Abstract
The precise phenotype and biology of acute myeloid leukemia stem cells remain controversial, in part because the “gold standard” immunodeficient mouse engraftment assay fails in a significant fraction of patients and identifies multiple cell-types in others. We sought to analyze the clinical utility of a novel assay for putative leukemia stem cells in a large prospective cohort. The leukemic clone’s most primitive hematopoietic cellular phenotype was prospectively identified in 109 newly-diagnosed acute myeloid leukemia patients, and analyzed against clinical risk groups and outcomes. Most (80/109) patients harbored CD34+CD38− leukemia cells. The CD34+CD38− leukemia cells in 47 of the 80 patients displayed intermediate aldehyde dehydrogenase expression, while normal CD34+CD38− hematopoietic stem cells expressed high levels of aldehyde dehydrogenase. In the other 33/80 patients, the CD34+CD38− leukemia cells exhibited high aldehyde dehydrogenase activity, and most (28/33, 85%) harbored poor-risk cytogenetics or FMS-like tyrosine kinase 3 internal tandem translocations. No CD34+ leukemia cells could be detected in 28/109 patients, including 14/21 patients with nucleophosmin-1 mutations and 6/7 acute promyelocytic leukemia patients. The patients with CD34+CD38− leukemia cells with high aldehyde dehydrogenase activity manifested a significantly lower complete remission rate, as well as poorer event-free and overall survivals. The leukemic clone’s most immature phenotype was heterogeneous with respect to CD34, CD38, and ALDH expression, but correlated with acute myeloid leukemia risk groups and outcomes. The strong clinical correlations suggest that the most immature phenotype detectable in the leukemia might serve as a biomarker for “clinically-relevant” leukemia stem cells. ClinicalTrials.gov: NCT01349972.
Introduction
More than two decades ago, Lapidot et al. reported that acute myeloid leukemia (AML) cells capable of engrafting immunodeficient mice expressed a CD34+CD38− normal hematopoietic stem cell (HSC) phenotype.1 These so-called leukemia stem cells (LSCs) gave rise to partially differentiated progeny that constituted the bulk of the leukemia, but possessed only limited proliferative potential.2 More recently, leukemic cells of varying surface phenotypes, even within the same patient, have been shown to be capable of engrafting immunodeficient mice, the generally accepted “gold standard” for LSC activity.3,4 However, this traditional approach for LSC identification has proven to be somewhat elusive. Not only is the assay cumbersome and non-quantitative,5 but in a significant fraction of AML patients no leukemia cell subset will engraft5–10 even using the newer mouse models.11 This inability to confirm the identity of LSCs in many patients is at least in part the reason that the clinical relevance of LSCs remains uncertain.12
Regardless of their tumorigenic potential in immunodeficient mice, leukemic cells that persist after therapy [i.e. minimal residual disease (MRD)] are arguably the most clinically important.13,14 We recently showed that MRD during complete remission (CR) was enriched for CD34+CD38− leukemic cells, and their presence after therapy was highly associated with subsequent clinical relapse.13 Others found that CD34+CD38− leukemia cell frequency correlated with prognosis.7,15 Thus, accumulating evidence now suggests that initial clinical responses likely reflect the behavior of the bulk leukemia, while long-term survival/cure requires the eradication of LSCs.7,13–15 We also showed that the leukemic CD34+CD38− cells from most patients, particularly those with core-binding factor (CBF) AMLs, could be separated from normal HSCs by their expression of aldehyde dehydrogenase 1 (ALDH). Normal HSCs exhibited high ALDH expression (CD34+CD38−ALDHhigh), while the putative LSCs expressed intermediate levels (CD34+CD38−ALDHint).13 These findings have recently been confirmed.16,17
Clinical outcomes in AML are highly diverse with some patients curable with standard therapies, others initially refractory to all known therapies, and the majority eventually relapsing and succumbing to the disease after initially achieving CRs. While patient factors such as age and performance status may influence the heterogeneous outcomes, the underlying biology - currently best reflected by cytogenetic and molecular markers - is the major determinant. AML’s highly diverse biology suggests that the LSCs are also heterogeneous. Accordingly, our previous report identified two patients, both primary refractory to induction, whose putative LSCs demonstrated high ALDH expression indistinguishable from normal HSCs.13 We could not detect any CD34+ leukemia cells in two other patients.13 Other groups have also described heterogeneous CD34 and ALDH expression in AMLs.8,16–20
Since no leukemia subset from many patients will engraft immunodeficient mice,5–11 and no leukemic CD34+CD38− cells can be identified in some patients,4,13,15,16,21 other means for LSC identification are needed to allow for their study clinically.14 Based on our smaller study of mostly CBF AML patients,13 we hypothesized that the most primitive hematopoietic cell phenotype that could be found in leukemia cells might have important clinical relevance. Thus, we prospectively assessed the leukemia’s most immature phenotype in a multi-institutional randomized clinical trial comparing two induction therapies in patients lacking favorable-risk cytogenetics: standard cytarabine-based “7+3” therapy22 and a novel regimen called FLAM (flavopiridol, cytarabine, mitoxantrone).23,24 To fully assess heterogeneity of the leukemic clone’s most immature phenotype, we also studied patients who initially agreed to the trial but were ultimately ineligible because they were found to have favorable-risk cytogenetics. Here we find that the most primitive hematopoietic cellular phenotype present in leukemia cells is not only heterogeneous for CD34, CD38, and ALDH expression, but also that this phenotypic heterogeneity correlates with both AML risk groups and outcomes. Moreover, the robust clinical correlations suggest that the most immature phenotype detectable in the leukemia might serve as a biomarker for “clinically-relevant” LSCs.
Methods
Patients
Patients aged 18–70 with newly-diagnosed AML, excluding CBF AMLs and APL, were eligible for this multicenter clinical study (clinicaltrials.gov NCT01349972).24 Patients were randomized 2:1 to FLAM or the standard “7+3” regimens, respectively.24 Patients who achieved complete or partial responses to the first cycle were eligible to receive a second cycle of FLAM or high-dose cytarabine (HiDAC), and/or could undergo allogeneic bone marrow transplantation (alloBMT) as per physician discretion. Johns Hopkins patients who were study ineligible because their cytogenetics proved favorable were also included in this analysis. Informed consent for participation in NCT01349972, as well as for the bone marrow donations by the patients not treated on trial, was obtained in accordance with the Declaration of Helsinki as approved by the Johns Hopkins Institutional Review Board.
Isolation of cells
Specimens were collected between April 2011 and April 2013. Marrow mononuclear (MMNC) and CD34+ cell subsets were identified and isolated as previously described.13,25 At least 500,000 cells from each AML specimen were then stained with Aldefluor (Aldagen, Durham, NC, USA) to assess ALDH activity according to the manufacturer’s instructions utilizing diethylaminobenzaldehyde (DEAB) controls. Next, cells were labeled with monoclonal phycoerythrin-conjugated anti-CD34 and allophycocyanin (APC)-conjugated anti-CD38 (BD Biosciences, San Jose, CA, USA) and analyzed with a MoFlo cell sorter (Beckman Coulter, Brea, CA, USA). Gating for CD34 and CD38 populations was based on clearly distinguishable populations, or in the absence of such, the negative antibody control.25 A representative example of gating is shown in Online Supplementary Figure S1.
Fluorescence in situ hybridization (FISH) and molecular analyses
For patients with cytogenetic abnormalities detectable by FISH, 250–1000 cell aliquots were sorted directly onto slides and fixed with 3:1 methanol-glacial acetic acid (Sigma-Aldrich, St. Louis, MO, USA). FISH was performed and analyzed by the Johns Hopkins Kimmel Cancer Center Cytogenetics Core, using probes specific for the patients’ known cytogenetic abnormalities per manufacturer’s guidelines (Abbot Molecular, Des Plaines, IL, USA) as we previously described.13 Real-time polymerase chain reaction for FLT3 internal tandem duplication (ITD) (qPCR) and NPM1 mutations (reverse transcriptase-qPCR) was performed by Johns Hopkins Molecular Hematopathology Laboratory.
Data analysis
The AML’s most immature phenotype was scored in a blinded fashion by RJJ, BP, and SM as we previously described.13 Any differences in scoring were to be decided by a simple majority, but there was complete concordance on all observations. The samples were then de-identified by the Johns Hopkins Kimmel Cancer Center Specimen Accessioning Core for statistical analysis. Clinical outcomes were determined by the NCT01349972 clinical study team24 blinded to the AML phenotypic data. Event-free survival (EFS) was defined as the date of treatment to the occurrence of persistent AML, relapse, or death. Poor-risk cytogenetics [> 3 clonal abnormalities, -5, 5q-, -7, -7q, t(3;3), inv 3, non-t(9;11) 11q23 excluding t(6;9), t(9;22)] and molecular abnormalities (FLT3-ITD mutation) were classified according to the European LeukemiaNet reporting system.26
Statistical analysis
P-values for differences in categorical data were determined by Fisher’s exact tests or T-tests, and for differences in outcome, stratified by treatment arm (FLAM vs. 7+3), by Mantel-Haenszel tests. Overall survival (OS) and EFS were estimated using the Kaplan-Meier method. Differences in OS and EFS according to the leukemic clone’s most primitive hematopoietic cellular phenotypes were analyzed with hazard ratios (HR) from Cox proportional hazards models that adjust for treatment arm, and tested for significance using likelihood ratio tests. Analyses were completed using R version 3.1.1.27
Results
Patient characteristics
The leukemia clone’s most primitive hematopoietic cellular phenotype was assessed in all patients entered in NCT0134997224 with adequate bone marrow specimens for analyses. Of the 147 patients entered in the clinical trial, bone marrow samples from 98 patients were analyzed. The main reason for patients not being analyzed was the absence of a research sample because not enough cells could be obtained with the diagnostic marrow (43 patients). The specimen arrived in the laboratory but was not adequate for analysis in 4 patients, and consent for the laboratory study was withdrawn in 2 patients. Over the same time frame, seven patients with CBF AML and 14 with APL were newly diagnosed and treated at Johns Hopkins. Bone marrow samples from 4 of the CBF patients and 7 of the APL patients were available for analysis. The clinical characteristics of the 98 patients on trial and the 11 favorable-risk patients not eligible for the trial are shown in Table 1.
Table 1.
Clinical characteristics of patients studied. Clinical Trial NCT01349972 Patients.
The leukemia’s most immature phenotype was heterogeneous
We defined the most immature phenotype present in the AML based on CD34, CD38, and ALDH expression, as we previously described.25,28,29 As CD34+CD38−ALDHhigh HSCs16,28,30 differentiate into more committed progenitors, both CD34 and ALDH expression decrease while CD38 expression increases.29,31–33 Thus, CD34+CD38−ALDHint, CD34+CD38+, and CD34− cells were considered increasingly more differentiated phenotypes. The leukemic versus normal origin of the hematopoietic phenotypes was determined by cytogenetic (FISH) or molecular (FLT3-ITD or NPM1) markers when present.
CD34+ cells comprised a median of 12% (range 0.07 – 81%) of total MMNCs from the 98 patients in NCT01349972. In 22/98 of the patients, the AML phenotype was clinically determined to be CD34− by standard flow cytometry criteria:7,16,34 i.e., CD34+ cells represented < 1% of the MMNCs (Table 1, Online Supplementary Figure S2A). In all 22 patients with < 1% CD34+ cells in the MMNCs, the small fraction (mean + SEM − 0.52+0.08) of CD34+ cells was completely CD38-ALDHhigh (Table 2, Figure 1A), and displayed low forward (FSC) and side (SSC) scatter on flow cytometry (data not shown). Only a small percentage (2.2+1.6%) of the CD34+CD38−ALDHhigh cells contained the leukemia-specific marker present in the five CD34− leukemias with cytogenetics detectable by FISH (Table 2). Likewise, when an AML with < 1% CD34+ cells was FLT3-ITD or NPM1-mutated (14/22 patients), the CD34+ cells did not harbor the mutation (Figure 1B).
Table 2.
Characterization of the most immature phenotype present in the leukemia by CD34, CD38, and ALDH.
Figure 1.
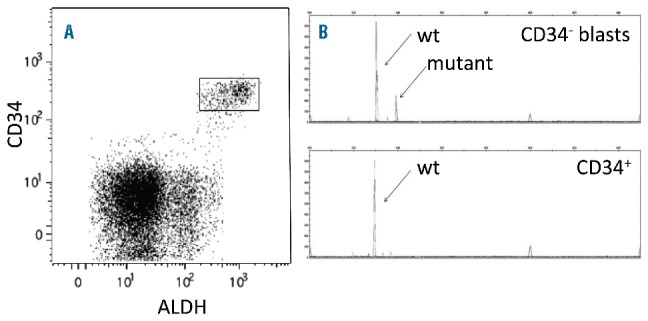
Assessment of CD34+ cells from an NPM1 and FLT3-ITD mutated AML patient with < 1% CD34+ cells. (A) Representative flow cytometric staining pattern of ALDH activity by CD34 is displayed on MMNCs from patient. All the CD34+ cells are CD34+CD38−ALDHhigh. The CD34+ALDHhigh cells are shown in rectangle. (B) FLT3-ITD status of isolated cell fractions. The CD34−blasts harbored the FLT3-ITD mutation, while the CD34+ cells exclusively displayed the 330bp wild-type gene.
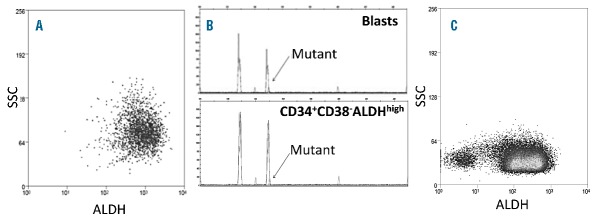
CD34+ cells comprised a mean of 25.3+3.1% of MMNCs in the 76 patients from NCT01349972 who harbored CD34+ leukemia cells; the CD34+CD38− cells comprised 44.8+3.4% of the CD34+ cells in these patients. In 43 of these 76 patients, the majority (65.1+3.4%) of CD34+CD38−cells were ALDHint (Table 2, Online Supplementary Figure S2B), while the ALDHhigh population represented 1.7+0.5% of the CD34+CD38− cells (Figure 2A, Table 2). In the 11/43 cases with leukemia-specific cytogenetics scorable by FISH, we confirmed that the CD34+CD38−ALDHint cells were predominantly leukemic (Table 2). In contrast, the small number of CD34+CD38−ALDHhigh cells predominantly lacked the FISH marker that characterized the leukemia (Table 2). Likewise, when AMLs with prominent CD34+CD38−ALDHint populations exhibited FLT3-ITD mutations (3 patients) or were NPM1-mutated (4 cases), the CD34+CD38−ALDHint cells exhibited the mutation while the CD34+CD38−ALDHhigh cells did not (Figure 2B). The CD34+CD38−ALDHhigh HSCs exhibited much lower FSC (data not shown) and SSC than the CD34+CD38−ALDHint AML cells (Figure 2A). These data are consistent with the CD34+CD38−ALDHhigh cells representing normal HSCs as we previously demonstrated.13 The 4 CBF patients also displayed prominent leukemic CD34+CD38− ALDHint populations harboring, and small CD34+CD38− ALDHhigh fractions lacking, the FISH abnormality (Tables 1, 2).
Figure 2.
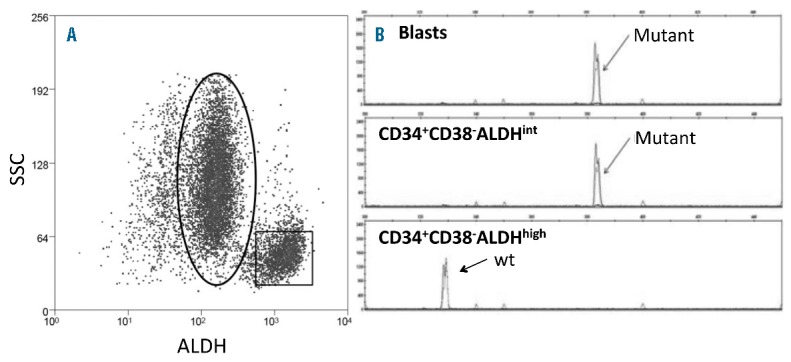
Prominent ALDHint population of CD34+CD38− cells from a patient with FLT3-ITD AML. (A) Representative flow cytometric staining pattern of ALDH activity by side scatter (SSC) is displayed for CD34+CD38− cells isolated from patient. (B) FLT3-ITD status of isolated cell fractions. The CD34+CD38−ALDHint population (oval) harbored the FLT3-ITD mutation, while the CD34+CD38−ALDHhigh cells (square) exclusively displayed the 330bp wild-type gene.
Only ALDHhigh CD34+CD38− cells were present in 26 of the 76 patients in NCT01349972 with > 1% CD34+ cells (Tables 1, 2 and Figure 3A). In the 14 patients with leukemia-specific mutations scorable by FISH, the CD34+CD38−ALDHhigh population contained mostly (78+6.7%) leukemic cells (Table 2). Similarly, this population was mostly leukemic in those patients with AML-specific mutations (6 patients with FLT3-ITD and 2 with NPM1 mutations) (Figure 3B). The CD34+CD38−ALDHhigh leukemia cell population contained many more cells, and also exhibited much higher FSC (data not shown) and SSC on flow cytometry, than CD34+CD38−ALDHhigh HSC populations (Figure 2A) as others have also found.7 In 7 of the 76 patients with CD34+ AML cells, two nearly equal-sized (or dual) CD34+CD38−ALDHint (34.4+3.3 of CD34+CD38-cells) and CD34+CD38−ALDHhigh (40.2+5.2% of CD34+CD38− cells) populations were seen (Figure 3C, Tables 1, 2). Adequate numbers of cells were sorted for FISH in 4 of these 7 patients, and both the CD34+CD38−ALDHint andCD34+CD38−ALDHhigh populations were leukemic (Table 2).
Figure 3.
Prominent ALDHhigh populations of CD34+CD38− cells. (A) Representative flow cytometric staining pattern of ALDH activity by side scatter (SSC) is displayed for CD34+CD38− cells isolated from patient. The CD34+CD38−ALDHhigh cells represented essentially all of the total CD34+CD38− cells. (B) FLT3-ITD status of isolated cell fractions. The CD34+CD38−ALDHhigh population harbored the FLT3-ITD mutation. (C) Representative flow cytometric staining pattern of ALDH activity by side scatter (SSC) is displayed for CD34+CD38− cells isolated from a patient with dual CD34+CD38−ALDHint and CD34+CD38−ALDHhigh populations.
Absence of detectable CD34+ AML cells is associated with NPM1 mutations or APL
Of the 22 patients in NCT01349972 with < 1% CD34+ cells in the MMNCs, 14 harbored NPM1 mutations compared to 8 of the 76 patients with CD34+ AML cells (Table 1; Online Supplementary Figure S2A, S2B, P<0.001). Of the 12 patients with NPM1 mutations as the sole abnormality, no CD34+ leukemia cells could be detected in 11 (Online Supplementary Figure S2A) and one harbored CD34+ CD38−ALDHint leukemia cells (Online Supplementary Figure S2B) (P<0.002). The only two patients in the series with t(9;11) were among the other 8 non-NPM1-mutated patients in this CD34− group (P<0.001), as were 4 patients with normal cytogenetics (Online Supplementary Figure S2A). Only one CD34− patient harbored poor-risk cytogenetics, and 3 of the CD34− NPM1-mutated patients also manifested FLT3-ITD mutations (Online Supplementary Figure S2A). Of the 8 CD34+NPM1-mutated patients, 6 had a predominant population of CD34+CD38−ALDHint (5 had additional detectable mutations) and 2 (both with complex cytogenetics) had CD34+CD38−ALDHhigh leukemia cells (Online Supplementary Figure S2B).
Of the 7 APL patient specimens available for study, 6 also had < 1% (0.15+0.04) CD34+ cells (Tables 1, 2). These 6 patients showed exactly the same pattern as the other AMLs with <1% CD34+: i.e., the CD34+ cells were exclusively CD38−ALDHhigh and lacked the t(15;17) by FISH (Table 2). CD34+ cells comprised 27.3% of the MMNCs in the other APL patient (Table 2); very few (0.9%) of the CD34+ cells from this patient were CD38−, and they all lacked t(15;17) by FISH. In contrast, the CD34+CD38+ cells did harbor the translocation.
CD34+CD38−ALDHhigh leukemia cells are associated with poor-risk AML
Of the 26 patients in NCT01349972 displaying a predominant CD34+CD38−ALDHhigh leukemic population, 17 had poor-risk cytogenetics and an additional 4 patients had FLT3-ITD mutations (Table 1). All 7 of the patients with dual CD34+CD38−ALDHint and CD34+CD38− ALDHhigh leukemia populations also harbored poor-risk cytogenetics: 4 had highly complex cytogenetic changes, two del 7q, and one del 5q (Table 1). Thus, 28/33 (85%) patients with CD34+CD38−ALDHhigh AML cells harbored poor-risk genetic markers, while only 4 of the 22 (18%) patients with <1% CD34+ cells and 16 out of 43 (37%) patients with predominant CD34+CD38−ALDHint populations harbored poor-risk cytogenetics or FLT3-ITD mutations (P<0.0001). The patients with CD34+CD38−ALDHhigh AML cells were also more likely to have AML arising out of myelodysplastic syndrome or myeloproliferative disease (18/33, 55%) than the CD34+CD38−ALDHint and CD34− groups (21/65, 32%, P=0.04).
The leukemias’ most primitive hematopoietic cell phenotype correlates with outcomes
Not surprisingly, given the strong association with poor-risk genetics, patients harboring CD34+CD38−ALDHhigh leukemic populations displayed relative drug resistance. There was a significantly lower CR rate for patients harboring CD34+CD38−ALDHhigh leukemic populations when compared to patients with CD34+CD38−ALDHint or no CD34+ cells AML cells (Table 3, P=0.007). The CR rates for the patients with CD34+CD38−ALDHhigh leukemic populations were similar with FLAM (11/24, 46%) and 7+3 (4/9, 44%). However, there was a trend for more CRs on the FLAM arm (36/45, 80%) than on the 7+3 arm (12/20, 60%) in the other 65 patients (P=0.1).
Table 3.
Clinical outcomes of patients in NCT01349972 by leukemia’s most primitive hematopoietic cellular phenotype.
We next studied if the most immature phenotype present in the leukemia also showed a correlation with survival. OS was significantly different according to most immature leukemia phenotype present in the leukemia (P=0.02, Table 3, Figure 4A), with patients harboring CD34+CD38−ALDHhigh AML cells demonstrating the worst OS. There was also a significant difference in EFS according to the most primitive leukemia phenotype (P<0.001, Table 3, Figure 4B). The EFS probability at 1-year was 61% (95% CI, 41–90%), 45% (95% CI 29–69%), and 19% (95% CI 8–47%) for patients without detectable CD34+ leukemia cells and those with CD34+CD38−ALDHint and CD34+CD38−ALDHhigh leukemia cells, respectively (P<0.001).
Figure 4.
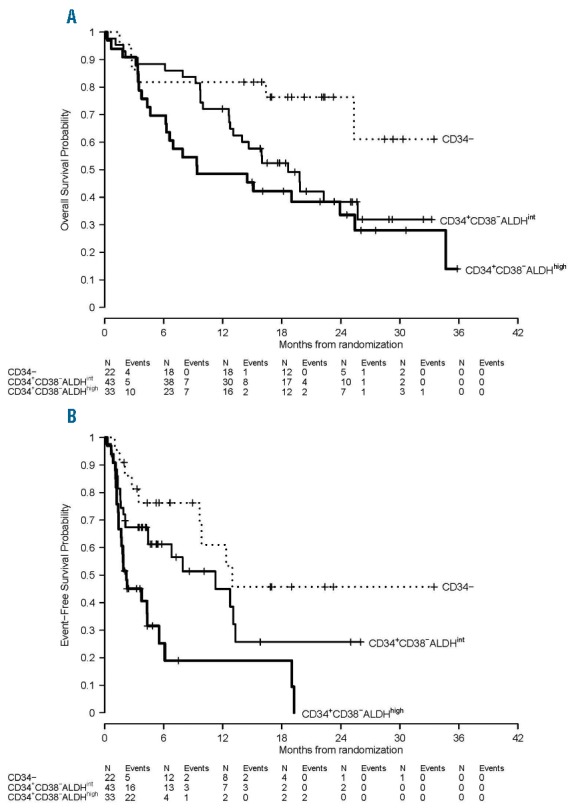
(A) OS and (B) EFS in clinical trial NCT01349972 by the most immature phenotype detectable in leukemia cells. With a median followup of 22 (range 12–36) months, OS (P=0.02) and EFS (P<0.001) were significantly different according to the leukemias’ most primitive hematopoietic phenotype.
As others have found a strong correlation between just leukemic CD34+CD38− cell numbers (without using ALDH expression) at diagnosis and outcome,7,15 we analyzed the prognostic impact of total CD34+CD38−numbers. There was a trend for total CD34+CD38− cell numbers at diagnosis to correlate with outcome. For patients with detectable CD34+ AML cells in trial NCT01349972, CD34+CD38− cells represented 5.6+1.5% of the MMNCs for those who entered a CR compared to 11+3% in those who did not (P=0.08, t-test). Of those patients with < 5% CD34+CD38− cells, 23% remained event-free compared to 9.5% with > 5% CD34+CD38− cells (P=0.2, Fisher’s exact test). The mean frequency of CD34+CD38− cells was the same in both the ALDHint and ALDHhigh groups at 7.6+1.8% and 7.5+2.5%, respectively.
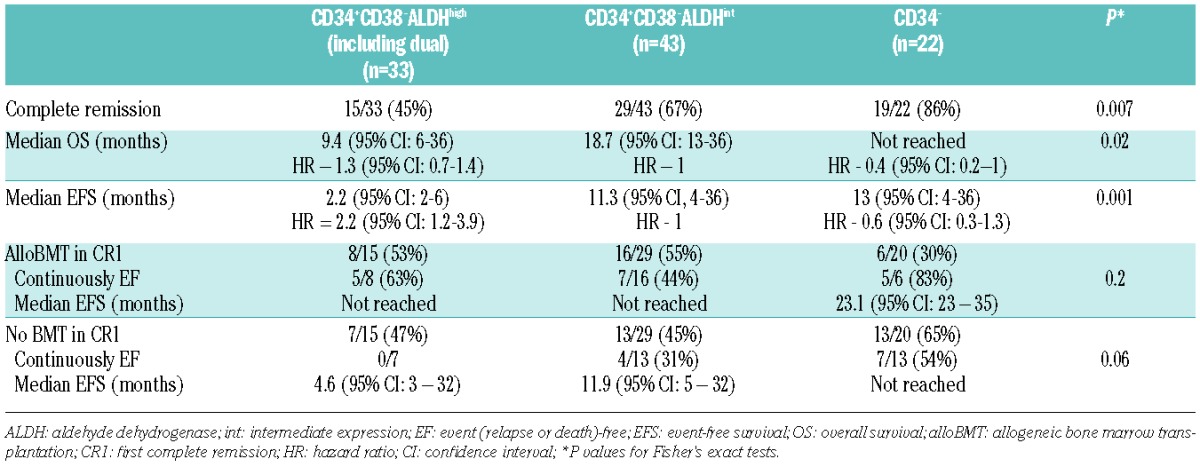
The type of postremission therapy was not specifically mandated on this trial, and many of the patients went onto alloBMT (Table 3). AlloBMT was very effective in all patients in NCT01349972, regardless of their most primitive leukemia phenotype. Of the patients with ALDHhigh leukemia cells, 8/15 who achieved a CR underwent alloBMT in CR1 and 5 remain alive and disease-free (Table 3). Similarly, 16 of the CD34+CD38−ALDHint and 6 of the CD34− patients underwent alloBMT in CR1, and 7 and 5 patients remain alive and disease-free, respectively (Table 3, P=0.2). In contrast, the outcomes of the patients who did not receive alloBMT in CR1, with most receiving cytarabine-based consolidation therapy, differed significantly by the most primitive phenotype present in leukemia cells. Seven patients with CD34+CD38−ALDHhigh leukemia cells did not undergo alloBMT in CR1, and all relapsed including 3 with normal cytogenetics and wild-type FLT3/NPM1 (Table 3). In contrast, 4/13 (3/9 with normal cytogenetics and wild-type FLT3/NPM1) patients with CD34+CD38−ALDHint leukemia cells and 7/13 (1 of 2 with normal cytogenetics and wild-type FLT3/NPM1) CD34− patients who did not undergo alloBMT remain alive and disease-free in CR1 (Table 3, P=0.06).
Discussion
The failure of CRs to reliably translate into cures in AML35,36 can be explained by the LSC paradigm. However, the true clinical relevance of LSCs remains the focus of considerable debate.3–20,37 Several groups have shown that CD34+CD38− leukemia cell numbers present at diagnosis have strong prognostic significance, providing support for a clinical relevance for LSCs.7,15 Patients with increased numbers of CD34+CD38− at diagnosis in clinical trial NCT01349972 showed a trend toward worse outcomes. Our inability to show a stronger clinical correlation between CD34+CD38− leukemia cell numbers at diagnosis and outcome may relate to the exclusion of favorable-risk cytogenetic-risk groups from the study. We also did not use the same methodology as others who showed a stronger correlation; we analyzed only total CD34+CD38− numbers, while others further refined the CD34+CD38− subset to include the expression of leukemic stem cell associated markers7 or CD123.15 We did find that the most immature hematopoietic cellular phenotype present in leukemia cells was heterogeneous, ranging from CD34− to that of primitive HSCs (i.e., CD34+CD38−ALDHhigh), but was relatively consistent across AML risk groups. Perhaps most importantly, the strong association between the leukemic clone’s most immature phenotype and outcome in this prospective patient cohort supports further testing of this clinical biomarker in future studies.
The vast majority of AML patients (80/109) in our series harbored CD34+CD38− leukemia cells, as initially reported by Lapidot et al.1 Moreover, we confirmed our prior data13 that the majority of non poor-risk AMLs, including all of the CBF patients, harbored CD34+CD38− leukemia cells that could be separated from normal HSCs by their lower ALDH activity. However, 33 out of 98 (34%) of patients from NCT01349972 harbored CD34+CD38−ALDHhigh leukemia cells. This group of patients was more likely to harbor poor-risk cytogenetics or FLT3-ITD mutations, and had a statistically lower chance of achieving CRs than the other AML patients. Importantly, the presence of CD34+CD38−ALDHhigh leukemia cells was associated with a significantly lower EFS and OS, even when no unfavorable genetic or cytogenetic abnormalities could be identified. Even though patients with CD34+CD38−ALDHhigh LSCs did poorly overall, 5/8 of those patients who got to alloBMT remain alive and disease-free. Several groups have also described ALDHhigh LSCs in a fraction of AML patients who appeared to have a worse prognosis.8,17–20
No CD34+ leukemia cells could be detected in 22/98 patients from NCT01349972. As others have also described,7,16,34 the CD34+ cells in these patients represented <1% of the cells at diagnosis, were exclusively CD38−ALDHhigh, and lacked the leukemic mutation. Thus, the CD34+ cells in such patients likely represented residual normal HSCs. NPM1 mutations were detected in 14 (64%) of the 22 AML patients who lacked detectable CD34+ cells, and 11/12 AML patients with NPM1 mutation as a sole abnormality were in this group. No CD34+ cells were detected in 6/7 APL patients, as others have also found.38 The one APL patient in this series with CD34+ AML cells only had the t(15;17) detected in the CD34+CD38+ cells. Other groups have reported that CD34 expression is a bad prognostic factor for both NPM1-mutated AMLs39 and APLs;40–42 the small numbers of these patients in our cohort may have hindered demonstrating similar statistical significance.
The phenotype of the LSCs in NPM1-mutated AML has been somewhat controversial. Two other groups also found that most NPM1-mutated AMLs were CD34−, with the CD34+ cells lacking leukemia mutations.4,16 However, Martelli et al. found that the NPM1 mutation was present in CD34+CD38− cells, and these cells generated AML in immunodeficient mice.43 Interestingly, CD34+ cells represented >1.5% of the MMNCs in all the NPM1-mutated AMLs transplanted into mice in that report.43 We also found the mutation in the CD34+CD38− cells from all 8 NPM1-mutated AML patients with >1% CD34+ MMNCs. Of note, 7 of these patients had cytogenetic or FLT3-ITD mutations in addition to NPM1. Thus, it appears that the most immature leukemic cell in NPM1-mutated AMLs can be either CD34+ or CD34−; it is possible that the differences can be explained by the fact that Martelli et al. did not perform immunodeficient mouse transplants with any of the 18 patients in their series harboring < 1% CD34+ cells.43
Despite our inability to detect AML cells by PCR in the small population of CD34+ cells present in the diagnostic marrows of the CD34− AMLs, a very small number (2.2%) of CD34+CD38−ALDHhigh cells had the FISH marker that characterized the AML (Table 2). Zeijlemaker et al. recently suggested that although the vast majority of AML patients with < 1% CD34+ cells in their diagnostic marrow lacked CD34+ AML cells, a small number did harbor neoplastic CD34+ cells.21 It is similarly possible that CD34+CD38−ALDHhigh AML cells may be present at very low levels in the patients whose leukemias’ most immature phenotype appeared to be CD34+CD38−ALDHint; however, based on the low FSC/SSC of these CD34+CD38−ALDHhigh cells, we believe that these small leukemic populations by FISH represent flow sorting contamination. We also previously found that the CD34+CD38−ALDHhigh cells present in AMLs harboring large CD34+CD38−ALDHint populations only produced normal hematopoiesis when transplanted into immunocompromised mice.13 Importantly, the phenotype of the most primitive hematopoietic cells found to harbor predominately leukemia-specific mutations correlated with AML risk groups and outcomes.
Our data raise the possibility that the most immature phenotype present in leukemia may be a function of the stage of hematopoietic differentiation at which the leukemogenic mutation develops. Those AMLs harboring leukemia cells sharing a phenotype with primitive normal HSCs (CD34+CD38−ALDHhigh) had the worst prognosis, while CBF and intermediate-risk AMLs’ most primitive phenotype was that of more differentiated hematopoietic progenitors (CD34+CD38−ALDHint). The most immature hematopoietic phenotype found in the most favorable-risk AMLs, APLs and those with NPM1-mutations as sole abnormalities, expressed even more differentiated phenotypes: CD34+CD38+ and CD34−CD38+.
These findings suggest that the leukemia clones’ most primitive hematopoietic cellular phenotype might serve as a biomarker for risk-stratifying patients at diagnosis. About 30–40% of AML patients lack any cytogenetic or usual genetic prognostic factors,44 and even when present such prognostic factors may not be available for days or weeks. The most immature phenotype present in leukemia cells can be readily determined in essentially all patients by flow cytometry within hours of diagnosis. Rapid risk-stratification may be particularly useful for patients harboring CD34+CD38−ALDHhigh leukemia cells, which appear to identify high-risk patients often refractory to induction chemotherapy. Although the phenotype of CD34+CD38−ALDHhigh leukemia cells is the same as normal HSCs, the flow cytometric pattern of the CD34+CD38−ALDHhigh population at AML diagnosis allows the primitive leukemic phenotype to be clearly distinguished from HSCs even in the absence of cytogenetic or genetic markers. The ALDHhigh cells represent the vast majority of the CD34+CD38− cells and had higher FSC/SSC at diagnosis when leukemic (Figures 3A, Table 2), while the low FSC/SSC ALDHhigh HSCs represented only a very small percentage (on average 1–2%) of the total CD34+CD38− cells (Figure 2A, Table 2). Others have published similar findings.7 Should studies confirm the adverse prognosis of a CD34+CD38−ALDHhigh leukemia phenotype, rapid identification of such patients could allow them early access to clinical trials studying novel induction approaches. Moreover, a CD34+CD38−ALDHhigh leukemic phenotype could be used to guide patients toward alloBMT when no prognostic cytogenetic or genetic abnormalities are present.
Acknowledgments
The authors thank the patients who contributed research samples, investigators who enrolled patients on this clinical trial and graciously shared patient samples (Matthew C. Foster: University of North Carolina, Mark R. Litzow: Mayo Clinic-Rochester, MN, Lawrence E. Morris: The Blood and Marrow Transplant Group at Northside Hospital, Stephen Strickland: Vanderbilt University Medical Center, Jeffrey E. Lancet: H. Lee Moffitt Cancer and Research Institute, Prithviraj Bose: Virginia Commonwealth University, M. Yair Levy: Texas Oncology, Baylor Charles A. Simmons Cancer Center, and Raoul Tibes: Mayo Clinic-Scottsdale, AZ, USA), the Cancer Therapy Evaluation Program (L. Austin Doyle, John J. Wright, Richard F. Little) at the NCI for sponsoring and supporting the clinical study, and the research staff at the Johns Hopkins Kimmel Cancer Center who assisted in specimen procurement. J.F.Z. is a recipient of a 2013 Young Investigator Award, in memory Dr. John R. Durant, and a 2014–2017 LLS Special Fellow in Clinical Research Award.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/5/607
Funding
This work was supported by the Leukemia & Lymphoma Society (LLS) (TRP R6459-13, R.J.J. and J.M.G.), and the National Institutes of Health [grants P01 CA015396 (R.J.J.), U01 A70095 (J.E.K.), 5T32 HL007525 (J.M.G.), and P30 CA006973].
References
- 1.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. [DOI] [PubMed] [Google Scholar]
- 3.Sarry JE, Murphy K, Perry R, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2011;121(1):384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taussig DC, Vargaftig J, Miraki-Moud F, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(−) fraction. Blood. 2010;115(10):1976–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce DJ, Taussig D, Zibara K, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107(3):1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung AM, Chow HC, Kwong YL, Liang R, Leung AY. FLT3/internal tandem duplication subclones in acute myeloid leukemia differ in their engraftment potential in NOD/SCID mice. Leuk Res. 2010;34(1):119–122. [DOI] [PubMed] [Google Scholar]
- 7.Terwijn M, Zeijlemaker W, Kelder A, et al. Leukemic stem cell frequency: a strong biomarker for clinical outcome in acute myeloid leukemia. PLoS One. 2014;9(9):e107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung AM, Wan TS, Leung JC, et al. Aldehyde dehydrogenase activity in leukemic blasts defines a subgroup of acute myeloid leukemia with adverse prognosis and superior NOD/SCID engrafting potential. Leukemia. 2007;21(7):1423–1430. [DOI] [PubMed] [Google Scholar]
- 9.Monaco G, Konopleva M, Munsell M, et al. Engraftment of acute myeloid leukemia in NOD/SCID mice is independent of CXCR4 and predicts poor patient survival. Stem Cells. 2004;22(2):188–201. [DOI] [PubMed] [Google Scholar]
- 10.Ailles LE, Gerhard B, Kawagoe H, Hogge DE. Growth characteristics of acute myelogenous leukemia progenitors that initiate malignant hematopoiesis in nonobese diabetic/severe combined immunodeficient mice. Blood. 1999;94(5):1761–1772. [PubMed] [Google Scholar]
- 11.Feuring-Buske M, Gerhard B, Cashman J, Humphries RK, Eaves CJ, Hogge DE. Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia. 2003;17(4):760–763. [DOI] [PubMed] [Google Scholar]
- 12.Rombouts WJ, Martens AC, Ploemacher RE. Identification of variables determining the engraftment potential of human acute myeloid leukemia in the immunodeficient NOD/SCID human chimera model. Leukemia. 2000;14(5):889–897. [DOI] [PubMed] [Google Scholar]
- 13.Gerber JM, Smith BD, Ngwang B, et al. A clinically relevant population of leukemic CD34+CD38 − cells in acute myeloid leukemia. Blood. 2012;119(15):3571–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiaur G, Gerber J, Jones RJ. Concise review: Cancer stem cells and minimal residual disease. Stem Cells. 2012;30(1):89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vergez F, Green AS, Tamburini J, et al. High levels of CD34+CD38low/−CD123+ blasts are predictive of an adverse outcome in acute myeloid leukemia: a Groupe Ouest-Est des Leucemies Aigues et Maladies du Sang (GOELAMS) study. Haematologica. 2011;96(12):1792–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuurhuis GJ, Meel MH, Wouters F, et al. Normal hematopoietic stem cells within the AML bone marrow have a distinct and higher ALDH activity level than co-existing leukemic stem cells. PLoS One. 2013; 8(11):e78897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang VT, Buss EC, Wang W, et al. The rarity of ALDH(+) cells is the key to separation of normal versus leukemia stem cells by ALDH activity in AML patients. Int J Cancer. 2015;137(3):525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce DJ, Taussig D, Simpson C, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23(6):752–760. [DOI] [PubMed] [Google Scholar]
- 19.Ran D, Schubert M, Pietsch L, et al. Aldehyde dehydrogenase activity among primary leukemia cells is associated with stem cell features and correlates with adverse clinical outcomes. Exp Hematol. 2009;37(12):1423–1434. [DOI] [PubMed] [Google Scholar]
- 20.Ran D, Schubert M, Taubert I, et al. Heterogeneity of leukemia stem cell candidates at diagnosis of acute myeloid leukemia and their clinical significance. Exp Hematol. 2012;40(2):155–165. [DOI] [PubMed] [Google Scholar]
- 21.Zeijlemaker W, Kelder A, Wouters R, et al. Absence of leukaemic CD34 cells in acute myeloid leukaemia is of high prognostic value: a longstanding controversy deciphered. Br J Haematol. 2015;171(2):227–238. [DOI] [PubMed] [Google Scholar]
- 22.Estey EH. How to manage high-risk acute myeloid leukemia. Leukemia. 2012;26(5):861–869. [DOI] [PubMed] [Google Scholar]
- 23.Karp JE, Blackford A, Smith BD, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34(7):877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeidner JF, Foster MC, Blackford AL, et al. Randomized multicenter phase 2 study of flavopiridol (alvocidib), cytarabine, and mitoxantrone (FLAM) versus cytarabine/daunorubicin (7+3) in newly diagnosed acute myeloid leukemia. Haematologica. 2015;100(9):1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber JM, Qin L, Kowalski J, et al. Characterization of chronic myeloid leukemia stem cells. Am J Hematol. 2011;8631–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 27.R: R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Viena, Austria: 2014. [Google Scholar]
- 28.Ghiaur G, Yegnasubramanian S, Perkins B, Gucwa JL, Gerber JM, Jones RJ. Regulation of human hematopoietic stem cell self-renewal by the microenvironment’s control of retinoic acid signaling. Proc Natl Acad Sci USA. 2013;110(40):16121–16126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerber JM, Gucwa JL, Esopi D, et al. Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget. 2013;4(5):715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Storms RW, Trujillo AP, Springer JB, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96(16):9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87(1):1–13. [PubMed] [Google Scholar]
- 32.Jones RJ, Barber JP, Vala MS, et al. Assessment of aldehyde dehydrogenase in viable cells. Blood. 1995;85(10):2742–2746. [PubMed] [Google Scholar]
- 33.Jones RJ, Collector MI, Barber JP, et al. Characterization of mouse lymphohematopoietic stem cells lacking colony-forming activity. Blood. 1996;88(2):487–491. [PubMed] [Google Scholar]
- 34.van der Pol MA, Feller N, Roseboom M, et al. Assessment of the normal or leukemic nature of CD34+ cells in acute myeloid leukemia with low percentages of CD34 cells. Haematologica. 2003;88(9):983–993. [PubMed] [Google Scholar]
- 35.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 37.Becker MW, Jordan CT. Leukemia stem cells in 2010: current understanding and future directions. Blood Rev. 2011;25(2):75–81. [DOI] [PubMed] [Google Scholar]
- 38.Turhan AG, Lemoine FM, Debert C, et al. Highly purified primitive hematopoietic stem cells are PML-RARA negative and generate nonclonal progenitors in acute promyelocytic leukemia. Blood. 1995; 85(8):2154–2161. [PubMed] [Google Scholar]
- 39.Dang H, Chen Y, Kamel-Reid S, Brandwein J, Chang H. CD34 expression predicts an adverse outcome in patients with NPM1-positive acute myeloid leukemia. Hum Pathol. 2013;44(10):2038–2046. [DOI] [PubMed] [Google Scholar]
- 40.Lee JJ, Cho D, Chung IJ, et al. CD34 expression is associated with poor clinical outcome in patients with acute promyelocytic leukemia. Am J Hematol. 2003;73(3):149–153. [DOI] [PubMed] [Google Scholar]
- 41.Breccia M, De Propris MS, Stefanizzi C, et al. Negative prognostic value of CD34 antigen also if expressed on a small population of acute promyelocitic leukemia cells. Ann Hematol. 2014;93(11):1819–1823. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad EI, Akl HK, Hashem ME, Elgohary TA. The biological characteristics of adult CD34+ acute promyelocytic leukemia. Med Oncol. 2012;29(2):1119–1126. [DOI] [PubMed] [Google Scholar]
- 43.Martelli MP, Pettirossi V, Thiede C, et al. CD34+ cells from AML with mutated NPM1 harbor cytoplasmic mutated nucleophosmin and generate leukemia in immunocompromised mice. Blood. 2010;116(19):3907–3922. [DOI] [PubMed] [Google Scholar]
- 44.Walker AR, Marcucci G. Management of patients with cytogenetically normal acute myeloid leukemia who have neither favorable nor unfavorable markers. J Natl Compr Canc Netw. 2014;12(4):527–534. [DOI] [PubMed] [Google Scholar]