Erythropoiesis is arranged in erythroblastic islands (Ery-Is),1 the specialized niche known for more than 50 years2 in which erythroid precursors proliferate and differentiate. However, the significance of erythropoiesis in anemia, the leading symptom of myelodysplastic syndromes (MDS), has remained unclear. Evaluating bone marrow biopsies (BMBs) from patients with MDS, we detected alterations of Ery-Is in a significant proportion of patients, and this was independently associated with severe anemia and poor prognosis of disease, a novel observation.
We evaluated the diagnostic BMBs from a total of 317 patients with newly diagnosed MDS according to the French-American-British (FAB) classification.3 Patients were monitored and received best supportive care in more than 20 hematology centers. Diagnosis according to FAB classification was: refractory anemia (RA), n=109; RA with ring sideroblasts (RARS), n=36; RA with excess of blasts (RAEB), n=82; RAEB in transformation (RAEB/t), n=40; chronic myelomonocytic leukemia (CMML), n=50; World Health Organization (WHO)4 RA with ring sideroblasts (RARS), n=11; refractory cytopenia with unilineage dysplasia (RCUD), n=32; MDS with isolated del(5q), n=19; MDS, unclassified (MDS U), n=3; refractory cytopenia with multilineage dysplasia (RCMD), n=66; RAEB-1, n=37; RAEB-2, n=37; CMML-1, n=34; CMML-2, n=27; RARS with thrombocytosis (RARS-T) or myelodysplastic/myeloproliferative neoplasms, unclassified (MDS/MPN), n=18; AML with MDS-related changes, n=14; therapy-related MDS (t-MDS), n=19. Fifty-two subjects with normal healthy marrow served as control (for further details see Online Supplementary Appendix).
Ery-Is are composed of a central macrophage surrounded by and binding to nucleated erythroid cells (NEC).5,6 Normoblasts were marked using anti-hemoglobin-A (HbA), HbA- NEC using anti-glycophorin-C and -CD71 antibodies (for details see Online Supplementary Appendix). To overcome difficulties in separating confluencing or adjacent Ery-Is (Online Supplementary Table S2), dimension and numerical density of Ery-Is were determined indirectly by a statistical approach. Each biopsy section was divided into 20 areas (“A”) of equal size, and each area into 100 adjacent virtual squares (“sq”) of 30.5 mm², each comprising approximately 10 nucleated cells (measuring oculars), amounting to a total count of approximately 20,000 nucleated cells per biopsy section. Squares with 50% or more NEC were determined as “sq+”. Each area “A” served as a block with similar proportions of marrow zones (proliferation/differentiation zones) to eliminate their possible influence (block design). Numerical density and dimension of Ery-Is were determined by analysis of variance components7 assuming that differences in the sq+ counts between areas “A” result from clustering of NEC within Ery-Is (randomly-positioned clusters model), defining Ery-Is as clusters of one or more adjacent sq+, each with at least 5 NEC. The validity of this model was checked by a compound Poisson model, handling the total counts of NEC as the product of the number of sectioned NEC per Ery-I and the number of Ery-Is being hit by a marrow section. The results from both statistical approaches were indistinguishable (r=0.998, 156 randomly selected cases) and could be reproduced by a second investigator in a blinded evaluation procedure (Online Supplementary Table S2).
Since the interaction between macrophage and NEC appears to be essential for the differentiation of erythropoiesis,1,5,8,9 Ery-Is were also evaluated for the existence of a central macrophage marked by anti-CD68 (PGM110 all cases) and anti-CD169 antibody8 (10% of cases) (Online Supplementary Table S2).
Healthy marrow differed significantly from MDS in all the alterations of erythropoiesis (Online Supplementary Table S3). Impaired maturation of erythropoiesis, dysplastic features in more than 10% of NEC and apoptosis in more than 1% of NEC were not observed in healthy marrow; however, these occurred in 92.0%, 60.3% and 49.5% of MDS patients. Differences with respect to Ery-Is were initially less obvious since Ery-Is were detectable in MDS as well as in healthy marrow (Figure 1A–F). In healthy marrow, Ery-Is changed significantly according to age: in subjects older than 60 years of age, their numerical density progressively declined accompanied by a progressive increase in dimension (P<0.0005) (Figure 1C and F, and Figure 2A and B), similar to MDS. Senescence, an increased risk of clonal hematopoiesis in normal elderly individuals with mutations similar to those observed in MDS,11,12 or aging of the hematopoietic stem cell niche13,14 might be an explanation. In MDS, however, the alterations of Ery-Is did not depend on patient age (P>0.05) (Table 1 and Figure 1C and F). They significantly exceeded that observed in aging healthy marrow and were associated with progressive anemia (P<0.000005) (Table 1), whereas the alterations of Ery-Is in aging healthy marrow were not associated with anemia; all persons with healthy marrow showed normal Hb values of peripheral blood (P>0.1). These differences between MDS and healthy marrow were independent of patient sex and age (Table 1 and Figure 1A–F).
Figure 1.
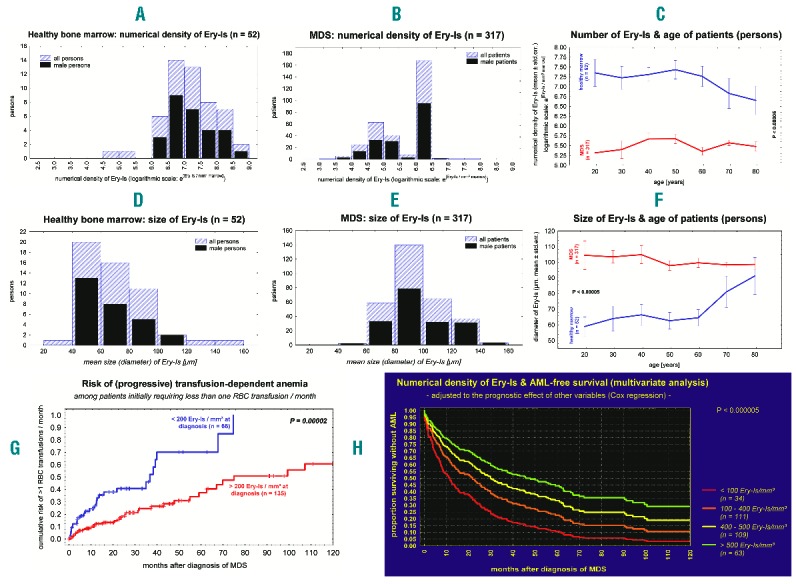
Alterations of Ery-Is in myelodysplastic syndromes (MDS) compared to healthy bone marrow (BM) and their prognostic relevance. In MDS, the numerical density of Ery-Is within marrow was significantly lower than in healthy BM (A–C) whereas Ery-Is were enlarged (D–F; P<0.000001). These differences were independent of patient sex (A, B, D, E; P>0.05). In healthy marrow, numerical density and size of Ery-Is changed significantly with age (C, F; P<0.001; blue lines) whereas the alterations observed in MDS were independent of age (C, F; P>0.05; red lines). In healthy marrow as well as in MDS, the size of Ery-Is (mean diameter) correlated inversely with their numerical density within marrow (P<0.000001). The risk of transfusion-dependent anemia and acute myeloid leukemia (AML)-free survival time significantly depended on the numerical density of Ery-Is within BM (G, H; P<0.00002).
Figure 2.
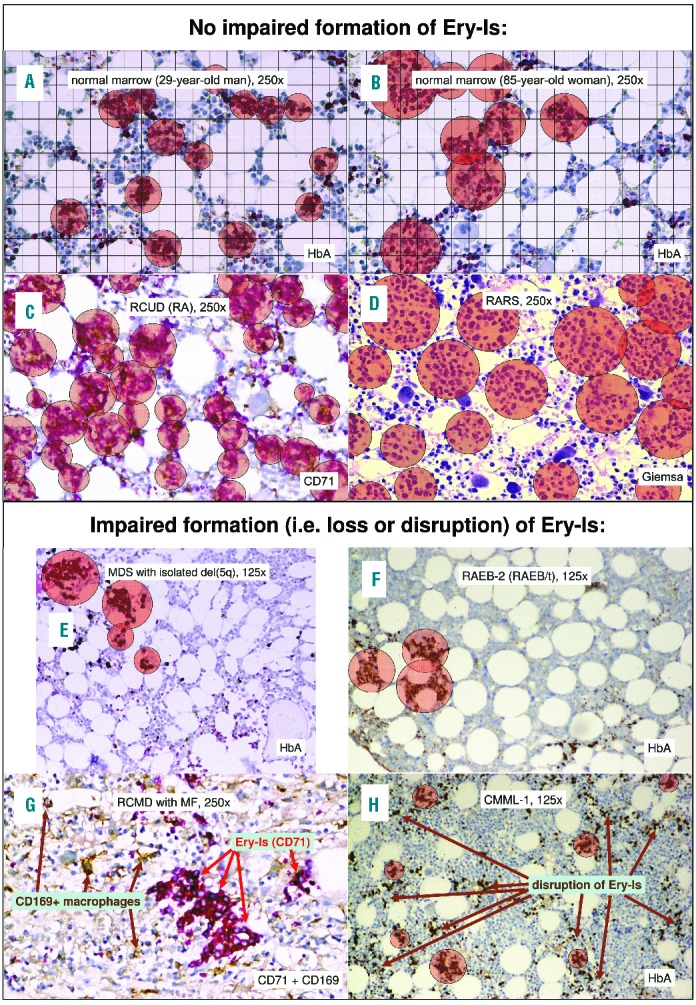
Alterations of Ery-Is and myelodysplastic syndromes (MDS) subtype. Alterations of Ery-Is differed significantly from those observed in healthy marrow from younger subjects (A) but resembled those observed in older subjects with healthy marrow (B). Alterations of Ery-Is varied significantly between different subtypes of MDS considering size, numerical density, loss and disruption of Ery-Is (C-H; P<0.000001). Significant loss or disruption of Ery-Is resulting in a decrease in the numerical density of Ery-Is to values lower than the 2.5% percentile observed in healthy marrow were detected in all subtypes except for refractory anemia with ring sideroblasts (RARS) (D) with the highest risk in MDS with del(5q), refractory anemia with excess of blasts (RAEB-2), MDS with myelofibrosis (MDS-MF), chronic myelomonocytic leukemia (CMML) and acute myeloid leukemia (AML) with MDS-related changes (E–H). In more than 50% of MDS patients, impaired formation of Ery-Is was associated with a dissociation of central macrophages from more than 10% of Ery-Is (G); this was independent of whether anti-CD68 (PGM1, all cases) or anti-CD169 (10% of cases) antibody was applied to identify the central macrophages (G).
Table 1.
Influences on numerical density and size of Ery-Is, the degree of anemia, and prognosis of myelodysplastic syndromes (MDS) dependent on the findings on the day of diagnosis: only variables remaining significant in multivariate analyses were presented (the results from univariate analyses and an overview of all the variables taken into consideration are listed in the Online Supplementary Tables S4–S6). Multivariate Poisson and linear regression analyses, analysis of variance components, and Cox proportional hazards regression; stepwise regression; βi = regression coefficient (Poisson regression); Δμm=change in mean diameter in the case of a subtype, a karyotype aberration, or a one-log increase of the numerical density of Ery-Is. ΔHb=mean change in the hemoglobin (Hb) level of peripheral blood (g/dL) in the case of alterations of erythropoiesis, occurrence of MF, or a del(5q); VC=estimated proportion (in %) of variance in the deviation of the Hb value of peripheral blood from the normal Hb level of the control group attributable to a variable. HR: hazard ratio.
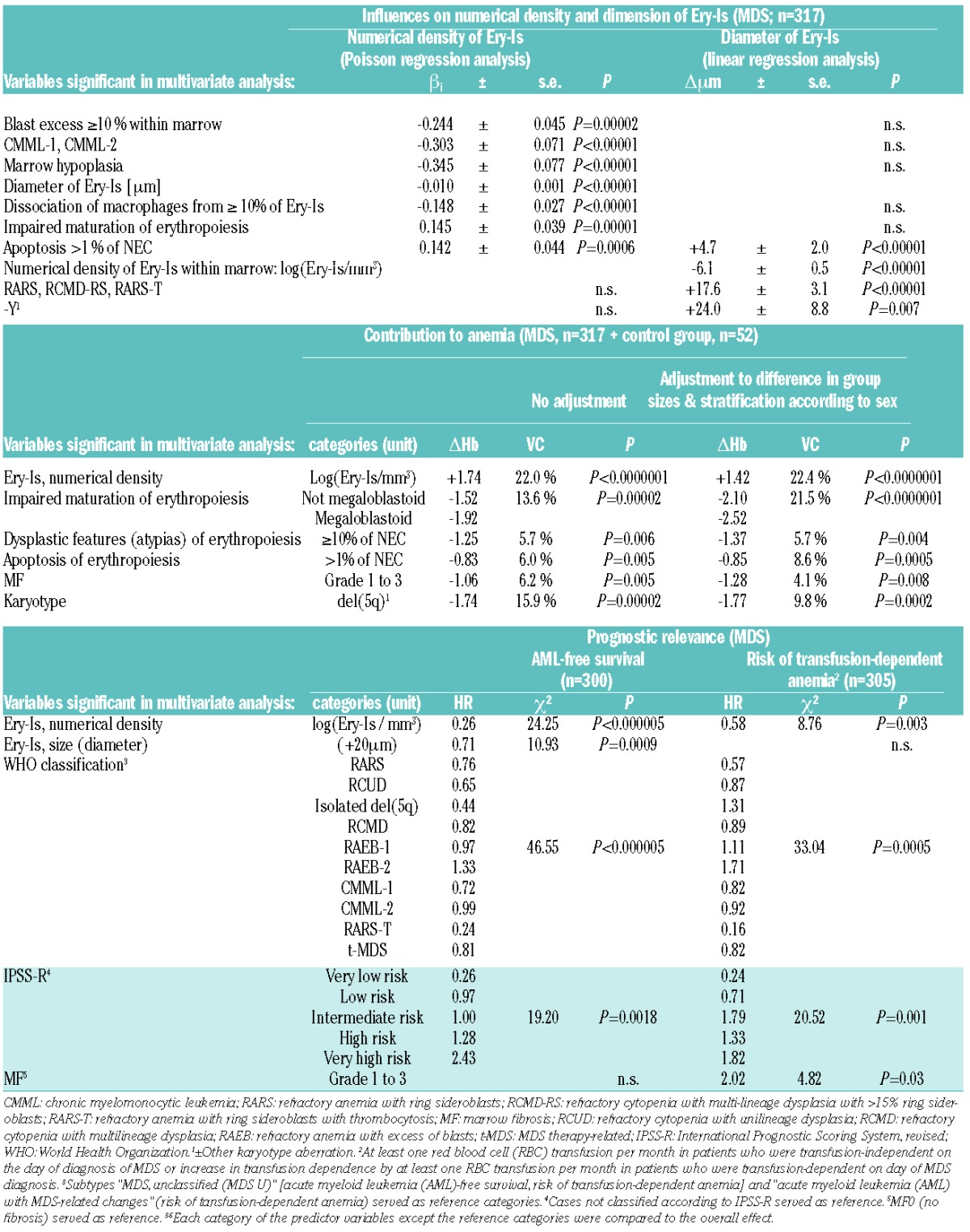
The Ery-Is in patients with more than 15% ring sideroblasts within erythropoiesis, a loss of the Y chromosome, or more than 1% apoptotic NEC were significantly larger than in other MDS patients (Table 1 and Figure 2D). However, enlargement of Ery-Is that exceeded 100 mm in mean diameter in approximately one-third of patients was not an independent predictor of anemia or transfusion dependence (P>0.05) (Table 1).
Impaired formation of Ery-Is with reduction in their number due to loss or disruption of this entity was observed in 45.7% of patients (Figure 1B and Figure 2E–H). In 30.3%, impaired formation of Ery-Is resulted in a marked reduction in their numerical density down to values lower than the 2.5% percentile of healthy marrow, a feature associated with a marked increase in monopoiesis (CMML), excess of blasts of 10% or more within marrow, occurrence of MF, or marrow hypoplasia (Table 1 and Figure 2F–H), whereas impaired maturation or increased apoptosis rate of erythropoiesis were associated with increased numbers of Ery-Is within marrow (Table 1 and Figure 2C and D). In 8% of MDS patients, impaired formation of Ery-Is was the only obvious alteration of erythropoiesis.
In 72.6% of patients, a central macrophage was lacking in 10% or more of Ery-Is (Figure 2G); this was independently associated with reduced numbers of Ery-Is within marrow (P<0.00005) (Table 1). A further difference to healthy marrow. In healthy marrow, the number of Ery-Is-associated macrophages and the number of Ery-Is within marrow were similar (P>0.1). Association of (usually iron-presenting) macrophages with Ery-Is indistinguishable (i.e. P>0.05) from that in healthy marrow was observed in 27.4% of MDS patients.
Impaired formation resulting in reduced numbers of Ery-Is showed the most significant influence on the Hb level of blood contributing to 20%-25% of the degree of anemia, independently of all other alterations of erythropoiesis (P<0.00001) (Table 1); this was the only alteration of erythropoiesis predicting the risk of (progressive) red blood cell (RBC) transfusion dependence independently of the type of disease, the risk group (IPSS-R),15 and the occurrence of MF (Table 1 and Figure 1G), a further novel observation. Hematopathological evaluations of BMBs in MDS patients should, therefore, include a statement on the numerical density of Ery-Is within marrow, especially in cases with a marked reduction of this entity.
In summary, Ery-Is appear to be significantly altered in many patients with MDS, and these alterations seem to play a central role in the evolution of severe transfusion-dependent anemia in this type of disease. The background to this may be a differentiation defect at the level of an early erythroid precursor cell, responsible for the production of Ery-Is, and/or an impaired interaction between macrophages and NEC,8 thus impairing the formation of Ery-Is, as suggested by our observation of a dissociation of macrophages from a small proportion (≥10%) of Ery-Is in the majority of patients.
Impaired formation of Ery-Is may be the only pathological change in erythropoiesis in a minority of MDS patients. In the majority of MDS patients, the numerical density of Ery-Is seems to be normal or increased, and the association of increased numbers of Ery-Is with an impaired maturation or increased apoptosis of NEC may indicate a compensatory role of an increased formation of Ery-Is, explaining the increase in erythropoiesis in many of these patients. Disruption or loss of Ery-Is, as ob-served in more than 40% of patients, however, hinder a compensatory increase in Ery-Is production, and in 30% of patients, loss of Ery-Is appears to be the most obvious pathological alteration of erythropoiesis prior to the occurrence of severe transfusion-dependent anemia. Failure of this compensatory mechanism seems to be an independent unfavorable prognostic factor in patients receiving best supportive care, not only with respect to the risk of transfusion-dependence, but also to the risk of AML and shortened survival time (Table 1 and Figure 1H), another novel observation. Considering this background, the relevance of Ery-Is and their alterations to anemia and prognosis in MDS appear to have been, until now, underestimated.
Our observations were based on patients treated with best supportive care, thus representing the natural course of disease. Further evaluation of alterations of Ery-Is and their significance in patients receiving disease-modifying therapy regimes are required before these observations can be translated into clinical practice. According to our observations, the erythropoietic niche seems to be worthy of consideration in the development of therapy and prognostic scoring systems for patients with MDS.
Acknowledgments
The Authors would like to thank the numerous clinical colleagues in Germany who supported these evaluations by sending and validating the clinical and hematologic data of their patients recruited into this study. We furthermore thank Mahtab Taleb-Naghsh, Regina Lohmann, Marita Markwart, Nicole Cramer, and Michael Engel for their excellent technical assistance and Ms Gillian Teicke for proof-reading this manuscript.
Footnotes
The online version of this letter has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessis M. [L’ îlot érythroblastique. Unité functionelle de la moelle osseuse]. Rev Hematol. 1958;13(1):8–11. [PubMed] [Google Scholar]
- 3.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51(2):189–199. [PubMed] [Google Scholar]
- 4.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press Lyon; 2008. [Google Scholar]
- 5.Rhodes MM, Kopsombut P, Bondurant MC, Price JO, Koury MJ. Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood. 2008;111(3):1700–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee G, Lo A, Short SA, et al. Targeted gene deletion demonstrates that the cell adhesion molecule ICAM-4 is critical for erythroblastic island formation. Blood. 2006; 108(6):2064–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson CR. Estimation of variance and covariance components. Biometrics. 1953;9(2):226–252. [Google Scholar]
- 8.Chow A, Huggins M, Ahmed J, et al. CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat Med. 2013;19(4):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falchi M, Varricchio L, Martelli F, et al. Dexamethasone targeted directly to macrophages induces macrophage niches that promote erythroid expansion. Haematologica. 2015;100(2):178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falini B, Flenghi L, Pileri S, et al. PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol. 1993; 142(5):1359–1372. [PMC free article] [PubMed] [Google Scholar]
- 11.Busque L, Patel JP, Figueroa ME, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44(11):1179–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura-Ishizu A, Suda T. Aging of the hematopoietic stem cells niche. Int J Hematol. 2014;100(4):317–325. [DOI] [PubMed] [Google Scholar]
- 14.Vas V, Senger K, Dörr K, Niebel A, Geiger H. Aging of the microenvironment influences clonality in hematopoiesis. PLoS One. 2012; 7(8):e42080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 20;120(12):2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]


