Current therapies for anemia associated with high hepcidin have limited success. Humanized monoclonal antibodies targeting hemojuvelin decrease hepcidin and improve anemia in three preclinical models. The antihemojuvelin antibodies were successfully applied to a mouse and a rat model of ACD (anemia of chronic disease), and to a genetic mouse model of IRIDA (iron refractory iron deficiency anemia). The hemojuvelin antibodies are effective in suppressing hepcidin and increasing hemoglobin (Hb) in inflammatory and non-inflammatory states caused by excess hepcidin and represent new therapeutic candidates for patients suffering from ACD and IRIDA.
Hepcidin is a peptide hormone that plays a major role in iron homeostasis as it inhibits the release of iron into the circulation from duodenal enterocytes, macrophages and hepatocytes.1 Therefore, increased hepcidin expression causes anemia while low hepcidin leads to iron overload. Hepcidin expression is stimulated via the bone morphogenetic receptor (BMPR) and co-receptor, repulsive guidance molecule c/ hemojuvelin (RGMc/HJV), neogenin and interleukin (IL)-6 signaling pathways. The RGM family consists of three members: a, b and c. RGMc/HJV controls systemic iron homeostasis,2 while RGMa and b are involved in neural network formation and stabilization.3,4 RGMa and RGMc/HJV are 47% identical.5 RGMc/HJV will from now on be referred to as HJV in this manuscript.
Two clinical manifestations of inappropriately high hepcidin include anemia of chronic disease (ACD) and iron refractory iron deficiency anemia (IRIDA). ACD is common in patients suffering from a variety of persistent inflammatory diseases, where hepcidin together with inflammatory cytokines contribute to the complex pathophysiology of the disease, while low serum iron and iron-restricted erythropoiesis contribute to morbidity.6,7 On the other hand, in IRIDA patients high hepcidin is caused by mutations affecting the TMPRSS6 gene encoding the transmembrane serine protease, Matriptase-2,8,9 that down-regulates hepcidin by cleaving HJV.10 HJV cleavage interferes with BMP binding to the BMPR and decreases the hepcidin transcription.11
Current therapies for ACD include blood transfusions, erythropoietin stimulating agents or parenteral iron injections, however, they are associated with potential hazards and limited success.12–14 IRIDA is generally refractory to oral iron treatment but shows a slow response to intravenous iron injections and partial correction of the anemia.15 Therefore, novel therapies are needed. The lack of safe and effective therapies for diseases associated with high hepcidin and the essential role of HJV in systemic iron homeostasis make HJV an excellent therapeutic target for hepcidin suppression.
Two humanized anti-RGM monoclonal antibodies (mAbs), h5F9.23 and h5F9-AM8 designed to target HJV were successfully applied to a mouse and a rat model of ACD, and to a genetic mouse model of IRIDA.
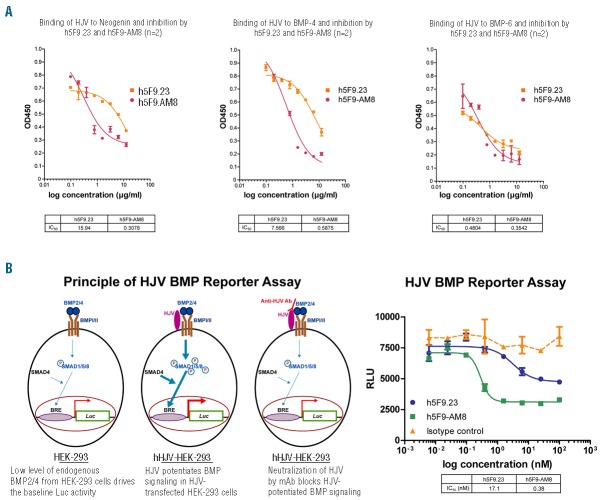
The mAbs react with human, rat and mouse HJV and in vitro inhibit the interaction between HJV and the ligands of the BMP receptors (BMP-4 and BMP-6) and neogenin in a binding assay (Figure 1A, Online Supplementary Table S1). To evaluate the inhibitory effect of the mAbs on the BMP pathway, a luciferase reporter assay was used. HEK-293 cells transfected with HJV showed increased luciferase reporter gene expression, which was strongly decreased by treatment with h5F9.23 or h5F9-AM8 (Figure 1B). In the reporter assay, h5F9.23 inhibited luciferase expression with an IC50 of 17.1 nM whereas h5F9-AM8 did so with an IC50 of 0.38 nM.
Figure 1.
Specificity and activity of anti-HJV mAbs in vitro. (A) The monoclonal HJV antibodies inhibit the interaction with Neogenin, BMP-4 and BMP-6 in a receptor binding assay. Number of replicate experiments n=2. (B) The monoclonal HJV antibodies effectively neutralize the effect of HJV in a luciferase reporter assay. Bone morphogenetic protein (BMP), BRE (BMP response element), relative light units (RLU), luciferase (Luc), mothers against decapentaplegic, drosophila homolog of (SMAD). Number of replicate experiments n=3. Data in Figure 1 is presented as mean±SEM.
The first in vivo characterization of the mAbs, including toxicology studies, was carried out in healthy rats and cynomolgus monkeys and results demonstrated that the mAbs have a long–lasting effect and an excellent safety profile.16 Herein, the mAbs were tested in a rat model of chronic arthritis17 and a mouse model of aseptic inflammation18 as well as in a non-inflammatory model of high hepcidin, the Tmprss6−/− mouse.19 In the rat model, arthritis with normocytic, normochromic anemia and low serum iron manifested 3 weeks after a single ip injection of the peptidoglycan-polysaccharide (PGPS-10).17 At this time point, treatment started once per week for 4 weeks with a 20 mg/ kg iv dose of mAbs. Weekly Hb measurements demonstrated that the h5F9-AM8 mAb already significantly improved Hb levels after the second injection and the effect lasted for around 2 weeks (Figure 2A). Complete blood counts from this study are summarized in Online Supplementary Table S2. To evaluate whether h5F9-AM8 suppresses inflammation-induced Hamp mRNA in another species, we used a murine inflammatory model.
Figure 2.
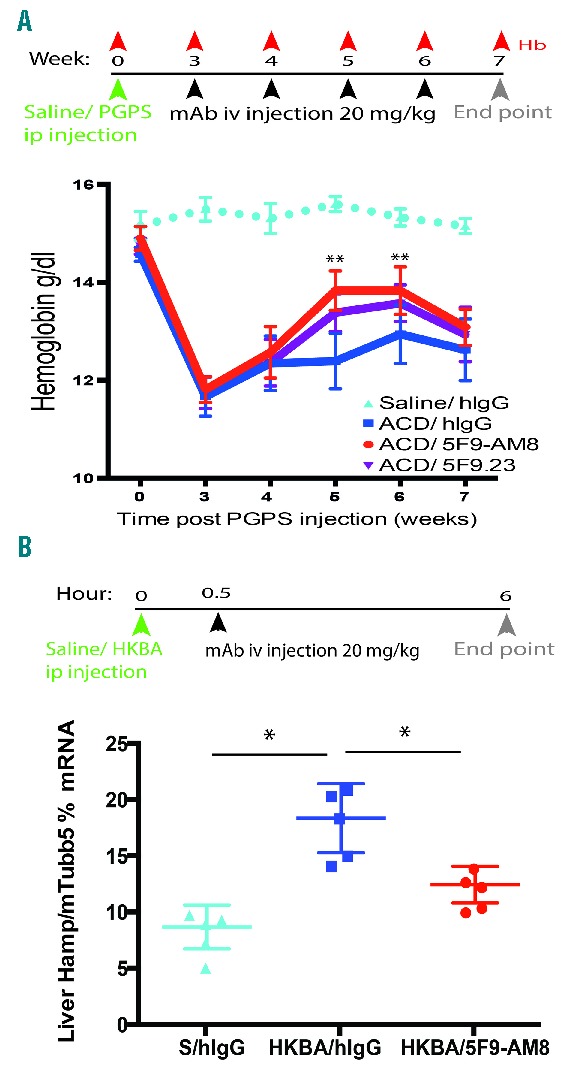
Anti-HJV antibody improves hemoglobin and decreases Hamp in inflammatory animal models of high hepcidin. (A) Schematic summarizing the rat ACD peptidoglycan-polysaccharide (PGPS)-10 protocol and hemoglobin profile over the course of the experiment. Recovery of hemoglobin was observed in rats treated with h5F9.23 and h5F9-AM8, peaking after the second and third mAb injections (n=16–18/group). Data analyzed with Least Squares means of the change from baseline were estimated using Mixed Model, **P<0.001. (B) Schematic summarizing the heat-killed Brucela abortus antigen (HKBA) experimental protocol. h5F9-AM8 significantly decreased liver Hamp mRNA expression in mice with inflammation, *P<0.05 (n=6/group). Mouse tubulin 5 (mTubb5). Data is presented as mean±SEM.
A partial improvement of anemia was previously reported in the heat-killed Brucela abortus (HKBA) model following anti-hepcidin antibody treatment.18,20 Follow-up studies characterizing the HKBA model demonstrated that mice develop both acute and chronic inflammation, with an early rise in hepcidin mRNA and a peak in mRNA expression 6 hours following a HKBA injection. To evaluate the effect of h5F9-AM8 on inflammation-induced Hamp mRNA expression, h5F9-AM8 was administered 30 minutes after a HKBA injection and liver Hamp mRNA was measured after 6 hours. Our results also show the increase in Hamp mRNA 6 hours after the HKBA injection and, importantly, h5F9-AM8 significantly decreased hepcidin mRNA compared to hIgG-treated mice (Figure 2B). These data further support the notion that anti-HJV antibodies decrease inflammation-induced hepcidin expression.
To directly test whether h5F9-AM8 antagonizes the decrease in hemoglobin caused by hemojuvelin-induced hepcidin expression in the non-inflammatory, genetic mouse model of IRIDA,19 Tmprss6−/− mice were used. Remarkably, a single iv injection of 20 mg/kg h5F9-AM8 increased Hb in Tmprss6−/− compared to hIgG-treated Tmprss6−/− mice. Hb recovery peaked 2 weeks after antibody injection and slowly declined over 8 weeks (Figure 3A). We observed no histopathological abnormalities in the spleen such as iron pigmentation, peritonitis, necrosis, fibrosis, follicle atrophy, follicle regeneration, regenerative hematopoiesis; or in the liver such as periportal iron pigmentation, peritonitis, fibrosis, focal necrosis and Kupffer cell activation following mAb treatment.
Figure 3.
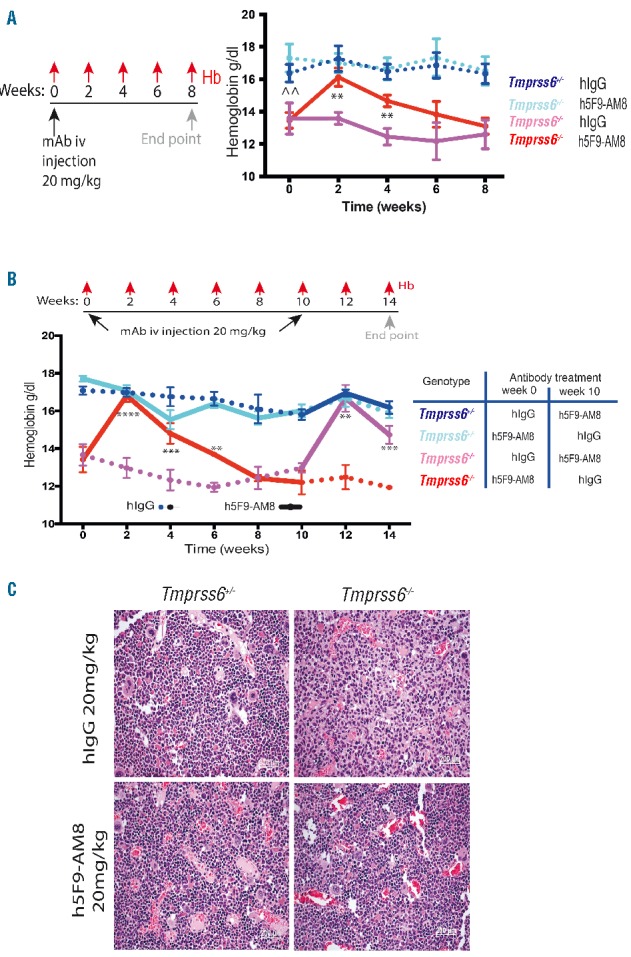
Anti-HJV antibody increases hemoglobin and recovers erythropoiesis in a non-inflammatory high hepcidin model. (A) Schematic summarizing experimental protocol (n=10). At 0 weeks hemoglobin levels of the homozygous Tmprss6−/− was significantly lower than the heterozygous Tmprss6+/−. At week 2–6 the Tmprss6−/− mice had a significant increase in hemoglobin levels in comparison to the Tmprss6−/− hIgG mice. (B) Cross-over experiment (n=5). Homozygous Tmprss6−/−mice treated first by the humanized istotype control mAb hIgG were treated at week 10 with 20 mg/kg iv h5F9-AM8. Anemia was corrected again in weeks 12 and 14. Change from baseline data over time were analyzed using mixed model (fitting change from baseline by timepoint and using baseline as a covariate), **P<0.01, ***P<0.001, ****P<0.0001. Statistical analysis shown is for comparisons between Tmprss6−/− mice treated with hIgG and h5F9-AM8. Statistical analysis for comparisons between Tmprss6+/– and Tmprss6−/− mice treated with hIgG at time 0 is shown by ^^, P<0.01. Data in A and B are presented as mean±SEM. (C) Cells representing various stages of erythropoiesis in the bone marrow (H&E stained sections) are not present in Tmprss6−/− mice treated with hIgG. The bone marrow shows hypocellularity for this lineage. Following treatment with h5F9-AM8 at the 14 week time point, this status was improved and erythropoiesis was reactivated to a normal level. Images taken at 20× magnification, scale bars 50mm.
In a separate experiment, mice were injected first with h5F9-AM8 (or hIgG). After 10 weeks, the treatment groups were crossed-over such that the mice previously treated with hIgG were injected with h5F9-AM8, and vice versa (Figure 3B). Hb levels were monitored every 2 weeks over 14 weeks. In Tmprss6−/− mice, Hb levels increased by 2 weeks and remained elevated for up to 6 weeks. By 8 weeks Hb was back down to pre-injection levels. In animals treated with hIgG for the first 8 weeks, Hb levels remained low. The injection of h5F9-AM8 at week 10 induced a rapid increase in Hb in the Tmprss6−/− mice and this Hb increase was also observed in week 14 (Figure 3B, Online Supplementary Table S3). No histopathological abnormalities were observed regarding spleen and liver iron, and neither was iron accumulation observed in either organ. Bone marrow histopathology demonstrated that treatment with h5F9-AM8 can rescue erythropoiesis in homozygous Tmprss6−/− mice, while Tmprss6−/− mice treated with hIgG display a moderate to marked decrease of erythropoiesis (14 week time point) (Figure 3C). Although the efficiency of the antibody is limited by its short half-life in mice (4 days), it provides sufficient time to improve the hemoglobinization of erythrocytes.
This report demonstrates in pre-clinical models that anti-HJV antibodies have the potential to modulate hepcidin and improve Hb in inflammatory and non-inflammatory conditions. Furthermore, the anti-HJV antibodies appear to be non-toxic and, therefore, may be new therapeutic candidates for patients suffering from ACD and IRIDA.
Acknowledgments
We thank Mary Leddy, Philip Bardwell, Suju Zhong, and Chee-Ho Choi for the generation of anti-RGM antibodies. Many thanks also to Andrea Egly, Isabel Trommer and Alice Tischer for their outstanding support. We also thank Carlos Lopez-Otin for providing the Tmprss6 mutant mice, and the staff of the laboratory animal resources (LAR) at EMBL for their dedicated work, especially Klaus Schmitt for his outstanding technical support. We also thank V. Devanarayan for his statistical expertise.
Footnotes
The online version of this letter has a Supplementary Appendix.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. [DOI] [PubMed] [Google Scholar]
- 3.Niederkofler V, Salie R, Sigrist M, Arber S. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J Neurosci. 2004;24(4):808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samad TA, Srinivasan A, Karchewski LA, et al. DRAGON: a member of the repulsive guidance molecule-related family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive properties. J Neurosci. 2004;24(8):2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller BK, Yamashita T, Schaffar G, Mueller R. The role of repulsive guidance molecules in the embryonic and adult vertebrate central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1513–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001;91(12):2214–2221. [PubMed] [Google Scholar]
- 7.Nissenson AR, Goodnough LT, Dubois RW. Anemia: not just an innocent by stander? Arch Intern Med. 2003;163(12):1400–1404. [DOI] [PubMed] [Google Scholar]
- 8.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Falco L, Totaro F, Nai A, et al. Novel TMPRSS6 mutations associated with iron-refractory iron deficiency anemia (IRIDA). Hum Mutat. 2010;31(5):E1390–1405. [DOI] [PubMed] [Google Scholar]
- 10.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nili M, Shinde U, Rotwein P. Soluble repulsive guidance molecule c/hemojuvelin is a broad spectrum bone morphogenetic protein (BMP) antagonist and inhibits both BMP2- and BMP6-mediated signaling and gene expression. J Biol Chem. 2010;285(32):24783–24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson JW. The anemia of inflammation/malignancy: mechanisms and management. Hematology Am Soc Hematol Educ Program. 2008;159–165. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116(20):4045–4059. [DOI] [PubMed] [Google Scholar]
- 14.Del Vecchio L, Pozzoni P, Andrulli S, Locatelli F. Inflammation and resistance to treatment with recombinant human erythropoietin. J Ren Nutr. 2005;15(1):137–141. [DOI] [PubMed] [Google Scholar]
- 15.De Falco L, Sanchez M, Silvestri L, et al. Iron refractory iron deficiency anemia. Haematologica. 2013;98(6):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boser P, Seemann D, Liguori MJ, et al. Anti-repulsive Guidance Molecule C (RGMc) Antibodies Increases Serum Iron in Rats and Cynomolgus Monkeys by Hepcidin Downregulation. AAPS J. 2015;17(4):930–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theurl I, Schroll A, Sonnweber T, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118(18):4977–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasu BJ, Cooke KS, Arvedson TL, et al. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115(17):3616–3624. [DOI] [PubMed] [Google Scholar]
- 19.Folgueras AR, de Lara FM, Pendas AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–2545. [DOI] [PubMed] [Google Scholar]
- 20.Cooke KS, Hinkle B, Salimi-Moosavi H, et al. A fully human antihepcidin antibody modulates iron metabolism in both mice and nonhuman primates. Blood. 2013;122(17):3054–3061. [DOI] [PubMed] [Google Scholar]