The efficacy of rituximab alone in the treatment of B-cell non-Hodgkin lymphoma (NHL) has been well estab-lished since 1999. Furthermore, the addition of rituximab to conventional chemotherapy improved survival outcome.1–3
Lenalidomide (Celgene Corp., Summit, NJ, USA) a potent immunomodulatory agent, has shown efficacy in patients with relapsed or refractory indolent NHL.4 The combination of these agents showed synergistic effects in vitro and in animal models.5,6 Thus, in 2009, we started a Phase II study to test the combination of rituximab and lenalidomide in patients with marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), and small lymphocytic lymphoma (SLL). The study started under the patronage of the Intergruppo Italiano Linfomi (IIL) and was completed after the merge of the IIL in the Fondazione Italiana Linfomi (FIL). This study was an investigator-initiated, open label, multicenter, phase II trial, conducted from 2009 to 2013 at 16 Italian institutions (INFL08/RV-LYM-PI 378, clinicaltrials.gov identifier 01830478, EudraCT number 2008-001591-80). Eligibility criteria were: age 18 years or older and biopsy-proven diagnosis of indolent non-follicular lymphoma, including MZL, LPL and SLL, according to the World Health Organization Classification of Tumors of Hematopoietic and Lymphoid Tissue.7 Patients with extranodal gastric MZL were excluded. The inclusion criteria were: relapsed after 2 or 3 prior lines of rituximab-containing immunotherapy with measurable disease; Eastern Cooperative Oncology Group performance status of 2 or under, and adequate organ function.
All patients provided written informed consent The study protocol was approved by the ethics committee of each participating institution. The study was conducted according to the provisions of the Declaration of Hel-sinki, the International Conference on Harmonization, and the Guidelines for Good Clinical Practice.
Patients received oral lenalidomide 20 mg/day; days 1–21. Rituximab (375 mg/m2) was administered once on day 14 of every course. Treatment was repeated every 28 days for up to 6 courses. To avoid tumor flare, patients with SLL started lenalidomide at a dose of 10 mg/day, with a monthly 5 mg increase, up to 20 mg/day in the absence of toxicity.
The primary end points were safety and efficacy. Efficacy was evaluated in terms of the overall response rate (ORR) and of tumor control rate (TCR). ORR was defined as the ratio between the number of patients that achieved complete response (CR), CR unconfirmed (CRu), and partial response (PR) over all eligible patients. TCR was defined as the ratio between the number of patients who achieved at least stable disease (SD) over all the eligible patients. Both ORR and TCR were evaluated at four weeks after the end of treatment. Tumor response was defined according to the 1999 Cheson criteria.8 Safety was based on laboratory parameters and adverse events. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE version 3.0, http://ctep.cancer.gov/reporting/ctc.html); toxicity was reported on an individual patient basis.
Secondary end points were progression-free survival (PFS) duration of remission (DoR) and overall survival (OS).
For this study, we planned to monitor efficacy with the Bayesian sequential analysis,9 with a reference ORR of 45%. We screened 44 patients to achieve a final total of 40 eligible patients, considering about 10% loss of accrued patients.
Survival was evaluated with the Kaplan-Meier method, and group comparisons were performed with the log rank test.
Safety analyses included all patients who received at least 1 dose of study drug.
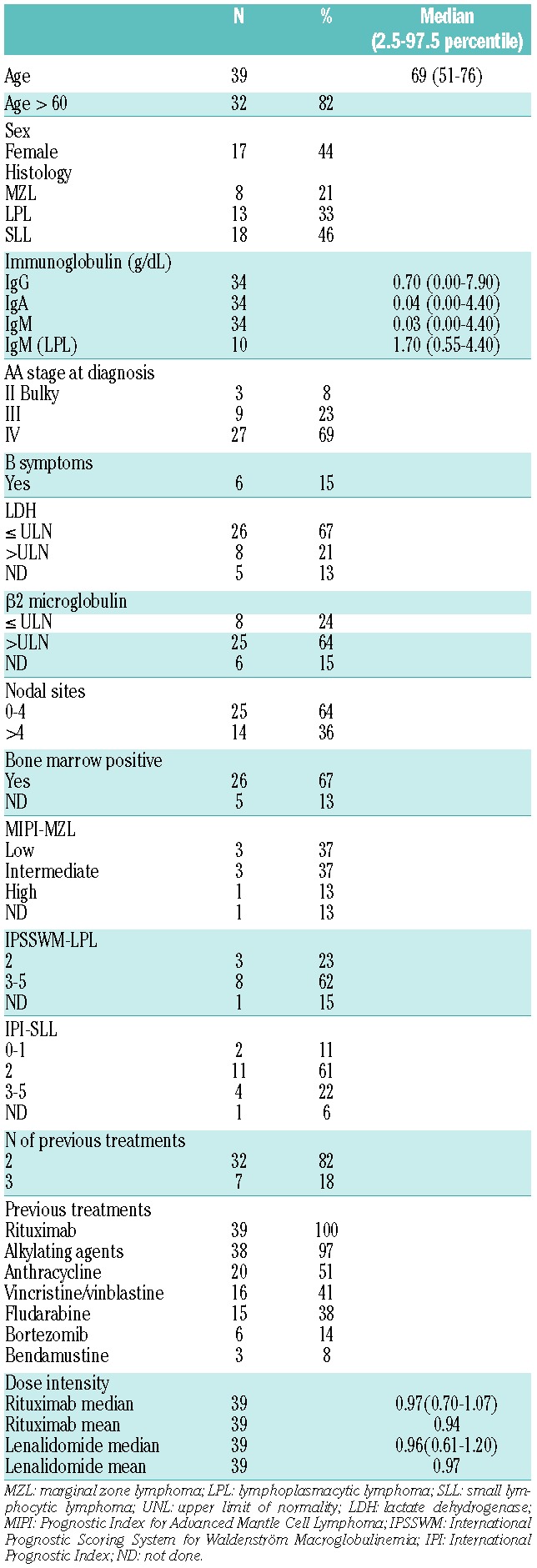
Between 2009 and 2011, 44 patients with relapsed MZL, LPL, or SLL were screened. Five patients were excluded from any response or survival outcome evaluation: 4 had declined consent before or at the beginning of the first cycle of therapy; 1 was excluded after a histology-based revision of the diagnosis. Thirty-nine patients were considered eligible and were included in the intent to treat analysis. Base-line clinical characteristics and prior therapies were recorded (Table 1).
Table 1.
Base-line characteristics, prior treatments and dose intensity (n=39).
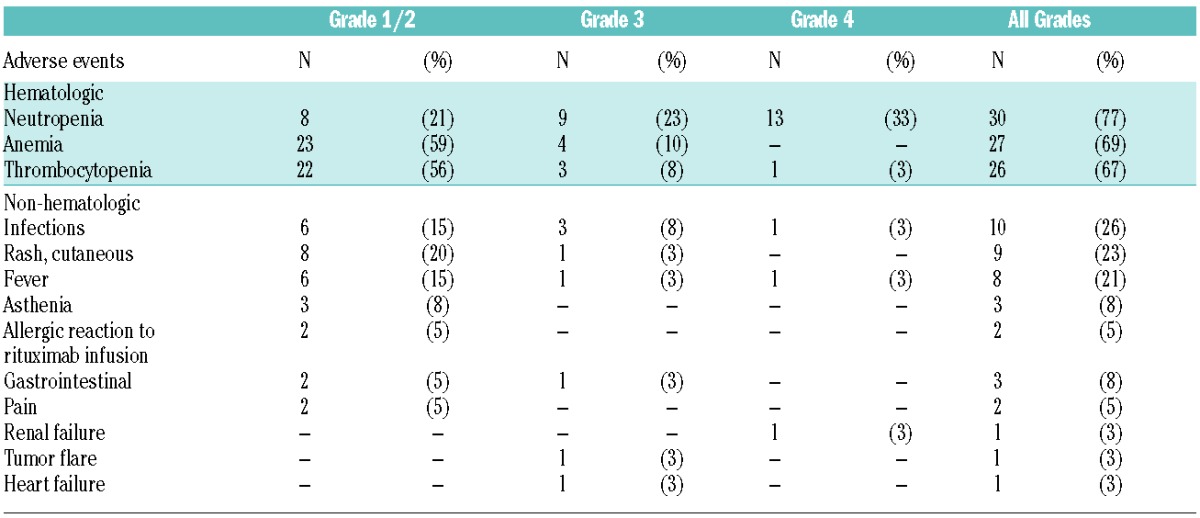
Toxicity was evaluable in 39 patients (Table 2) and treatment was generally well tolerated. The most common serious adverse events (grade >2) were neutropenia (56%), thrombocytopenia (10%), anemia (10%), and infection (10%). The median duration of neutropenia, WHO grade over 2, was 20 days (range 7–28 days). We observed 11 (28%) cases of grade 3 non-hematologic toxicity, including 4 cases of infection (10%), 2 cases of fever (5%), 1 case of tumor flare (3%), 1 case of dyspnea (3%), 1 case of renal failure (3%), 1 case of cutaneous rash (3%), and 1 case of heart failure (3%). No patient died of toxicity during the treatment. Over Grade 2 neutropenia was more frequent between cycles 2 to 5, and about 30% of patients required G-CSF support during cycles 2 to 6.
Table 2.
The most common adverse events associated with lenalidomide plus rituximab, graded according to the National Cancer Institute common toxicity criteria classification (NCI CTCAE version 3.0, http://ctep.cancer.gov/reporting/ctc.html); n=39.
Fifteen patients had reductions in the lenalidomide dose, 10 from 20 to 10 mg/day and 5 from 20 to 5 mg/day; furthermore, 2 patients started lenalidomide and rituximab after a one-week delay.
Out of 39 patients, 21 patients achieved CR, CRu, or PR; the ORR was 54% (95%CI: 37–70) and the TCR was 72% (95%CI: 55–85). In particular, 5 (13%, 95%CI: 4–27), 2 (5%, 95%CI: 1–17), and 14 (36%, 95%CI: 21–53) patients achieved CR, CRu, and PR, respectively. Seven patients (18%, 95%CI: 7–34) achieved SD, and 6 patients (15%, 95%CI: 6–30) showed disease progression. Five patients (13%, 95%CI: 4–27) withdrew from the study before the first 3 cycles of therapy without response assessment and were considered early failure or withdrawal. After a median follow up of 35 months (range 6–73 months), we observed 14 (36%) deaths. The median PFS was 22 months (95%CI: 11–39) and the median DoR was 34 months (95%CI: 14 months to not assessable). The median OS was 49 months (95%CI: 35 months to not assessable). At two years, estimated OS, PFS, and DoR, were 78% (95%CI: 58%–89%), 45% (95%CI: 27%–62%), and 76% (95%CI: 48%–90%), respectively (Figure 1).
Figure 1.
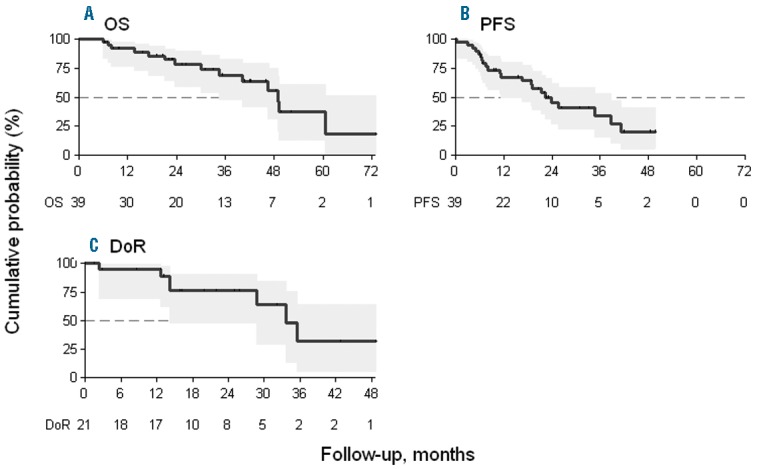
Kaplan-Meier estimates of (A) overall survival (OS) and (B) progression-free survival (PFS) of the 39 patients included in the study. (C) Duration of remission (DoR) of the 21 patients who obtained complete (CR) or partial (PR) responses at the end of therapy.
The evaluation of treatment response by histology showed an ORR of 75%, 46%, and 50% for MZL, LPL and SLL, respectively (P=0.492).
By histology, the estimated PFSs at two years were 75% (95%CI: 31%–93%) for MZL, 54% (95%CI: 15%–82%) for LPL, and 28% (95%CI: 9%–51%) for SLL; the log rank test showed no significant difference between these survival rates (P=0.144). The estimated DoR at two years by histology was 100% for MZL, 100% for LPL, and 51% (95%CI: 16%–82%) for SLL; the log rank test between MZL plus LPL curve and SLL curve was marginally statistically significant (P=0.056).
In a Phase II study, lenalidomide monotherapy induced sustained responses in patients with recurrent/refractory indolent NHL,5 and the median DoR was longer than 16.5 months. The CALGB 50401 Phase II study randomized 89 patients with recurrent follicular lymphoma to lenalidomide alone or in combination with rituximab. The combination was more active than lenalidomide alone, showing ORRs of 76% versus 53%, CR rates of 39% versus 20%, and event-free survivals (EFSs) of 2.0 years versus 1.2 years, respectively.10 A recently published11 Phase II study tested lenalidomide plus rituximab for treating relapsed/refractory indolent NHL. The results showed an ORR of 74%, with 44% CR, and a DoR of 15.4 months. These two combination studies included 73 patients with relapsed/refractory indolent lymphoma. Among patients, the large majority had follicular lymphoma (FL); only 3 patients had SLL, and one had MZL.
Our study included 8, 13, and 18 patients with MZL, LPL, and SLL, respectively. Patients with MZL and LPL showed better responses, DoRs, and EFSs than patients with SLL, and were consistent with results from a large Phase II study conducted with previously untreated patients with advanced-stage indolent NHL who were treated with the lenalidomide and rituximab combination;12 the ORRs based on histology were 98%, 89%, and 80% in FL, MZL, and SLL, respectively. Similar results were found in a study presented at the 2014 ASCO meeting,13 which used the same combination of lenalidomide and rituximab, a CR of 72% was achieved in 57 patients with previously untreated FL. Our results demonstrated that patients with MZL and LPL who responded to the combination treatment had long-lasting responses. The combination treatment appeared to have less efficacy in SLL. In conclusion, our findings show that the combination treatment of lenalidomide and rituximab was also active in heavily pre-treated patients with indolent NHL
The efficacy of this combination, in terms of either the percentage and quality of responses or the DoR, was very good in patients with MZL. Also, we found that the DoR was good in patients with LPL. Of note, no previous study has published the effects of this combination in patients with relapsed LPL. Our results showed a mild, predictable toxicity profile, substantially similar to those observed in previous studies on previously untreated patients.12–13 Our findings should be interpreted with caution because they reflect results of a multicenter Phase II study that included a relatively low number of patients. However, the high efficacy and relatively low toxicity of the combination treatment in patients with indolent NHL were previously shown in other studies, both in patients who had relapsed and in previously untreated patients.11–13 If these results are confirmed in Phase III trials,11–13,14 such as the pivotal NCT 01476787 trial, we may move towards a chemotherapy-free approach in the management of indolent NHL, which would finally turn a dream into reality.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Mclaughlin P, Grillo-Lopez AJ, Link BK, et al. Anti-Cd20 Monoclonal Antibody Therapy For Relapsed Indolent Lymphoma: Half Of Patients Respond To A Four-Dose Treatment Program. J Clin Oncol. 1998;16(8):2825–2833. [DOI] [PubMed] [Google Scholar]
- 2.Sacchi S, Pozzi S, Marcheselli L, et al. Introduction of rituximab in front-line and salvage therapies has improved outcome of advanced-stage follicular lymphoma patients. Cancer. 2007;109(10):2077–2082. [DOI] [PubMed] [Google Scholar]
- 3.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood. 2014; 123(19):2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140(1):36–45. [DOI] [PubMed] [Google Scholar]
- 5.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol. 2009;27(32):5404–5409. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84(9):553–559. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization: Classification of tumors of Haematopoietic and Lymphoid Tissues. Lyon, France, IARC Press; 2008. [Google Scholar]
- 8.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 9.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14(4):357–379. [DOI] [PubMed] [Google Scholar]
- 10.Leonard JP, Sin-Ho Jung SH, Jeffrey J, et al. Randomized Trial of Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With Recurrent Follicular Lymphoma: CALGB 50401 (Al-liance). J Clin Oncol. 2015;33(31):3635–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014;165(3):375–381. [DOI] [PubMed] [Google Scholar]
- 12.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15(12):1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin P, Jung SH, Johnson JL, et al. CALGB 50803 (Alliance): A phase II trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma. J Clin Oncol. 2014;32: 5s (Suppl) (Abstract 8521). [Google Scholar]
- 14.Buske C. Towards a chemotherapy-free approach in indolent lymphoma. Lancet Oncol. 2014;15(12):1281–1282. [DOI] [PubMed] [Google Scholar]


