Abstract
Natural killer cells are key cells of the innate immune system. Natural killer cell receptor repertoires are diversified by a stochastic expression of killer-cell-immunoglobulin-like receptors and lectin-like receptors such as NKG2 receptors. All individuals harbor a subset of natural killer cells expressing NKG2A, the inhibitory checkpoint receptor for HLA-E. Most neoplastic and normal hematopoietic cells express HLA-E, the inhibitory ligand of NKG2A. A novel anti-human NKG2A antibody induced tumor cell death, suggesting that the antibody could be useful in the treatment of cancers expressing HLA-E. We found that immunodeficient mice, co-infused with human primary leukemia or Epstein-Barr virus cell lines and NKG2A+ natural killer cells, pre-treated with anti-human NKG2A, were rescued from disease progression. Human NKG2A+ natural killer cells reconstituted in immunodeficient mice after transplantation of human CD34+ cells. These natural killer cells are able to kill engrafted human primary leukemia or Epstein-Barr virus cell lines by lysis after intraperitoneal administration of anti-human NKG2A. Thus, this anti-NKG2A may exploit the anti-leukemic action of the wave of NKG2A+ natural killer cells recovering after hematopoietic stem cell transplants or adoptive therapy with natural killer cell infusions from matched or mismatched family donors after chemotherapy for acute leukemia, without the need to search for a natural killer cell alloreactive donor.
Introduction
Natural killer (NK) cells play a critical role in host defense against infections and tumors by secreting cytokines and killing infected or transformed cells. Activation of NK-cell effector functions is regulated by activating and inhibitory receptors that recognize ligands on potential target cells. NK cell-mediated killing is efficient when target cells abundantly express stress- or transformation-induced ligands for activating NK receptors, and few or no major histocompatibility complex (MHC)-class I molecules, which are ligands for inhibitory receptors on NK cells. In humans, a family of killer cell immunoglobulin-like receptors (KIR) bind distinct subgroups of human leukocyte antigen (HLA) class I allotypes. KIR are clonally expressed on NK cells, creating a repertoire of NK cells with specificities for different HLA class I molecules. Due to extensive genetic polymorphisms, there are significant variations in the repertoire of KIR+ NK cells among individuals in the population. Another inhibitory receptor, with broad specificity, the CD94-NKG2A complex, recognizes HLA-E, a non-classical MHC class I molecule. CD94-NKG2A and its HLA-E ligand exhibit very limited polymorphism. CD94-NKG2A is expressed primarily on NK cells that do not express an inhibitory KIR for a self-HLA class I, so it fills gaps in the KIR repertoire. However, some NK cells co-express CD94-NKG2A and one or more inhibitory KIR with different MHC class I specificities.1–3 The NKG2A receptor is also expressed on T cells.
Individuals harbor NK cells in their repertoire that may express, as the only inhibitory receptor, a single KIR that is inhibited by one self-MHC class I KIR ligand. Target cells that lack this KIR ligand do not block NK cell activation, and are killed. The clinical relevance of such missing self-recognition was demonstrated in adult patients with acute myeloid leukemia (AML) and in children with acute lymphoblastic leukemias (ALL).4–9 Haploidentical stem cell transplantation from KIR ligand mismatched donors (NK alloreactive donors) was associated with a reduced risk of relapse and increased survival rates.4–8 Unfortunately, NK alloreactive donors cannot be identified for about 50% of patients who express each of the main three groups of KIR ligands (HLA-C group 1 and 2 and Bw4 specificity) which block all the NK cells in the donor repertoire. To extend the benefits of NK cell alloreactivity to these patients another strategy had to be found. A human anti-KIR monoclonal antibody (lirilumab) was generated to bind to all KIR2D inhibitory receptors specific for groups 1 and 2 HLA-C alleles. In vitro and murine model studies showed that lirilumab efficiently promoted NK cell alloreactivity and killing of otherwise resistant HLA-C group 1+ or group 2+ targets, such as normal and tumor cells.10–13 Phase I clinical trials demonstrated that the anti-inhibitory KIR mAb is safe.14 Phase II clinical trials with lirilumab are ongoing.
Another approach has been to generate and explore the role of an anti-human NKG2A antibody. Every individual possesses NKG2A+ NK cells which are always blocked by HLA-E. Since HLA-E is expressed by most normal and neoplastic hematopoietic cells, these are protected from killing by CD94-NKG2A+ NK cells.1–3
Stem cell transplantation remains the only curative treatment option for many patients with acute leukemia. Interestingly, in the immediate post-transplant period, most reconstituting NK cells are NKG2A+.15 Nguyen and Godal have already demonstrated in vitro that anti-NKG2A antibody treatment is able to reconstitute NKG2A+ NK cell lysis against acute leukemia cells.16,17 Administering an anti-NKG2A monoclonal antibody could strengthen many of the benefits of NK cell alloreactivity and potentiate the anti-leukemic action of NK cells recovering after hematopoietic transplants or of NK cell infusions from matched or mismatched family donors without the need to search for an NK alloreactive donor.
We have generated a novel, humanized anti-NKG2A therapeutic monoclonal antibody that is being developed for treatment of solid tumors such as ovarian cancer and hematologic malignancies. In this study, we investigated the potential clinical role of this new therapeutic monoclonal antibody in vitro and in humanized mouse models.
Methods
Therapeutic anti-NKG2A monoclonal antibody
The murine anti-human NKG2A monoclonal antibody clone Z270 was generated and characterized as previously described.18 Details of the generation and characterization of humanized Z270 will be reported elsewhere. In brief, the murine Z270 monoclonal antibody was humanized by grafting the Kabat complementarity determining regions onto a human acceptor framework, and expressed in Chinese hamster ovary cells. Recombinant humanized clones were screened to identify those that retained binding to CD94-NKG2A with similar affinity as the original murine monoclonal antibody. Clones were then counter-screened on CD94-NKG2C and CD94-NKG2E, to ensure specificity for CD94-NKG2A. The selected humanized clone, designated humZ270, or IPH2201, was expressed as an IgG4 with a single point mutation in the Fc heavy chain to prevent formation of half-antibodies.
Cell isolation
All neoplastic cells were obtained from patients’ bone marrow aspirates or peripheral blood. All the normal lympho-hematopoietic cell types were obtained from healthy donors. Patients and donors provided prior written informed consent to the use of their biological material in accordance with the Declaration of Helsinki.
Neoplastic cells (if >95% of all cells) were obtained from peripheral blood or marrow samples after Ficoll-Hypaque gradient separation.
Human T and B cells and monocytes were purified from peripheral blood mononuclear cells on a Ficoll-Hypaque gradient and enriched by human T and B isolation kits or anti-CD14+ microbeads, respectively, and immunomagnetic selection (Miltenyi Biotec, Bergisch Gladbach, Germany). Dendritic cells were obtained as described elsewhere.19
Human NK cells were purified from peripheral blood mononuclear cells on a Ficoll-Hypaque gradient, then enriched by a human NK isolation kit and immunomagnetic selection (Miltenyi Biotec). Single KIR+/NKG2A− NK cells were cloned and used as controls for NK cell alloreactivity assay as previously described.7 NKG2A+/KIR− NK cells were depleted of KIR2DL1/2/3+ and KIR3DL1+ cells using anti-KIR2DL2/L3/S2 (clone CH-L, IgG2b) (BD Biosciences San José, CA, USA), anti-KIR2DL1 (clone #143211, IgG1) (R&D Systems Inc., Minneapolis, MN, USA) and KIR3DL1 (Miltenyi Biotech) phycoerythrin (PE)-conjugated monoclonal antibodies and negative selection by anti-PE immunomagnetic microbeads (Miltenyi Biotech). NKG2A+/KIR− NK cells were stimulated by 1% phytohemagglutinin (Biochrom, Berlin, Germany) and 250 IU/mL interleukin-2 (Novartis Farma S.p.A., Origgio, Italy), and expanded for up to 7 days. At the end of culture, before their use, the final purity of the NKG2A+ NK cells was >95%.
CD34+ stem cells were obtained from healthy donors’ peripheral blood after mobilization with granulocyte colony-stimulating factor, leukapheresis and positive selection by immunomagnetic microbeads conjugated with anti-human CD34+ monoclonal antibody (Miltenyi Biotec).
Epstein-Barr virus cell lines
HLA-E+ Epstein-Barr virus (EBV)-transformed B-cell lines, which were resistant to NKG2A+ NK cell lysis, were a kind gift from the European Collection for Biomedical Research (ECBR). Anti-human HLA-E-PE (IgG1, clone 3D12, eBioscience, San Diego, CA, USA) was used to estimate HLA-E expression on EBV cell lines and all the other normal and neoplastic human hematopoietic cells by flow cytometry.
In vitro cytotoxicity assays
NKG2A+/KIR− NK cells were pre-treated with humanized anti-human NKG2A antibody or with an isotype control antibody (10 μg/1×106 cells/mL). Single KIR+/NKG2A− and KIR−/NKG2A+ NK cells were screened for alloreactivity by standard 51Cr release cytotoxicity assays at an increasing effector-to-target (E:T) ratio (from 1:1 to 20:1) against KIR ligand mismatched HLA-E+ B and T cells, monocytes, dendritic cells, EBV cell lines, chronic lymphatic leukemia (CLL) cells, T-cell ALL, AML and multiple myeloma (MM) cells.
Mouse models
Colonies of non-obese diabetic - severe combined immunodeficiency (NOD-SCID) mice and NOD-scidIL2rgtm (NSG) mice were bred at the University of Perugia Animal House. Breeders were obtained from Jackson Laboratory (Bar Harbor, Maine, USA).
All experiments were performed in accordance with the National Ethic Approval Document for animal experimentation.
Female 10-week old mice were irradiated with 3.5 Gy. The next day NOD-SCID mice received an intravenous co-infusion of primary AML cells (12×106) or EBV-transformed B-cell line (12×106) and NKG2A+ non-alloreactive, interleukin-2-activated NK cells (1×106) that had been pre-treated with anti-human NKG2A monoclonal antibody (10 μg/1×106 cells/mL) at the E:T ratio of 1:12. Isotype control antibody-pretreated NK cells were infused in control mice at the E:T ratio of 1:12.
Mice that succumbed to leukemia or EBV lymphoproliferative disease were assessed for AML or EBV organ infiltration by flow cytometry analysis with a specific panel of anti–human monoclonal antibodies which previously characterized the neoplastic cells (see below).
In a model of engrafted disease, we infused the same mouse strain with AML or EBV cell lines. When bone marrow engraftment was around 20–30%, mice were given escalating doses of interleukin-2-activated NKG2A+ NK cells that had been pre-treated with anti-human NKG2A monoclonal antibody (10 μg/1×106 cells/mL) (from 1 to 10×106 per mouse, intravenously). Mice that died of leukemia or lymphoma were assessed for AML or EBV organ infiltration by flow cytometry analysis using a specific panel of anti-human monoclonal antibodies (see below).
In other mouse models, the day after irradiation, female 10-week old NSG mice were given 10×106 human CD34+ hematopoietic stem cells intravenously. At day 20 mice were infused intravenously with 5×106 HLA-E+ EBV cells or AML cells. When CD34+ stem cells had differentiated into CD56+/CD3−/NKG2A+ NK cells, mice received an intraperitoneal administration of 200, 250 or 300 µg anti-human NKG2A monoclonal antibody. Control mice were left untreated or treated with the same doses of isotype control antibody.
From day 40 onwards mice were evaluated for EBV or AML engraftment with a combination of anti-human CD45 monoclonal antibody and monoclonal antibody specific for AML or the EBV cell line (anti-CD20, anti-CD19, anti-IgM, anti-kappa, anti-lambda, anti-CD23, anti-CD3, anti-CD33, anti-CD34, anti-CD56, anti-CD117, anti-CD8, CD4, CD34 monoclonal antibodies, eBioscience).
Mice that succumbed to EBV lymphoproliferative disease or leukemia were assessed for EBV or AML organ infiltration by flow cytometry analysis with a combination of anti-human monoclonal antibodies (see above). Mice that survived were sacrificed after 100 days, and tumor organ infiltration analyzed with the same antibody combination.
Statistical analyses
The Student t test was used to compare variables and was applied by Graphpad Prism 5. The Kaplan-Meier method was used to evaluate murine survival. All P values are two-sided and considered statistically significant at P values <0.05.
Results
In vitro treatment with anti-human NKG2A antibody triggers NKG2A+ natural killer cell lysis of HLA-E+ hematopoietic lineage targets
In order to assess the susceptibility of normal and neoplastic hematopoietic lineage targets to alloreactive NK cell lysis, we generated single inhibitory KIR+NKG2A− NK cell clones and evaluated their ability to kill KIR ligand-lacking targets such as B and T cells, monocytes, dendritic cells, EBV cell lines, CLL, T-ALL, AML and MM cells. These normal and neoplastic hematopoietic lineage cells expressed HLA-E and were resistant to NKG2A+ NK cells. Figure 1 shows that most acute leukemias express HLA-E. All HLA-E+ lympho-hematopoietic cell types were targets of alloreactive NK cell killing when they did not express the appropriate inhibitory KIR ligand for the single inhibitory KIR receptor expressed by alloreactive NK cell clones (Figure 2A). We pre-treated NKG2A+ NK cells with anti-human NKG2A antibody and assessed their ability to kill otherwise resistant HLA-E+ hematopoietic lineage cells. Treatment with anti-NKG2A monoclonal antibody converted NKG2A+KIR− NK cells into cells that were functionally “alloreactive” against HLA-E+ lympho-hematopoietic cells i.e. killed B and T cells, monocytes, dendritic cells, EBV cell lines, CLL, T-ALL, AML and MM cells. The most effective lysis was obtained with an E:T ratio of 15:1 (Figure 2B).
Figure 1.
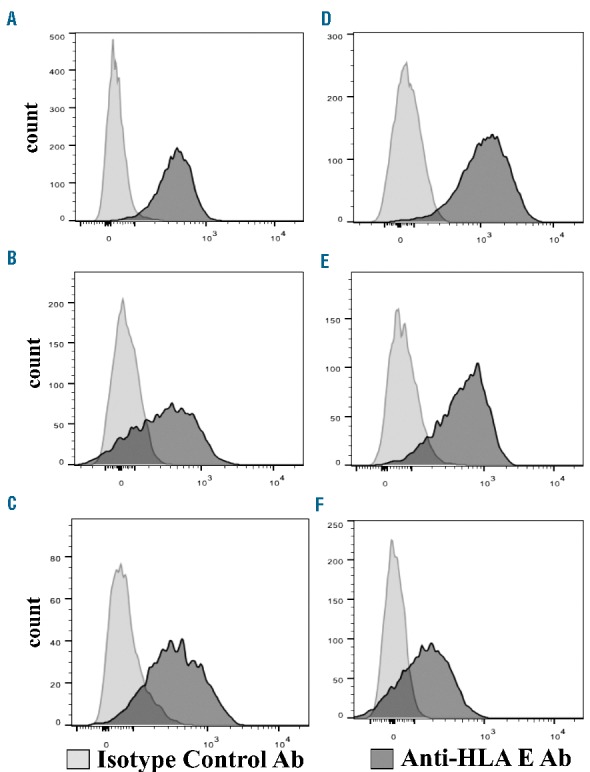
HLA-E expression on acute leukemia cells. (A–C) HLA-E expression on AML cells from three patients. (D–F) HLA-E expression on ALL cells (1 T-ALL and 2 B-ALL) from three patients.
Figure 2.
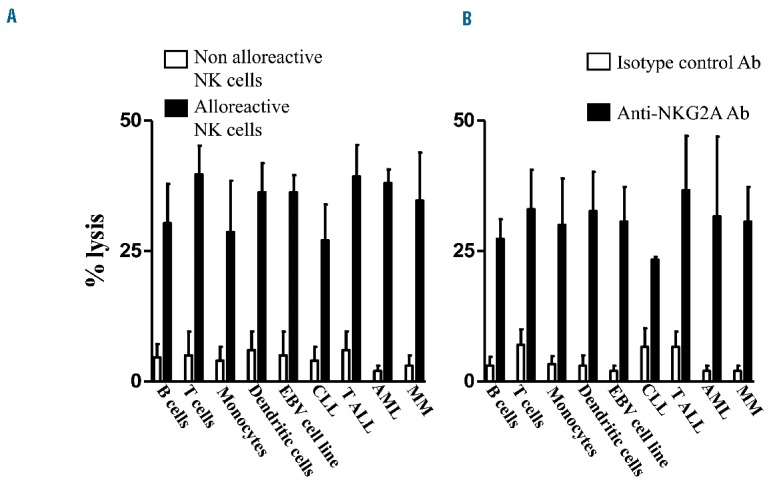
In vitro treatment with anti-human NKG2A monoclonal antibody reconstitutes NKG2A+ NK cell lysis against HLA-E+ normal and neoplastic lymphohematopoietic cells. (A) Percentage lysis of KIR ligand-mismatched HLA-E+ B and T cells, monocytes, dendritic cells, EBV cell lines, CLL, T-ALL, AML and MM cells mediated by single KIR+ alloreactive NK clones at the E/T 15:1 in a standard 51Cr release cytotoxicity assay. (B) Percentage lysis of HLA-E+ B and T cells, monocytes, dendritic cells, EBV cell lines, CLL, T-ALL, AML and MM cells mediated by activated and cultured in IL2 NKG2A+/KIR− NK cells at the E:T of 15:1 after treatment with anti-human NKG2A monoclonal antibody (10 μg/1×106 cells/mL) in a standard 51Cr release cytotoxicity assay. Lysis mediated by NKG2A+ NK cells after treatment with anti-human NKG2A monoclonal antibody is comparable to lysis mediated by single KIR+ alloreactive NK cell clones. Each cytotoxicity assay was repeated with three targets for each category of cells and the mean ± SD is shown.
Each cytotoxicity assay was repeated with three targets for each category of cells and the mean ± standard deviation is shown.
In vivo treatment with the anti-human NKG2A antibody eradicates HLA-E+ leukemia and lymphoma
In order to evaluate the in vivo efficacy of anti-NKG2A monoclonal antibody at triggering NKG2A+ NK cells to kill neoplastic cells, we developed xenogenic murine models of human neoplastic disease. NOD-SCID mice that received the HLA-E+ EBV-cell lines or AML cells died of high-grade lymphoma or AML. In these mice, co-infusion of human NKG2A+ non-alloreactive NK cells did not prevent engraftment of EBV or AML cells and mice died of the diseases.
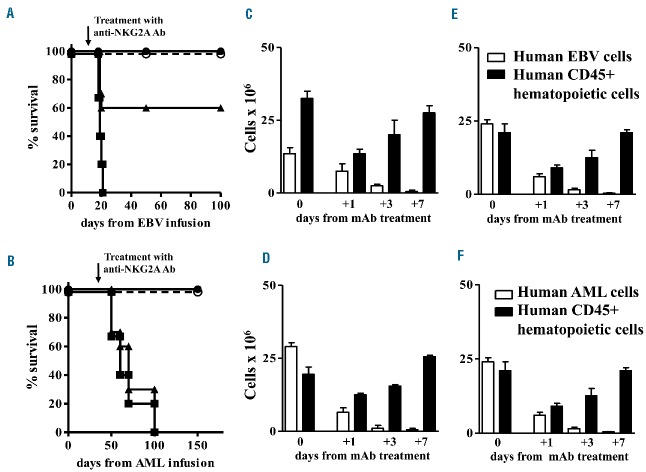
In contrast, infusion of NKG2A+ NK cells that had been pre-treated with anti-NKG2A monoclonal antibody, prevented engraftment of human EBV cell lines and AML cells and mice survived without symptoms or signs of tumor localization (Figure 3A). In fact, mice were sacrificed 100 days after cell infusion and cytofluorimetric analysis confirmed the absence of neoplastic infiltration. We pooled results from eight experiments with four mice per group for each experiment.
Figure 3.
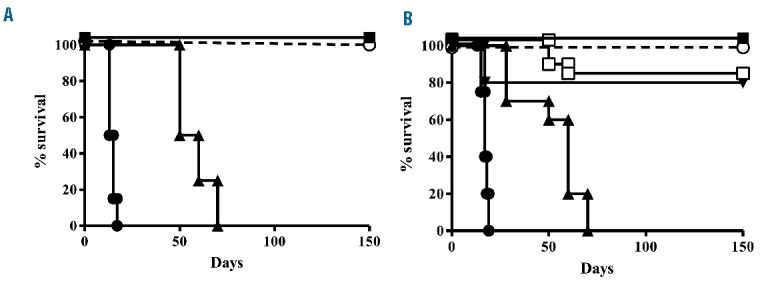
Pre-treatment of human NKG2A+ NK cells with the anti-human NKG2A monoclonal antibody prevents engraftment of human EBV cell lines and AML cells and cures engrafted disease in NOD-SCID mice. (A) One million NKG2A+/KIR− NK cells were pre-treated with anti-human NKG2A monoclonal antibody (10 μg) and co-infused with EBV cell line (■) or AML (○) expressing HLA-E at an E:T of 1:12. Control mice were co-infused with isotype control antibody-pretreated NKG2A+/KIR−NK cells and EBV cell line (●) or AML (▲) expressing HLA-E at an E:T of 1:12. Mice co-infused with human EBV cell lines or human AML cells and treated with isotype control antibody-pretreated NKG2A+ NK cells died of disease progression. The anti-human NKG2A monoclonal antibody pre-treatment prevented disease engraftment and all mice survived. We pooled results of eight experiments with four mice per group for each experiment. (B) Mice engrafted with AML or EBV cell lines (20–30% of bone marrow infiltration) were infused with escalating doses of NKG2A+ KIR− NK cells, pre-treated with anti-human NKG2A monoclonal antibody (10 μg/1×106 NK cells). Control mice were co-infused with isotype control antibody-pretreated NKG2A+ KIR− NK cells and EBV cell line or AML cells. At least 3×106 NKG2A+ KIR− NK cells pre-treated with anti-human NKG2A monoclonal antibody cured 80% of mice with EBV or AML. Treatment of engrafted mice with at least 4×106 pretreated NKG2A+NK cells rescued 100% of mice affected by EBV (■) or AML (○). Mice engrafted with human EBV cell lines (●) or human AML cells (▲) and infused with more than 4×106 isotype control antibody-pretreated NKG2A+ NK cells died of disease progression. The anti-human NKG2A monoclonal antibody pre-treatment cured engrafted diseases. We pooled results of eight experiments with four mice per group for each experiment.
NKG2A+ NK cell elimination of engrafted human AML or human EBV cell lines was evaluated in escalating dose experiments. At least 3×106 NKG2A+ NK cells per mouse, pre-treated with anti-NKG2A, were necessary to rescue 80% mice (Figure 3B). Repeating intraperitoneal doses of antibody did not improve the results because of autologous NK cell killing (fratricide effect).
In order to assess the ability of endogenously generated NKG2A+ NK cells to cure leukemia or lymphoma, we transplanted NSG mice with 10×106 human CD34+ hematopoietic cells. The transplanted CD34+ hematopoietic stem cells differentiated into various hematopoietic lineage cells, including NKG2A+ NK cells.20 Twenty days after the CD34+ cell infusion, mice received HLA-E+ EBV cell lines or AML tumor cells. On day 30 after the CD34+ cell infusion, when the numbers of NKG2A+ NK cells reached a plateau value in the bone marrow and spleen, we treated three groups of mice with 200, 250 or 300 µg of the anti-NKG2A monoclonal antibody. Control mice treated with isotype control monoclonal antibody, and mice that received 200 μg of anti-NKG2A monoclonal antibody, succumbed to EBV lympho-proliferative disease or leukemia. In contrast, mice that received 250 or 300 μg of anti-NKG2A monoclonal antibody survived (Figure 4A and 4B, respectively). NKG2A+ NK cells totally ablated the EBV cell line or AML cells in the bone marrow (Figure 4C and 4D, respectively) and spleen (Figure 4E and 4F, respectively). Thus, treatment with anti-human NKG2A monoclonal antibody enabled endogenously generated human NKG2A+ NK cells to kill lethal EBV lymphoproliferative disease or leukemia. We pooled results from three experiments with five mice per group for each experiment.
Figure 4.
In vivo treatment with the anti-human NKG2A monoclonal antibody rescues NSG mice engrafted with human CD34+ hematopoietic stem cells and HLA-E+ human AML cells or an EBV cell line. After 3.5 Gy total body irradiation, mice were infused with 10×106 human CD34+ hematopoietic stem cells. After 20 days they were infused with an EBV cell line or AML cells. When NKG2A+ NK cells differentiated from CD34+ cells, mice were treated with anti-human NKG2A monoclonal antibody. Mice that received 250 μg (○) or 300 μg (●) of anti-human NKG2A monoclonal antibody survived, control mice (isotype control antibody) (■) or mice that received 200 μg of the antibody (▲) succumbed to EBV lympho-proliferative disease (A) or AML (B). NKG2A+ NK cells ablated the EBV cell line in bone marrow* (C) and spleen (E) and AML cells in bone marrow* (D) and spleen (F). The normal human CD45+ hematopoietic population, which developed from CD34+ cells, was transiently depleted after administration of human anti-NKG2A antibody. We pooled results of three experiments with five mice per group for each experiment. * Bone marrow cell numbers are from two femurs per mouse.
In vivo treatment with the anti-NKG2A antibody transiently depletes non-neoplastic lympho-hematopoietic lineage cell
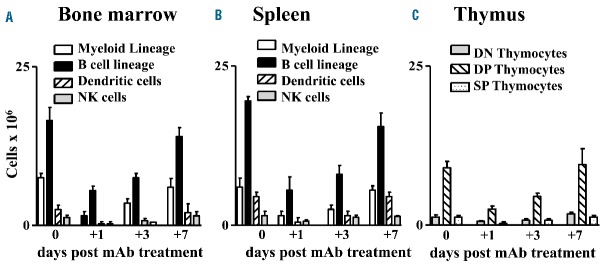
In order to evaluate the impact of anti-NKG2A monoclonal antibody on the various lympho-hematopoietic lineage cell subsets in vivo, NSG mice were transplanted with human CD34+ hematopoietic stem cells and the differentiated lympho-hematopoietic cell subpopulations were analyzed. One month after CD34+ cell infusion, human CD4+/CD8+ double positive thymocytes in the thymus, and myeloid lineage cells, B cells, NK cells and dendritic cells in the bone marrow and in the spleen reached plateau values (Figure 5). At this time point mice were treated with anti-human NKG2A monoclonal antibody. Monitoring human myeloid, B, and dendritic cell subpopulations in the bone marrow and spleen and human thymocytes at different times after anti-NKG2A treatment showed that all these hematopietic lineage cells were transiently depleted. They returned to pre-treatment values within 10 days (Figure 5). Analysis of the T-cell receptor repertoire in thymocytes revealed that it was polyclonal (data not shown). Thus, in vivo treatment with anti-human NKG2A monoclonal antibody did not induce persistent ablation of normal hematopoietic cells. We pooled results from three experiments with five mice per group for each experiment.
Figure 5.
Treatment with the anti-human NKG2A monoclonal antibody transiently depleted HLA-E+ autologous myeloid, B, T, NK and DC subpopulations in NSG mice engrafted with human CD34+ hematopoietic stem cells. After 3.5 Gy total body irradiation, mice were infused with human CD34+ hematopoietic stem cells. One month after, when NKG2A+ NK cells differentiated from CD34+ cells reached a plateau value, mice were treated with 300 μg of anti-human NKG2A monoclonal antibody. Transient depletion of human myeloid, B, dendritic, and NK cell subpopulations in the bone marrow* (A) and spleen (B) and double negative (DN), single CD8+ or CD4+, CD4+/CD8+ double positive (DP) thymocytes (C) was followed by recovery of all cell subsets within 10 days. We pooled results of three experiments with five mice per group for each experiment. *Bone marrow cell numbers are from two femurs per mouse.
Discussion
The present investigation into the clinical potential of a recently developed humanized anti-NKG2A antibody showed that it converted NKG2A+ NK cells into effector NK cells able to kill most HLA-E+ NK resistant lymphohematopoietic cells, including B and T lymphocytes, dendritic and myeloid cells, leukemic cells (CLL, T-ALL and AML), high-grade lymphoma and MM cells. We also demonstrated in mouse models that pre-treatment of NKG2A+ NK cells with anti-human NKG2A monoclonal antibody prevented engraftment of otherwise lethal EBV cell lines or AML cells.
Interestingly, the repertoire of each individual expresses a certain percentage of NKG2A+ NK cells and, after hematopoietic stem cell transplantation, a large population of reconstituting NK cells express the CD94-NKG2A inhibitory receptor.15 Consequently, the use of humanized anti-NKG2A antibody could enlarge the NK cell population that exerts an anti-tumor effect to the benefit of patients with hematologic malignancies.
Potential side effects such as autoreactivity against hematopoietic stem cells and subsequent cytopenia could develop, particularly after transplantation. To test this hypothesis, we transplanted mice with human CD34+ stem cells and then leukemic cells, which engrafted because the stem cells could not develop into mature T cells or alloreactive single KIR+NKG2A− NK cells.20 Human hematopoietic stem cells could, however, develop into NKG2A+ NK cells.20 Anti-NKG2A antibody treatment reconstituted NKG2A+ NK cell-mediated lysis of HLA-E+ engrafted leukemic cells, rescuing mice from death. The side effects appear slight as cytopenia of normal hematopoietic cells was transient and mice recovered quickly. The slight, transient cytopenia in the committed myeloid line may be due to either alloreactive NK cell fratricide or to CD34+ cell conservation. In fact, recurrent dosing does not seem to reduce the number of CD34+ cells as engraftment was always successful (data not shown). One might hypothesize that they are not a target of alloreactive NK cells.
Interestingly these in vitro and in vivo results are in accordance with previous findings that lirilumab bound to all KIR2D inhibitory receptors for groups 1 and 2 HLA-C alleles and blocked NK cell inhibitory recognition of self-HLA-C. It activated NK cell killing in vivo, eradicating tumors in mice.10–13 In fact, clinical trials of this fully human anti-KIR antibody as a single agent are ongoing in patients with acute leukemia.14
We might hypothesize about using the humanized anti-NKG2A antibody as an alternative to chemotherapy. Some studies demonstrated safety and a promising clinical role of haploidentical alloreactive NK cell infusions in combination with chemotherapy for the treatment of elderly or pediatric patients with high-risk acute leukemias.22,23 We speculate that humanized anti-NKG2A may be useful in similar settings, in order to reconstitute lysis by NKG2A+ NK cells obtained from non-alloreactive haploidentical or identical donors.
The role of the NKG2A receptor in autoimmune diseases is controversial. Activated NK cells with NKG2A down-regulation may play a role in the pathogenesis of psoriasis.24 However, since reconstituted NK cell lysis by means of the anti-NKG2A antibody is also directed against activated autologous T and B cells which mediate autoimmune diseases, the antibody might also be envisaged as therapy against human autoimmune diseases. In a murine model of rheumatoid arthritis, an anti-murine NKG2A (Fab) antibody selectively increased lysis of autologous TH17 and TFH cells, which are the mediators of rheumatoid arthritis. The antibody blockade of the inhibitory interaction between the NKG2A receptor and its Qa-1 ligand enhanced the NK cell-dependent elimination of pathogenic T cells, resulting in blockade of disease onset or progression.25
In vitro and in vivo findings suggest that the humanized anti-NKG2A antibody described here constitutes a unique, relatively safe, therapeutic approach to malignant hematologic and autoimmune diseases. Phase I/II clinical trials with anti-human NKG2A antibody are ongoing in patients with tumor types known to express HLA-E, including CLL (ClinicalTrials.gov:NCT02557516), head and neck cancer (ClinicalTrials.gov: NCT02331875) and ovarian cancer (ClinicalTrials.gov NCT02459301)26 in order to validate the present observations and provide hope for those 50% of patients with hematologic and solid malignancies who cannot find alloreactive NK cell donors.
Acknowledgments
The authors thank Dr Geraldine Anne Boyd for editorial assistance. LR is a Leukemia and Lymphoma Society Scholar in Clinical Research.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/5/626
References
- 1.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23(2): 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94(1):333–339. [PubMed] [Google Scholar]
- 5.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. [DOI] [PubMed] [Google Scholar]
- 6.Karre K. A perfect mismatch. [commentary] Science. 2002;295(5562):2029–2031. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110(1):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21(5):525–530. [DOI] [PubMed] [Google Scholar]
- 9.Pende D, Marcenaro S, Falco M, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113(13):3119–3129. [DOI] [PubMed] [Google Scholar]
- 10.Romagne F, Andre P, Spee P, et al. Pre-clinical characterization of 1-7F9, a novel human anti-KIR therapeutic antibody that augments NK-mediated killing of tumor cells. Blood. 2009;114(13):2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sola C, André P, Lemmers C, et al. Genetic and antibody-mediated reprogramming of natural killer cell missing-self recognition in vivo. Proc Natl Acad Sci USA. 2009;106(31):12879–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson DM, Jr Bakan CE, Zhang S, et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood. 2011;118(24):6387–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohrt HE, Thielens A, Marabelle A, et al. Anti-KIR antibody enhancement of anti-lymphoma activity of natural killer cells as monotherapy and in combination with anti-CD20 antibodies. Blood. 2014;123(5): 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vey N, Bourhis JH, Boissel N, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–4323. [DOI] [PubMed] [Google Scholar]
- 15.Stern M, De Angelis C, Urbani E, et al. Natural killer cell KIR repertoire reconstitution after haploidentical stem cell transplantation. Bone Marrow Transplant. 2010; 45(11):1607–1610. [DOI] [PubMed] [Google Scholar]
- 16.Godal R, Bachanova V, Gleason M, et al. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16(5):612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen S, Dhedin N, Vernant JP, et al. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–4142. [DOI] [PubMed] [Google Scholar]
- 18.Moretta A, Vitale M, Sivori S, et al. Human natural killer cell receptors for HLA-class I molecules. Evidence that the Kp43 (CD94) molecule functions as receptor for HLA-B alleles. J Exp Med. 1994;180(2):545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancusi A, Ruggeri L, Urbani E, et al. Haploidentical hematopoietic transplantation from KIR ligand-mismatched donors with activating KIRs reduces non relapse mortality. Blood. 2015;125(20):3173–82. [DOI] [PubMed] [Google Scholar]
- 20.André MC, Erbacher A, Gille C, et al. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J Immunol. 2010;185(5):2710–2720. [DOI] [PubMed] [Google Scholar]
- 21.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–3057. [DOI] [PubMed] [Google Scholar]
- 22.Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28(6):955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curti A, Ruggeri L, D’Addio A, et al. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients. Blood. 2011;118(12):3273–3279. [DOI] [PubMed] [Google Scholar]
- 24.Son SW, Kim EO, Ryu ES, et al. Upregulation of Fas and downregulation of CD94⁄NKG2A inhibitory receptors on circulating natural killer cells in patients with new-onset psoriasis. Br J Dermatol. 2009;161(2):281–288. [DOI] [PubMed] [Google Scholar]
- 25.Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H. Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc Natl Acad Sci USA. 2011;108(35):14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour L, Tinker A, Hirte H, Wagtmann N, Dodion P. Phase I and dose ranging, phase II studies with IPH2201, a humanized monoclonal antibody targeting HLA-E receptor CD94/NKG2A. Ann Oncol. 2015;26 (Suppl. 2):ii3–ii5. [Google Scholar]