Abstract
The tumor microenvironment is the cellular and molecular environment in which the tumor exists and with which it continuously interacts. In B-cell lymphomas, this microenvironment is intriguing in that it plays critical roles in the regulation of tumor cell survival and proliferation, fostering immune escape as well as the development of treatment resistance. The purpose of this review is to summarize the proceedings of the Second Annual Summit on the Immune Microenvironment in Hematologic Malignancies that took place on September 11–12, 2014 in Dublin, Ireland. We provide a timely overview of the composition and biological relevance of the cellular and molecular microenvironment interface and discuss the role of interactions between the microenvironment and neoplastic cells in a variety of B-cell lymphomas. In addition, we focus on various novel therapeutic strategies that target the tumor microenvironment, including agents that modulate B-cell receptor pathways and immune-checkpoints, chimeric antigen receptor T cells and immunomodulatory agents.
Introduction
Recent advances in the understanding of the pathogenesis of hematologic malignancies have focused attention on the role of the tumor microenvironment. In B-cell lymphomas, the cellular infiltrate intimately associated with the malignant lymphocytes, and the molecules that can be released or trapped within it, may aid tumor cell proliferation and survival as well as escape from immunosurveillance.1 Recognition of the microenvironment’s importance has paved the way for the development of exciting novel strategies that target the microenvironment and its interactions with neoplastic cells. In particular, drugs targeting B-cell receptor (BCR) signaling and programmed death-1 (PD-1) pathways as well as chimeric antigen receptor (CAR) T-cell therapy represent promising advances in lymphoma treatment. The purpose of this review is to summarize the proceedings of the Second Annual Summit on the Role of the Immune Microenvironment in B-cell Lymphomas that took place in Dublin, Ireland on September 11–12, 2014. The manuscript reflects the meeting’s structure: the first half is devoted to an overview of the tumor microenvironment in various lymphoma subtypes, and the remaining is a discussion of novel therapeutic approaches targeting the tumor microenvironment and practical aspects concerning the design and conduct of studies evaluating these agents.
Overview of the microenvironment in B-cell malignancies
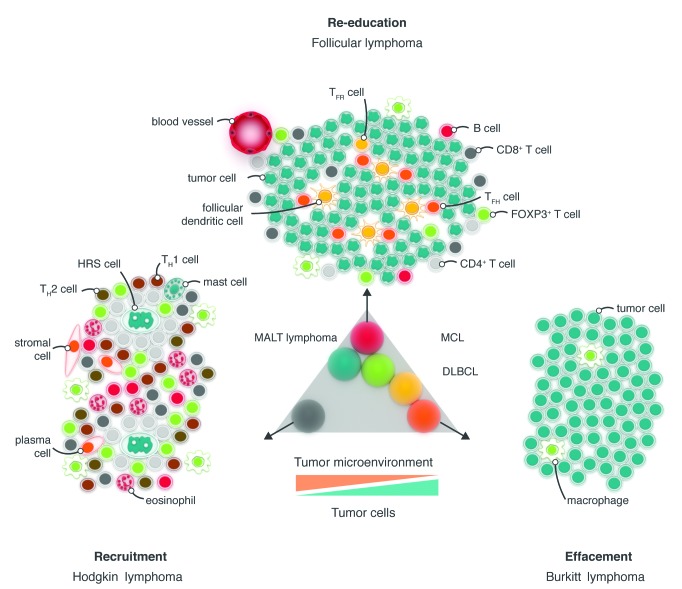
The tumor microenvironment of B-cell lymphomas is highly variable with regards to both spatial arrangement and composition of cells, including immune and inflammatory cells, blood and lymphatic vascular networks and the extracellular matrix. The cellular composition of the microenvironment generally mirrors that of the normal tissue at the site of development, the exception being classical Hodgkin lymphoma (see below). Tumor cells retain a degree of dependence on interactions with non-malignant cells and stromal elements of the tumor microenvironment for survival and proliferation.2 However, tumor cells also use these interactions to generate immunosuppressive mechanisms that promote tumor escape from immune surveillance and lead to disease progression.2–4 Increasing data indicate a critical role for the tumor microenvironment in mediating treatment resistance.5 The cellular composition and spatial characteristics of the microenvironment demonstrate significant heterogeneity depending on a number of factors, including the lymphoma subtype. Scott and Gascoyne have proposed three major models that divide up the broad range of tumor microenvironments described in B-cell lymphomas (Figure 1).2 The first, re-education, is typified by follicular lymphoma (FL), in which malignant cells retain dependence on the microenvironment for survival and proliferation signals; the second, recruitment, is observed in classical Hodgkin lymphoma (cHL) in which the infrequent Reed-Sternberg cells are surrounded by an extensive support milieu of nonmalignant cells that is distinct from the composition of normal lymphoid tissue; the third, effacement, is seen in Burkitt lymphoma (BL) and to some extent in diffuse large B-cell lymphoma (DLBCL), whereby genetic aberrations, such as translocation of MYC, within the malignant cell lead to autonomous, microenvironment-independent growth and survival.2 These tumors rely little on the microenvironment, which is sparse when compared to the microenvironment in cHL. Thus, the extent to which different histological subtypes of lymphoid malignancy are susceptible to agents targeting the immune microenvironment is likely to vary depending on the degree to which the tumor cells are dependent on external stimuli for growth or proliferation. In the following section, we provide an overview of the current understanding of the structure, composition and function of the tumor microenvironment in B-cell lymphomas and chronic lymphocytic leukemia (CLL).
Figure 1.
Schematic diagram of the typical microenvironment of the three B-cell lymphoma subtypes that represent the extremes of the spectrum of tumor microenvironment — recruitment, re-education and effacement. These lymphoma subtypes represent the range of tumor cell content, from ~1% in cHL to typically more than 90% in BL. The other B-cell lymphomas fall within this range, as shown for the most common B-cell lymphomas (center). Typically, the ratio of malignant cells to microenvironmental cells increases across the range, from cHL to BL, as shown. DLBCL, diffuse large B-cell lymphoma; FOXP3, forkhead box protein P3; HRS, Hodgkin Reed–Sternberg; MALT, mucosa-associated lymphoid tissue; MCL, mantle cell lymphoma; TFH, follicular T helper; TH, T helper; TFR, follicular regulatory T. Reproduced from Scott and Gascoyne2 with permission from Nature Publishing Group.
Aggressive lymphomas
Diffuse large B-cell lymphoma
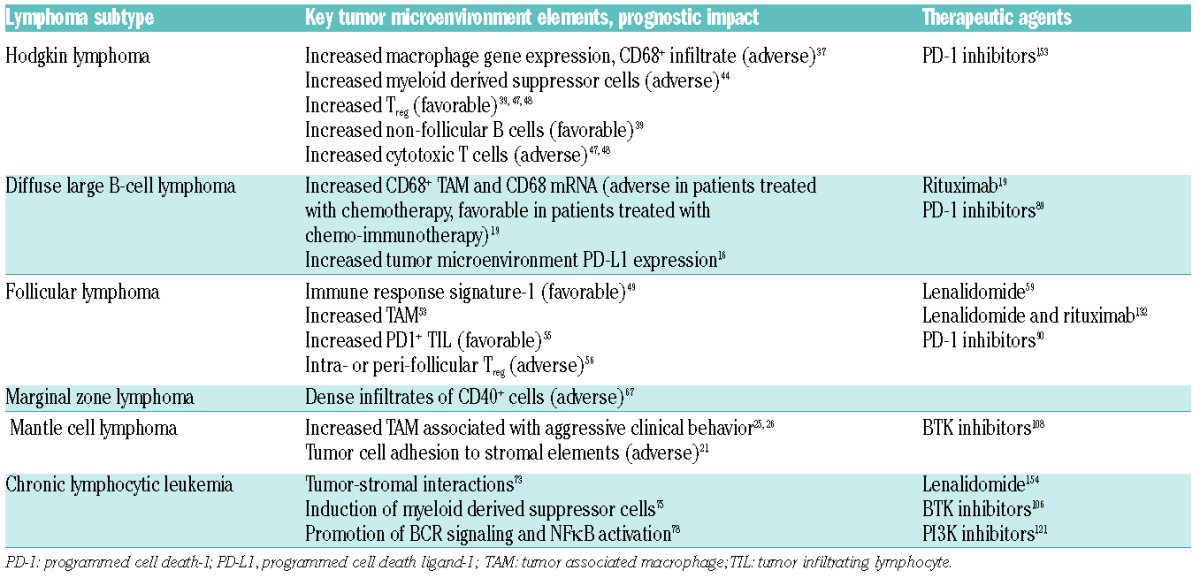
DLBCL is the most common type of non-Hodgkin lymphoma and is recognized as a heterogeneous disease with distinct molecular subtypes that are derived from different stages of B-cell differentiation.6,7 Alizadeh et al. first described gene expression profiling to define distinct subtypes of DLBCL: activated B cells and germinal center B cells.6 Seminal work by the Leukemia/Lymphoma Molecular Profiling Project further described two stromal signatures (termed stromal-1 and -2) in the tumor microenvironment, present in both activated and germinal center subtypes, which were predictive of outcome.8 Although key genetic lesions may explain some of this disparity, other factors, such as the microenvironment, likely play an important role. The contribution of the tumor microenvironment to the pathogenesis and tumor survival of DLBCL is poorly understood; however, several recent studies have yielded intriguing findings and shed some light on the microenvironment’s possible roles. One recent study in DLBCL demonstrated that 29% of cases have mutations or deletions resulting in inactivation of the β2-microglobulin gene (B2M) and 21% feature inactivations in the CD58 gene (CD58), two molecules that are critically involved in the immune recognition of tumor cells by circulating T-lymphocytes and natural killer (NK) cells, respectively.9 The immune escape from these important immune cells (circulating T-lymphocytes and NK cells) implicates the evasion of immune recognition as playing an important role in the pathogenesis of DLBCL. Thus, in the majority of cases of DLBCL these two gene alterations may be co-selected during lymphomagenesis to avoid cytotoxic circulating T-lymphocytes and NK cells.
Many studies have looked at the role of PD-1 and PD-L1, which are expressed in many aggressive B-cell lymphomas and have also been associated with mechanisms of immune evasion.3,10–12 The MHC class II transactivator CIITA is commonly fused to PD-L1 and PD-L2, which can result in a decrease in HLA-DR expression.10 A study by Steidl et al. looked at rearrangements of CIITA in B-cell lymphomas;10 combined with PD-L1 copy number gains and translocations independent of CIITA, this fusion resulted in T-cell exhaustion and immune escape. In addition, translocations and copy-number gains of PD-L1/2 appear to be a dominant mechanism of immune escape in primary mediastinal B-cell lymphoma (PMBL).13–15 Kiyasu et al. studied 1253 DLBCL biopsies and found tumor cell, but not microenvironmental, expression of PD-L1 was associated with adverse overall survival, a difference that was present even among the subgroup of patients treated with R-CHOP or similar regimens.16 Tumor PD-L1 expression was significantly associated with non-germinal center B-cell phenotype.
Other studies have investigated the role of chemokines and cytokines such as CCL22, CCL17, GAL-1 and TGF-β vis-à-vis how they recruit and/or retain immunosuppressive cells such as M2 macrophages, regulatory T cells (Tregs), and exhausted T cells, and in that way contribute to the pathogenesis of B-cell lymphomas.2,17,18 Riihijarvi et al. found that both CD68 mRNA levels and CD68+ tumor-associated macrophages, detected by immunohistochemistry, were adverse prognostic factors for overall survival among patients treated uniformly with chemotherapy in a prospective clinical trial.19 In contrast, among patients treated with chemo-immunotherapy, the impact of CD68+ tumor-associated macrophages was reversed, such that patients with high CD68+ tumor-associated macrophages had improved overall survival. This interesting observation led the authors to speculate that rituximab may alter the function of tumor-associated macrophages from having a pro-survival effect to an anti-tumor one.
Mantle cell lymphoma
The molecular hallmark of mantle cell lymphoma (MCL) is the t(11;14) translocation, which results in constitutive expression of cyclin D1, leading to cell cycle deregulation. However, extrinsic microenvironment-derived signals also play a role in the pathogenesis of this disease.20 MCL is biologically characterized by a tendency toward extranodal dissemination, mediated by attraction and retention through a highly regulated process involving chemokine gradients and adhesion molecules such as VLA-4, CCR7, CXCR5 and CXCR4.21 Through this mechanism, MCL cells interact with stromal cells such as fibroblasts and macrophages. Adhesion to stromal elements is an important mechanism of chemoresistance, and is likely a reason for the incurability of patients following chemotherapy.22 Another means by which MCL cells are protected from chemotherapy is through interleukin (IL)-6 secretion, which may be secreted by the MCL cells themselves or by bone marrow stromal cells.23 IL-6 activates the JAK/STAT3 and PI3K/Akt pathways, known to be key regulators of MCL growth and survival.
Relative to other lymphoma subtypes, the precise composition of the MCL tumor microenvironment is not well characterized. Macrophages have been described in MCL although, in contrast to FL and cHL, systematic evaluation of their prognostic or pathogenic implications is lacking.24 Studies in small series have suggested that increased numbers of macrophages are associated with aggressive clinical behavior.25,26 Two studies indicate that MCL cells induce microenvironmental changes to evade the host immune response. Firstly, intratumoral biopsies showed that CD4+CD25+Foxp3+ Tregs are present in MCL, where they likely contribute to a reduction of anti-tumor cytotoxicity.18 Secondly, PD-L1 (B7-H1) was shown to be expressed by MCL cell lines, in which it resulted in impaired T-cell proliferation after tumor exposure, inhibited specific anti-tumor T-cell responses and impaired T-cell-mediated tumor cell killing.27 The negative PI3K regulator PTEN is often inactivated by phosphorylation in MCL.28 This, along with antigenic stimulation via the BCR, resulted in constitutive activation of Syk, Btk and PI3k-Akt, which are critical in MCL disease progression and maintenance.29 Inhibition of Syk and Btk has been shown to inhibit BCR-mediated adhesion of MCL to bone marrow stromal cells and to increase apoptosis.30
Hodgkin lymphoma
The tumor microenvironment in cHL has been extensively studied, with four variant morphological patterns described: nodular sclerosing, mixed cellularity, lymphocyte-rich and lymphocyte-depleted. Neoplastic Hodgkin Reed-Sternberg (HRS) cells account for <5% of the tumor, with the remaining cells comprising B and T cells, eosinophils, neutrophils, mast cells, fibroblasts and macrophages.31 These cells are attracted by chemokines secreted by HRS cells such as CCL17 (TARC) and CCL12.32,33 HRS cells also secrete cytokines such as macrophage migration inhibition factor, which induces macrophage M2 polarization,34 and IL-9, which promotes mast cell differentiation (which in turn results in angiogenesis and fibrosis).35 Thus, HRS cells both attract and induce the differentiation of immune cells resulting in a tumor microenvironment favorable for tumor cell growth and survival.36
The importance of the tumor microenvironment in cHL was illustrated in studies by two independent groups who used gene expression profiling to demonstrate overexpression of genes associated with macrophages in biopsies taken from patients who experienced treatment failure.37 This tied in neatly with the findings of immunohistochemical studies, in which increased number of CD68+ cells in diagnostic biopsy specimens was prognostic of inferior progression-free survival and disease-specific survival in patients treated with doxorubicin, bleomycin, vinblastine and dacarbazine, independently of established clinical and laboratory parameters.38 The adverse prognostic impact of CD68 expression on overall survival was validated in another study from Barts Cancer Institute.39 CD68 is not specific for macrophages, as it stains other myeloid cells, and some fibroblasts.40 Increased numbers of CD163+ cells [whose expression is restricted to M2 polarized (immunosuppressive) macrophages] has been suggested by some studies to be a superior adverse prognostic marker.41–43 An interesting recent study showed that patients with Hodgkin lymphoma have higher numbers of circulating myeloid-derived suppressor cells in their peripheral blood than have healthy controls, and that increased levels of CD34+ myeloid-derived suppressor cells were predictive of inferior progression-free survival.44
With regard to lymphocyte subsets in the tumor microenvironment, increased numbers of non-follicular B cells are associated with favorable survival, indicating that they likely play an important role in the immunological control of cHL.39,45,46 Somewhat counter-intuitively, increased numbers of FOXP3+ Tregs have been associated with superior progression-free and overall survival.39,47,48 while increased numbers of granzyme B+ cytotoxic T cells have the opposite effect on survival.47,48 Although these findings require validation in larger, prospectively treated cohorts of patients, they suggest that Tregs have a contrasting function in cHL compared with solid tumors, such as direct suppression of HRS cells.
Indolent lymphomas
Follicular lymphoma
In FL and mucosal-associated lymphoid tissue (MALT) lymphoma, tumor cells appear to depend heavily on the microenvironment for survival and proliferation.2 Gene expression profiling of tumor infiltrating lymphocytes (TIL) in FL revealed two immune response signatures which predicted disparate clinical outcomes.49 Interactions between TIL and tumor cells can result in modulation of the immune response, which can have prognostic implications.50–54 For example, studies have shown that high numbers of PD1+ TIL are prognostically favorable, while patients with ≤5% PD1+ TIL had a higher risk of histological transformation to DLBCL.55 In another study from Vancouver, the follicular localization of Tregs was found to be an adverse prognostic factor for overall survival and transformation risk.56
Tumor-associated macrophages also appear to predict an unfavorable clinical course.52 Analysis of the gene expression profiles of CD4+ and CD8+ FL TIL revealed altered gene expression that resulted in impaired actin polymerization and immune synapse formation and decreased cytotoxicity and T-cell motility, leading to T-cell exhaustion and immunosuppression.57–60 This altered gene expression in TIL has prognostic significance with respect to overall survival and time to transformation.57 In terms of the potential therapeutic implications of these findings in T cells, an interesting study demonstrated that FL cells with T-cell immunological synapse dysfunction can be repaired with the immunomodulatory agent lenalidomide.59
Marginal zone lymphoma
Extranodal marginal zone lymphomas (MZL) of MALT provide a classical illustration of the role of the microenvironment in lymphomagenesis through B-cell antigen stimulation. Chronic infections may provide antigenic stimulation, which results in different manifestations of MZL at various anatomic sites. Examples include gastric MALT and Helicobacter pylori,61 splenic MZL and hepatitis C,62 ocular adnexal MZL and Chlamydophilia psittaci,63 and cutaneous MZL and Borrelia.64 Eradication of the implicated microorganism leads to lymphoma regression in many cases, supporting antigenic dependence.65 The occurrence of secondary genetic lesions, in particular t(11;18), has been associated with poor responses to eradication therapy for gastric MALT lymphoma, presumably due to the development of independence from the microenvironment for growth and survival.66 Although splenic MZL generally has an indolent course, up to one-third of patients experience rapid disease progression. Dense infiltrates of CD40+ cells within the bone marrow correlate with inferior prognosis, likely through interactions with CD40L with surrounding cells in the tumor microenvironment (including mast cells, helper T cells, dendritic cells, macrophages and B cells) resulting in immune cell activation through phosphorylation of STAT3 and resultant secretion of TNF/IL-6 – the net effect of which is the induction of a microenvironment favoring tumor growth and survival.67
Chronic lymphocytic leukemia
Studies examining tumor escape in CLL differ as to whether changes in expression of classical and non-classical human leukocyte antigens by tumor cells can modulate the interactions of NK- and T-cell subpopulations with target cells.68 In CLL, T-cell dysfunction is mediated by expression of inhibitory molecules such as CD200, CD270, PD-L1 and B7-H3 on tumor cells, with predominant influences mediated by PD-L1 expression.69,70 Expression of these molecules has been linked to a poor prognosis in patients with CLL.69 Interestingly, reducing expression of these genes in tumor cells can improve T-cell function. In addition, treatment of TIL with lenalidomide has been shown to reverse the signs of T-cell exhaustion and improve T-cell function.69
BCL-2 expression71 has been suggested to be in part controlled by miR-15/16 expression, but alternative microenvironmental interactions may be associated with BCL-2 upregulation and increased cell survival in CLL.72 Indeed, BCL-2 can be up-regulated by CD40/CD40L interactions, as shown by the increased expression upon culture with soluble CD40L. This interaction may potentially occur in the infiltrated lymphoid tissues and in particular in the proliferation centers where CD4+ T cells can be found in close proximity to leukemic B cells. Moreover, additional studies have shown that co-culture of CLL cells and stromal cells results in up-regulation of BCL-2 expression, thereby providing survival and drug-resistance signals to CLL cells.73 Investigations into the types of stromal cells that may mediate these interactions show that monocytes contribute to CLL survival and mediate expansion of CLL cells.74,75 Analyses in murine models show that depleting monocyte levels can decrease CLL burden in the mice.74 Similarly, the stimulation of surface receptors, including Toll-like receptors76 and BCR, is able to induce upregulation of BCL-2 and other anti-apoptotic molecules suggesting that a wide array of signals from the microenvironment can indeed be responsible for the regulation of apoptosis. All these signals translate into activation of downstream signaling pathways, including the MAPK and the NF-κB pathways, which contribute to the survival of leukemic cells. ERK is constitutively active in approximately 50% of CLL patients,77 likely due to the stimulation by anergizing antigenic elements, while SYK and NF-κB are upregulated in virtually all cases of CLL, with many patients having recurrent mutations within the NF-κB pathway78,79 in addition to induction by the microenvironment.
Table 1.
Overview of lymphoma subtypes, examples of impact of tumor microenvironment on outcome and novel agents of potential therapeutic relevance.
Novel therapies targeting the microenvironment
The following section focuses on several novel classes of agents that therapeutically exploit the dependence of lymphoma cells on microenvironmental stimuli as part of their mechanism of action.
Checkpoint inhibitors
PD-1 limits the response of activated T cells at sites of infection and prevents autoimmunity.80,81 Binding of PD-1 by its ligands PD-L1 and PD-L2 produces inhibitory signals that ultimately result in apoptosis of activated T cells, the so-called “immune checkpoint”.82 However, PD-1 is also present on other immune cells including Treg, B and NK cells. Thus, PD-1 blockade enhances anti-tumor cytotoxicity through increased NK-cell killing and Treg suppression.83,84 Tumor cells are able to exploit the pathway in a similar manner by expressing PD-L1 on TIL.85 In vitro experimental models indicate that PD-L1 expression by tumors results in the impairment of anti-tumor responses.86 Antibodies targeting the PD-1 axis thus “release the brakes” from effector T cells and promote anti-tumor cytotoxicity.87 Antibody-dependent cell-mediated cytotoxicity (ADCC) of tumor cells expressing PD-1 or PD-L1 does not appear to be a mechanism of action for these agents, as PD-1/PD-L1 surface expression by tumor cells or tumor microenvironment does not seem to be necessary for their activity.88 Various agents targeting the PD-1 axis are under development; however, preliminary data on three agents are currently available. The investigational agent pidilizumab is a humanized IgG1 monoclonal antibody directed against PD-1, which has been explored in phase II studies in DLBCL89 and FL.90 Pidilizumab increased in CD4+CD25+PD-L1+ activated T helper cells and PD-1 ligand-bearing monocytes in a phase II study in DLBCL,89 and in a phase II study of pidilizumab and rituximab in patients with FL a 41-gene signature representing immune activation correlated with improved progression-free survival.90 In both studies, pidilizumab was well tolerated and appeared to increase efficacy relative to historic controls. Pembrolizumab (humanized) and nivolumab (fully human), both investigational in hematologic malignancies, are IgG4 antagonistic anti-PD-1 monoclonal antibodies with outstanding activity in heavily pre-treated Hodgkin lymphoma.91,92 Preliminary results regarding nivolumab show promise in a variety of subtypes of non-Hodgkin lymphomas93 and phase II studies in multiple histological types are planned or underway.
Chimeric antigen receptor T-cell therapy
Much has been written about the success of investigational anti-CD19 CAR T-cell therapy in relapsed/refractory acute lymphoblastic leukemia, CLL and DLBCL.94–96 This technology uses gene-modified autologous T cells with antigen specificity for CD19, expressed mainly on the surface of B cells.97 CD19 represents a near optimal tumor-associated antigen to target, as its restricted expression minimizes off-target toxicity. One of the problems with CAR T-cell therapy is to overcome the immunosuppressive tumor microenvironment that includes M2 polarized macrophages, Tregs, and myeloid-derived suppressor cells.98 Investigators have approached this problem by modifying the CAR T-cell construct number in a number of customized ways, including the incorporation of pro-inflammatory cytokines such as IL-12,99 expression of dominant negative TGF-β,100 anti-apoptotic Fas-knockdowns101 and the expression of survival signals such as Bcl-xl.102 An alternate approach would be to combine CAR T cells with agents targeting the PD-1 axis to enhance the anti-tumor cytotoxicity.
B-cell receptor pathway inhibitors
B cells depend on signals mediated through the BCR to govern a variety of cellular processes including proliferation, apoptosis and differentiation.103 Deregulation of the BCR pathway is thought to be central to the pathogenesis of many B-cell lymphomas.104 The BCR signaling cascade involves numerous tyrosine kinases including Btk, Syk and PI3K, and small molecule inhibitors targeting these kinases have been developed.
Ibrutinib is a selective, small molecule that irreversibly binds to Btk.105 Ibrutinib has excellent activity in CLL,106,107 MCL108 and Waldenström macroglublinemia109 and has gained regulatory approval for the treatment of relapsed or refractory patients with these diseases and also for first-line therapy in patients with del(17p) CLL. Although the mechanism of action of ibrutinib involves direct effects on malignant B cells, including induction of apoptosis and disruption of cell adhesion and migration,110 the effects on the tumor microenvironment are also important. Btk regulates NK cell function in response to antigen presentation.111 However, ibrutinib also inhibits Itk, which is involved in NK cell effector function following FcR-mediated engagement.112 Interestingly, while some preclinical studies have shown that ibrutinib may antagonize ADCC induced by anti-CD20 monoclonal antibodies such as rituximab, in the clinical setting ibrutinib in combination with rituximab is highly active.113,114 More selective Btk inhibitors that spare Itk do not appear to have the same antagonism and may prove more effective in combinations. Through Itk inhibition, ibrutinib also influences T-cell polarization toward type 1 T helper cells and effector T cells.115 Preclinical work by Levy et al. at Stanford also suggests that ibrutinib potently enhances immunological tumor control when co-administered with a TLR9 agonist through stimulation of antigen-presenting cells in the tumor microenvironment.116 The same group also described how ibrutinib enhanced the T-cell anti-tumor activity of PD-L1 inhibitors, a finding with clear implications for combination studies.117 Btk plays a role in polarizing macrophages to an M1 (inflammatory) phenotype; as mice deficient in Btk are skewed towards M2 (immunosuppressive) polarization, which suggests a theoretical potential for ibrutinib to induce an unhelpful change in the microenvironment.118 However, we are unaware of data regarding macrophage polarization in ibrutinib-treated patients.
Several PI3K inhibitors with various isoform specificities are in development. The most advanced, idelalisib, is a selective inhibitor of the p110δ isoform of PI3K. It has demonstrated excellent clinical activity in patients with relapsed/refractory CLL/small lymphocytic lymphoma and FL, indications for which it has gained approval from both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).119–121 PI3Kδ is expressed by both normal and malignant lymphoid cells, and PI3k inhibition by idelalisib in vitro leads to induction of apoptosis.122 Like ibrutinib, idelalisib interferes with pro-survival microenvironment-derived signals, chemotaxis and adhesion.123,124 Its antagonism of ADCC induced by anti-CD20 monoclonal antibodies is weaker that that of ibrutinib in vitro.125 Idelalisib does not appear cytotoxic to T-cell subsets;126 however, the investigational dual PI3K p110γ and p110δ inhibitor duvelisib (IPI-145) reduces the viability of T and NK cells and impairs T-cell production of pro-inflammatory cytokines.127
Immunomodulatory drugs
Immunomodulatory drugs exert pleiotropic effects both directly on lymphoma cells and on the immune microenvironment. Lenalidomide (FDA-approved for multiple myeloma and relapsed MCL) has activity in a range of lymphoma subtypes both as a single agent128–131 and in combination with rituximab, particularly in MCL and FL.132–137 The molecular mechanism of action of lenalidomide has only recently been described in detail. Immunomodulatory drugs bind to the E3 ubiquitin ligase cereblon (CRBN), which is re-directed by lenalidomide to induce proteosomal degradation of the transcription factors Ikaros (IKZF1) and Aiolos (IKZF3).138–140 These transcription factors provide pro-survival signals for tumor cells and suppress IL-2 production. The binding of immunomodulatory drugs to CRBN therefore blocks survival signals to tumor cells and leads to increased IL-2 production and enhancement of T-cell co-stimulation.138 Furthermore, lenalidomide induces type 1 T helper cell polarization,141 reduces Treg cells, increases antigen presentation to effector T-cell populations,142 repairs the immune synapse between tumor cells and cytotoxic T cells,69 restores impaired T-cell motility and interferes with communication between endothelial and tumor cells, reducing neoangiogenesis.143 Lenalidomide also induces a change in the tumor microenvironment from an M2 macrophage immunosuppressive state to a pro-inflammatory state through polarization of macrophages toward an M1 phenotype.144 Lenalidomide augments the ADCC of anti-CD20 monoclonal antibodies145,146 and lowers the activation threshold of NK cells.147 The multitude of mechanisms by which lenalidomide is able to alter the tumor microenvironment into a hostile one for lymphoma provides a satisfactory explanation for the activity observed in the clinic – an excellent illustration of the potential benefits of targeting the lymphoma cell niche.
Future directions
Novel combinations
It is unlikely that any one agent or modulator of a single pathway will prove successful in inhibiting tumor cell survival over the long-term in B-cell lymphoproliferative diseases. Effective curative strategies will likely require optimal synergistic combinations of effective agents. However, the large number of possible combinations, limited resources and paucity of patients for clinical trials make it an imperative to prioritize and develop those combinations that are most likely to be curative. Designing logical combinations with strong pre-clinical rationales is, therefore, a priority of translational research in hematologic malignancies. Strategies that include the targeting of various steps of the cancer-immunity cycle148 will be imperative. For example, drugs targeting the PD-1 axis enhance the host anti-tumor response and may be logically used in combination with many of the aforementioned novel agents.148 Furthermore, “precision immunology” should consider the immunological milieu of both host and tumor. For example, highly immunogenic tumors (such as cHL) may benefit from rational strategies that include immunostimulatory combinations such as PD-1/PD-L1 inhibitors plus T-cell priming treatments.149 In contrast, immunologically inert lymphomas may be better approached with strategies such as CAR T cells in combination with agents such as monoclonal antibodies.150
Caution in developing such combination studies is required and vigilant monitoring for clinical or laboratory adverse events is essential. Two studies using the combination of lenalidomide, rituximab and idelalisib in relapsed/refractory FL were recently terminated due to an unexpected frequency and severity of hepatotoxicity, including two deaths.151,152 These episodes highlight the need to incorporate correlative studies into all multi-agent investigational protocols to survey for unexpected toxicities as well as to understand tumor biology and the reasons for treatment resistance better.
Monitoring the microenvironment during therapy
Although researchers typically obtain a snapshot of the microenvironment at the time of diagnostic biopsy, the development of processes that enable dynamic assessment is important. Although tumors with a circulating phase, such as CLL, are comparatively easy to assess at serial time-points from blood samples, obtaining biopsies during treatment poses major logistic challenges in most patients with lymphoma. To address this challenge, novel strategies that can assess circulating tumor DNA and mutational analyses in the peripheral blood are welcomed and should be incorporated in future studies aimed at developing therapies directed at the microenvironment.
Conclusion
Improved understanding of tumor biology and the role of the tumor microenvironment has led to advances in the diagnosis, classification, prognostication and novel treatment of patients with hematologic malignancies. In particular, translational research leading to drugs that target the interaction between the tumor microenvironment and malignant cells has provided many promising new approaches to cancer therapy. Ongoing dynamic and correlative studies of tumor biology and the contribution of the tumor microenvironment in the context of novel drug development should be encouraged to identify optimal therapies for various lymphomas and improve the curability of these diseases.
Acknowledgments
This manuscript was developed, in part, based on discussions from the Second Annual Summit on the Immune Microenvironment in Hematologic Malignancies that took place on September 11–12, 2014, in Dublin, Ireland. The Second Annual Summit was sponsored by a grant from AbbVie Inc., Acerta Pharma, Celgene Corporation, F. Hoffman-La Roche LTD, Infinity Pharmaceuticals, Inc., Pharmacyclics, Inc., and TG Therapeutics, Inc. Project management support for this manuscript was provided by BioConnections, LLC.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/5/531
References
- 1.Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott DW, Gascoyne RD. The tumour microenvironment in B cell lymphomas. Nat Rev Cancer. 2014;14(8):517–534. [DOI] [PubMed] [Google Scholar]
- 3.Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia. 2014;28(9):1784–1792. [DOI] [PubMed] [Google Scholar]
- 4.Brusa D, Serra S, Coscia M, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98(6):953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9(9):665–674. [DOI] [PubMed] [Google Scholar]
- 6.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 7.Dunleavy K, Roschewski M, Wilson WH. Precision treatment of distinct molecular subtypes of diffuse large B-cell lymphoma: ascribing treatment based on the molecular phenotype. Clin Cancer Res. 2014;20(20): 5182–5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz G, Wright G, Dave SS, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359(22):2313–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challa-Malladi M, Lieu YK, Califano O, et al. Combined genetic inactivation of beta2-microglobulin and CD58 reveals frequent escape from immune recognition in diffuse large B cell lymphoma. Cancer Cell. 2011;20(6):728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andorsky DJ, Yamada RE, Said J, et al. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232–4244. [DOI] [PubMed] [Google Scholar]
- 12.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062–2065. [DOI] [PubMed] [Google Scholar]
- 14.Dunleavy K, Steidl C. Emerging biological insights and novel treatment strategies in primary mediastinal large B-cell lymphoma. Semin Hematol. 2015;52(2):119–125. [DOI] [PubMed] [Google Scholar]
- 15.Twa DD, Mottok A, Chan FC, et al. Recurrent genomic rearrangements in primary testicular lymphoma. J Pathol. 2015;236(2):136–141. [DOI] [PubMed] [Google Scholar]
- 16.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66(11):5716–5722. [DOI] [PubMed] [Google Scholar]
- 18.Yang ZZ, Novak AJ, Stenson MJ, et al. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107(9):3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riihijarvi S, Fiskvik I, Taskinen M, et al. Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica. 2015;100(2):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger JA, Ford RJ. The microenvironment in mantle cell lymphoma: cellular and molecular pathways and emerging targeted therapies. Semin Cancer Biol. 2011;21(5): 308–312. [DOI] [PubMed] [Google Scholar]
- 22.Kurtova AV, Tamayo AT, Ford RJ, et al. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): importance for interactions with the stromal microenvironment and specific targeting. Blood. 2009;113(19):4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Yang J, Qian J, et al. Role of the microenvironment in mantle cell lymphoma: IL-6 is an important survival factor for the tumor cells. Blood. 2012;120(18): 3783–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen PL, Kurtin PJ, Donovan KA, et al. Bone marrow and peripheral blood involvement in mantle cell lymphoma. Br J Haematol. 1998;101(2):302–310. [DOI] [PubMed] [Google Scholar]
- 25.Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89(6):2067–2078. [PubMed] [Google Scholar]
- 26.Pittaluga S, Wlodarska I, Stul MS, et al. Mantle cell lymphoma: a clinicopathological study of 55 cases. Histopathology. 1995;26(1):17–24. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Qian J, Lu Y, et al. Immune evasion of mantle cell lymphoma: expression of B7-H1 leads to inhibited T-cell response to and killing of tumor cells. Haematologica. 2013;98(9):1458–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dal Col J, Zancai P, Terrin L, et al. Distinct functional significance of Akt and mTOR constitutive activation in mantle cell lymphoma. Blood. 2008;111(10):5142–5151. [DOI] [PubMed] [Google Scholar]
- 29.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–132. [DOI] [PubMed] [Google Scholar]
- 30.Bernard S, Danglade D, Gardano L, et al. Inhibitors of BCR signalling interrupt the survival signal mediated by the microenvironment in mantle cell lymphoma. Int J Cancer. 2015;136(12):2761–2774. [DOI] [PubMed] [Google Scholar]
- 31.Swerdlow SH, C E, Harris N, et al. WHO Classification of Tumors of the Hematopoeitic and Lymphoid Tissues. Lyon: IARC, 2008. [Google Scholar]
- 32.Plattel WJ, van den Berg A, Visser L, et al. Plasma thymus and activation-regulated chemokine as an early response marker in classical Hodgkin’s lymphoma. Haematologica. 2012;97(3):410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedvat CV, Jaffe ES, Qin J, et al. Macrophage-derived chemokine expression in classical Hodgkin’s lymphoma: application of tissue microarrays. Mod Pathol. 2001;14(12):1270–1276. [DOI] [PubMed] [Google Scholar]
- 34.Ma Y, Visser L, Roelofsen H, et al. Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood. 2008;111(4):2339–2346. [DOI] [PubMed] [Google Scholar]
- 35.Glimelius I, Edstrom A, Amini RM, et al. IL-9 expression contributes to the cellular composition in Hodgkin lymphoma. Eur J Haematol. 2006;76(4):278–283. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Sattarzadeh A, Diepstra A, et al. The microenvironment in classical Hodgkin lymphoma: an actively shaped and essential tumor component. Semin Cancer Biol. 2014;24:15–22. [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Aguilera A, Montalban C, de la Cueva P, et al. Tumor microenvironment and mitotic checkpoint are key factors in the outcome of classic Hodgkin lymphoma. Blood. 2006;108(2):662–668. [DOI] [PubMed] [Google Scholar]
- 38.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362(10):875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greaves P, Clear A, Coutinho R, et al. Expression of FOXP3, CD68, and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome. J Clin Oncol. 2013;31(2):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulford KA, Sipos A, Cordell JL, et al. Distribution of the CD68 macrophage/myeloid associated antigen. Int Immunol. 1990;2(10):973–980. [DOI] [PubMed] [Google Scholar]
- 41.Klein JL, Nguyen TT, Bien-Willner GA, et al. CD163 immunohistochemistry is superior to CD68 in predicting outcome in classical Hodgkin lymphoma. Am J Clin Pathol. 2014;141(3):381–387. [DOI] [PubMed] [Google Scholar]
- 42.Zaki MA, Wada N, Ikeda J, et al. Prognostic implication of types of tumor-associated macrophages in Hodgkin lymphoma. Virchows Arch. 2011;459(4):361–366. [DOI] [PubMed] [Google Scholar]
- 43.Tan KL, Scott DW, Hong F, et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: a correlative study from the E2496 Intergroup trial. Blood. 2012;120(16):3280–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romano A, Parrinello NL, Vetro C, et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin lymphoma patients treated up-front with a risk-adapted strategy. Br J Haematol. 2015;168(5):689–700. [DOI] [PubMed] [Google Scholar]
- 45.Panico L, Tenneriello V, Ronconi F, et al. High CD20+ background cells predict a favorable outcome in classical Hodgkin lymphoma and antagonize CD68+ macrophages. Leuk Lymphoma. 2014:1–7. [DOI] [PubMed] [Google Scholar]
- 46.Tudor CS, Distel LV, Eckhardt J, et al. B cells in classical Hodgkin lymphoma are important actors rather than bystanders in the local immune reaction. Hum Pathol. 2013;44(11):2475–2486. [DOI] [PubMed] [Google Scholar]
- 47.Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–1473. [DOI] [PubMed] [Google Scholar]
- 48.Kelley TW, Pohlman B, Elson P, et al. The ratio of FOXP3+ regulatory T cells to granzyme B+ cytotoxic T/NK cells predicts prognosis in classical Hodgkin lymphoma and is independent of bcl-2 and MAL expression. Am J Clin Pathol. 2007;128(6): 958–965. [DOI] [PubMed] [Google Scholar]
- 49.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21): 2159–2169. [DOI] [PubMed] [Google Scholar]
- 50.Alvaro T, Lejeune M, Salvado MT, et al. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol. 2006;24(34):5350–5357. [DOI] [PubMed] [Google Scholar]
- 51.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159–2169. [DOI] [PubMed] [Google Scholar]
- 52.de Jong D, Fest T. The microenvironment in follicular lymphoma. Best Pract Res Clin Haematol. 2011;24(2):135–146. [DOI] [PubMed] [Google Scholar]
- 53.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106(6):2169–2174. [DOI] [PubMed] [Google Scholar]
- 54.Glas AM, Knoops L, Delahaye L, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25(4):390–398. [DOI] [PubMed] [Google Scholar]
- 55.Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27(9):1470–1476. [DOI] [PubMed] [Google Scholar]
- 56.Farinha P, Al-Tourah A, Gill K, et al. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115(2):289–295. [DOI] [PubMed] [Google Scholar]
- 57.Kiaii S, Clear AJ, Ramsay AG, et al. Follicular lymphoma cells induce changes in T-cell gene expression and function: potential impact on survival and risk of transformation. J Clin Oncol. 2013;31(21):2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myklebust JH, Irish JM, Brody J, et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood. 2013;121(8):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114(21):4713–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramsay AG, Gribben JG. The kiss of death in FL. Blood. 2011;118(20):5365–5366. [DOI] [PubMed] [Google Scholar]
- 61.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, et al. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338(8776):1175–1176. [DOI] [PubMed] [Google Scholar]
- 62.Suarez F, Lortholary O, Hermine O, et al. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107(8):3034–3044. [DOI] [PubMed] [Google Scholar]
- 63.Ferreri AJ, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96(8):586–594. [DOI] [PubMed] [Google Scholar]
- 64.Kutting B, Bonsmann G, Metze D, et al. Borrelia burgdorferi-associated primary cutaneous B cell lymphoma: complete clearing of skin lesions after antibiotic pulse therapy or intralesional injection of interferon alfa-2a. J Am Acad Dermatol. 1997;36(2 Pt 2):311–314. [DOI] [PubMed] [Google Scholar]
- 65.Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342(8871): 575–577. [DOI] [PubMed] [Google Scholar]
- 66.Inagaki H, Nakamura T, Li C, et al. Gastric MALT lymphomas are divided into three groups based on responsiveness to Helicobacter pylori eradication and detection of API2-MALT1 fusion. Am J Surg Pathol. 2004;28(12):1560–1567. [DOI] [PubMed] [Google Scholar]
- 67.Franco G, Guarnotta C, Frossi B, et al. Bone marrow stroma CD40 expression correlates with inflammatory mast cell infiltration and disease progression in splenic marginal zone lymphoma. Blood. 2014;123(12):1836–1849. [DOI] [PubMed] [Google Scholar]
- 68.Guillaume N, Marolleau JP. Is immune escape via human leukocyte antigen expression clinically relevant in chronic lymphocytic leukemia? Focus on the controversies. Leuk Res. 2013;37(4):473–477. [DOI] [PubMed] [Google Scholar]
- 69.Ramsay AG, Clear AJ, Fatah R, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9): 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Bergwelt-Baildon M, Maecker B, Schultze J, et al. CD40 activation: potential for specific immunotherapy in B-CLL. Ann Oncol. 2004;15(6):853–857. [DOI] [PubMed] [Google Scholar]
- 72.Allegra D, Bilan V, Garding A, et al. Defective DROSHA processing contributes to downregulation of MiR-15/-16 in chronic lymphocytic leukemia. Leukemia. 2014;28(1):98–107. [DOI] [PubMed] [Google Scholar]
- 73.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanna B, McClanahan F, Zaborsky N, et al. Targeting dysfunctional myeloid cells delays disease development and improves immune fucntion in a CLL mouse model. Blood. 2014;124(21):3298. [Google Scholar]
- 75.Jitschin R, Braun M, Buttner M, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750–760. [DOI] [PubMed] [Google Scholar]
- 76.Fonte E, Apollonio B, Scarfo L, et al. In vitro sensitivity of CLL cells to fludarabine may be modulated by the stimulation of Toll-like receptors. Clin Cancer Res. 2013;19(2):367–379. [DOI] [PubMed] [Google Scholar]
- 77.Muzio M, Apollonio B, Scielzo C, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188–195. [DOI] [PubMed] [Google Scholar]
- 78.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mansouri L, Sutton LA, Ljungstrom V, et al. Functional loss of IkappaBepsilon leads to NF-kappaB deregulation in aggressive chronic lymphocytic leukemia. J Exp Med. 2015;212(6):833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. [DOI] [PubMed] [Google Scholar]
- 82.Seo SK, Seo HM, Jeong HY, et al. Co-inhibitory role of T-cell-associated B7-H1 and B7-DC in the T-cell immune response. Immunol Lett. 2006;102(2):222–228. [DOI] [PubMed] [Google Scholar]
- 83.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–5399. [DOI] [PubMed] [Google Scholar]
- 85.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moskowitz CH, Ribrag V, Michot J-M, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). Blood. 2014;124(21):290. [Google Scholar]
- 92.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lesokhin AM, Ansell SM, Armand P, et al. Preliminary results of a phase I study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. Blood (ASH Annual Meeting Abstracts). 2014;124(21):291. [Google Scholar]
- 94.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32(7):689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 99.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119(18):4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foster AE, Dotti G, Lu A, et al. Antitumor activity of EBV-specific T lymphocytes transduced with a dominant negative TGF-beta receptor. J Immunother. 2008;31(5):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dotti G, Savoldo B, Pule M, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105(12):4677–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eaton D, Gilham DE, O’Neill A, et al. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9(8):527–535. [DOI] [PubMed] [Google Scholar]
- 103.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2(12):945–956. [DOI] [PubMed] [Google Scholar]
- 104.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5(4): 251–262. [DOI] [PubMed] [Google Scholar]
- 105.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29):13075–13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med. 2015;372(15):1430–1440. [DOI] [PubMed] [Google Scholar]
- 110.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119(5):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ni Gabhann J, Spence S, Wynne C, et al. Defects in acute responses to TLR4 in Btk-deficient mice result in impaired dendritic cell-induced IFN-gamma production by natural killer cells. Clin Immunol. 2012;142(3): 373–382. [DOI] [PubMed] [Google Scholar]
- 112.Khurana D, Arneson LN, Schoon RA, et al. Differential regulation of human NK cell-mediated cytotoxicity by the tyrosine kinase Itk. J Immunol. 2007;178(6):3575–3582. [DOI] [PubMed] [Google Scholar]
- 113.Kohrt HE, Sagiv-Barfi I, Rafiq S, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123(12):1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15): 2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sagiv-Barfi I, Kohrt HE, Burckhardt L, et al. Ibrutinib enhances the antitumor immune response induced by intratumoral injection of a TLR9 ligand in mouse lymphoma. Blood. 2015;125(13):2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA. 2015;112(9):E966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ni Gabhann J, Hams E, Smith S, et al. Btk regulates macrophage polarization in response to lipopolysaccharide. PLoS One. 2014;9(1):e85834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-delta, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22):3406–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maffei R, Bulgarelli J, Fiorcari S, et al. Endothelin-1 promotes survival and chemoresistance in chronic lymphocytic leukemia B cells through ETA receptor. PLoS One. 2014;9(6):e98818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Roit FD, Engelberts PJ, Taylor RP, et al. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015;100(1):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dong S, Guinn D, Dubovsky JA, et al. IPI-145 antagonizes intrinsic and extrinsic survival signals in chronic lymphocytic leukemia cells. Blood. 2014;124(24):3583–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Witzig TE, Vose JM, Zinzani PL, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2011;22(7):1622–1627. [DOI] [PubMed] [Google Scholar]
- 129.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(30):4952–4957. [DOI] [PubMed] [Google Scholar]
- 130.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145(3):344–349. [DOI] [PubMed] [Google Scholar]
- 131.Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15(12):1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27(9):1902–1909. [DOI] [PubMed] [Google Scholar]
- 134.Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13(7):716–723. [DOI] [PubMed] [Google Scholar]
- 135.Kimby E, Martinelli G, Ostenstad B, et al. Rituximab plus lenalidomide improves the complete remission rate in comparison with rituximab monotherapy in untreated follicular lymphoma patients in need of therapy. Primary endpoint analysis of the randomized phase-2 trial SAKK 35/10. Blood. 2014;124(21):799. [Google Scholar]
- 136.Martin P, Jung S-H, Johnson JL, et al. CALGB 50803 (Alliance): a phase II trial of lenalidomide plus rituximab in patients with previously untreated follicular lymphoma. ASCO Meeting Abstracts. 2014;32(15_suppl):8521. [Google Scholar]
- 137.Ruan J, Martin P, Shah BD, et al. Sustained remission with the combination biologic doublet of lenalidomide plus rituximab as initial treatment for mantle cell lymphoma: a multi-center phase II study report. Blood (ASH Annual Meeting Abstracts). 2014;124(21):625. [Google Scholar]
- 138.Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee BN, Gao H, Cohen EN, et al. Treatment with lenalidomide modulates T-cell immunophenotype and cytokine production in patients with chronic lymphocytic leukemia. Cancer. 2011;117(17):3999–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Henry JY, Labarthe MC, Meyer B, et al. Enhanced cross-priming of naive CD8+ T cells by dendritic cells treated by the IMiDs® immunomodulatory compounds lenalidomide and pomalidomide. Immunology. 2013;139(3):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maffei R, Fiorcari S, Bulgarelli J, et al. Endothelium-mediated survival of leukemic cells and angiogenesis-related factors are affected by lenalidomide treatment in chronic lymphocytic leukemia. Exp Hematol. 2014;42(2):126–136 e121. [DOI] [PubMed] [Google Scholar]
- 144.Fiorcari S, Martinelli S, Bulgarelli J, et al. Lenalidomide interferes with tumor-promoting properties of nurse-like cells in chronic lymphocytic leukemia. Haematologica. 2015;100(2):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84(9):553–559. [DOI] [PubMed] [Google Scholar]
- 146.Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14(14): 4650–4657. [DOI] [PubMed] [Google Scholar]
- 147.Lagrue K, Carisey A, Morgan DJ, et al. Lenalidomide augments actin remodelling and lowers NK cell activation thresholds. Blood. 2015;126(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 149.Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20): 6446–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kochenderfer JN, Rosenberg SA. Chimeric antigen receptor-modified T cells in CLL. N Engl J Med. 2011;365(20):1937–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheah CY, Nastoupil LJ, Neelapu SS, et al. Lenalidomide, idelalisib, and rituximab are unacceptably toxic in patients with relapsed/refractory indolent lymphoma. Blood. 2015;125(21):3357–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith S, Pitcher BN, Jung SH, et al. Unexpected and serious toxicity observed with combined idelalisib, lenalidomide and rituximab in relapsed/refractory B cell lymphomas: Alliance A051201 and A051202 (Abstract 3091). Blood. 2014;124(21):3091. [Google Scholar]
- 153.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24(34):5343–5349. [DOI] [PubMed] [Google Scholar]