic outcomes by a single observer is also a potential weakness; however, consistent evaluation throughout the years can be also considered strength.
When a patient who chooses two-stage prosthetic reconstruction needs to undergo adjuvant radiotherapy, the reconstructive surgeon is presented with the dilemma of which is the better option to recommend. In other words, should patients undergo radiation to the TE accepting the higher rate of reconstructive failure to possibly achieve a better result or is a higher rate of successful reconstruction better albeit with a potentially inferior result? This decision-making should be shared between the physician and patient. The senior author's current approach is to have this discussion with patients to assess their priorities and patient reported outcomes may be informative in this scenario. For the past 5 years, all patients in the senior author's practice are also evaluated using the BREAST-Q©. Analysis of patients’ BREAST-Q© scores was performed to determine whether those patients undergoing a specific algorithm were more satisfied with outcomes. Interestingly, the scores for the six scales of the BREAST-Q© were not clinically different (less than a minimal importance difference of 5 points) between TE-XRT and implant-XRT patients.
This is the largest evaluation of prospectively collected surgical, aesthetic, and patient-reported outcomes in a homogeneous group of patients treated using the same surgical technique, but undergoing different timing of radiotherapy. The senior author has a large experience with implant reconstruction which likely contributed to obtaining the outcomes described. It is clear that any sequence of radiotherapy in two-stage prosthetic reconstruction negatively impacts the final aesthetic outcome as well as long-term implant survival. All these patients have advanced disease, often are not candidates for immediate autologous reconstruction, and using either algorithm maintain the option of salvage reconstruction with a flap.17 We believe that immediate reconstruction in this group of patients is still very worthwhile as studies demonstrate greater health related quality of life with breast reconstruction compared to mastectomy alone.18, 19 Based on the results of the study, it is important that patients are provided with the best information on potential outcomes in order to make an informed decision. Oncologic treatments continuously evolve and plastic surgeons need to adapt to these changes. The reconstructive approach and timing of radiation for this challenging cohort of patients will likely continue to evolve.
Footnotes
Disclosure
Dr. Cordeiro receives financial and material support from Allergan for participation in a multicenter clinical research study.
References
- 1.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. The New England journal of medicine. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. The New England journal of medicine. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 4.Albornoz CR, Bach PB, Mehrara BJ, et al. A paradigm shift in u.s. Breast reconstruction: increasing implant rates. Plastic and reconstructive surgery. 2013;131:15–23. doi: 10.1097/PRS.0b013e3182729cde. [DOI] [PubMed] [Google Scholar]
- 5.Cordeiro PG, Pusic AL, Disa JJ, et al. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plastic and reconstructive surgery. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 6.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plastic and reconstructive surgery. 2004;113:1617–1628. doi: 10.1097/01.prs.0000117192.54945.88. [DOI] [PubMed] [Google Scholar]
- 7.Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plastic and reconstructive surgery. 2013;132:511–518. doi: 10.1097/PRS.0b013e31829acc41. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plastic and reconstructive surgery. 2006;118:832–839. doi: 10.1097/01.prs.0000232397.14818.0e. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plastic and reconstructive surgery. 2006;118:825–831. doi: 10.1097/01.prs.0000232362.82402.e8. [DOI] [PubMed] [Google Scholar]
- 10.Spear SL, Baker JL., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plastic and reconstructive surgery. 1995;96:1119–1123. discussion 1124. [PubMed] [Google Scholar]
- 11.Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plastic and reconstructive surgery. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy CM, Klassen AF, Cano SJ, et al. Patient satisfaction with postmastectomy breast reconstruction: a comparison of saline and silicone implants. Cancer. 2010;116:5584–5591. doi: 10.1002/cncr.25552. [DOI] [PubMed] [Google Scholar]
- 13.Albornoz CR, Matros E, McCarthy CM, et al. Implant Breast Reconstruction and Radiation: A Multicenter Analysis of Long-Term Health-Related Quality of Life and Satisfaction. Annals of surgical oncology. 2014 doi: 10.1245/s10434-014-3483-2. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation therapy: 3-year follow-up. Plastic and reconstructive surgery. 2011;127:2154–2166. doi: 10.1097/PRS.0b013e3182131b8e. [DOI] [PubMed] [Google Scholar]
- 15.Nava MB, Pennati AE, Lozza L, et al. Outcome of different timings of radiotherapy in implant-based breast reconstructions. Plastic and reconstructive surgery. 2011;128:353–359. doi: 10.1097/PRS.0b013e31821e6c10. [DOI] [PubMed] [Google Scholar]
- 16.Cordeiro PG, Albornoz CR, McCormick B, et al. The Impact of Post-Mastectomy Radiotherapy on Two-Stage Implant Breast Reconstruction: An Analysis of Long Term Surgical Outcomes, Aesthetic Results and Satisfaction in 319 Irradiated and 1,814 Non-irradiated Breast Implants Over 13 Years (1998-2010). Plastic and reconstructive surgery. 2014 doi: 10.1097/PRS.0000000000000523. In Press. [DOI] [PubMed] [Google Scholar]
- 17.Albornoz CR, Cordeiro PG, Farias-Eisner G, et al. Diminishing Relative Contraindications for Immediate Breast Reconstruction. Plastic and reconstructive surgery. 2014 doi: 10.1097/PRS.0000000000000478. In Press. [DOI] [PubMed] [Google Scholar]
- 18.McCarthy CM, Mehrara BJ, Long T, et al. Chest and Upper Body Morbidity Following Immediate Postmastectomy Breast Reconstruction. Annals of surgical oncology. 2013 doi: 10.1245/s10434-013-3231-z. [DOI] [PubMed] [Google Scholar]
- 19.Eltahir Y, Werners LL, Dreise MM, et al. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic and reconstructive surgery. 2013;132:201e–209e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]