Abstract
Brentuximab vedotin was reported to be effective and safe against refractory/relapsed Hodgkin lymphoma in cohorts of between 12 to 102 patients. Herein we report our retrospective analysis of the French experience with brentuximab vedotin used alone to treat 240 refractory/relapsed Hodgkin lymphoma patients enrolled in a named patient program between 2011 and 2013. All patients had histologically documented CD30+ Hodgkin lymphoma; 74% had refractory disease or early relapses. After a median of 3 lines of chemotherapy, brentuximab vedotin was infused intravenously (1.8 mg/kg every 3 weeks). The primary endpoint was best response. Response at the end of treatment, its duration, survival data and toxicity profile were secondary endpoints. Patients received a median of 6 cycles; 68 underwent a consolidation thereafter. The best response was observed after a median of 4 cycles in 145 (60.4%) patients: 33.8% complete response/unconfirmed complete response, 26.7% partial response. Objective responses were observed as decreased (39.3%) in the 28 patients >60 years. The median response duration was 8.4 months. With median follow-up at 16.1 months, median progression-free survival was 6.8 months and this was significantly longer for patients transplanted after brentuximab vedotin (a median of 18,8 months); median overall survival was not reached. No death has been linked to brentuximab vedotin toxicity. The most common adverse events were peripheral sensory neuropathy (29.3%) and hematological toxicity. The results of this analysis support the previously reported brentuximab vedotin efficacy with manageable toxicity. Because of the short-term responses in most patients, a high-dose therapy with stem cell transplantation for responders should be considered as quickly as possible.
Introduction
Conventional chemotherapy (with radiation for localized disease) has made Hodgkin lymphoma (HL) a curable malignancy for most patients. However, approximately 20% of HL patients have refractory disease or relapse after currently available first-line treatments. Patients with chemosensitive HL after salvage therapy should receive high-dose chemotherapy supported by autologous stem cell transplantation (auto-SCT) as standard.1 However, about 50% of patients relapse after auto-SCT, underscoring the need for effective agents for post-autograft failures. Alternating non-cross-resistant chemotherapy lines, exploring the role of novel drugs and ensuring early referral for allogeneic SCT (allo-SCT) seem to be the best current options.
CD30 is a transmembrane receptor protein expressed on activated B or T lymphocytes and on the surface of malignant Hodgkin Reed-Sternberg cells. Through CD30–CD30 ligand interaction, eosinophils and mast cells may stimulate those malignant cells, promoting their survival.2 Brentuximab vedotin (BV, SGN-35) is a chimeric anti-CD30 antibody conjugated via a protease-cleavable linker to a microtubule-disrupting agent, monomethyl auristatin E. After binding to the CD30 cell-surface, that agent is internalized, traffics to the lysosome and is released to disrupt microtubules, inducing cell cycle arrest and apoptosis.3
In the phase I, dose-escalation trial on 12 relapsed/refractory CD30+ HL patients, the maximum tolerated BV dose was determined to be 1.8 mg/kg every 3 weeks.4 Tumor regression was observed in 50% of the patients. Subsequently, BV was tested in a phase II trial on 102 patients with relapsed/refractory HL who had already undergone auto-SCT.5 In that pivotal, phase II, multicenter trial, 75% of the patients achieved an objective response and 34% a complete response (CR). Others have reported their retrospective experiences with between 25 to 65 patients:6–9 overall response rate (ORR) varied between 60% and 72%, with the CR rate between 17% and 34%, the median response duration was 8 to 9 months, with a median progression-free survival (PFS) rate of between 5 and 7 months.
From 2011–2013, BV was available in France for a Millennium/Takeda Named Patient Program (NPP). Herein, we describe objective responses, adverse events, PFS and overall survival (OS) rates of 240 patients among the 313 enrolled in this NPP.
Methods
Study design
The BV NPP was open from January 2011 until October 2013. All patients had histologically confirmed CD30+ HL and all patients had relapsed after prior auto-SCT, or after two chemotherapy lines (if the patients were not SCT candidates due to age, insufficient stem cell collection or chemorefractory disease). BV, 1.8 mg/kg (capped at 100 kg of body weight) in 250 mL of 0.9% saline was infused intravenously over 30 minutes once every 3 weeks (for a maximum of 16 cycles).
The LYSA group analyzed the outcome of HL patients treated with BV during this NPP. Takeda provided the list of patients authorized to receive BV treatment. Inclusion criteria were diagnosis of relapsed/refractory CD30+ HL and treatment with BV in the NPP non-trial setting. We asked all participating centers that included more than one patient to complete a dedicated case report form (CRF). Among the 313 patients included in the NPP, 240 patients with completed CRFs from 89 French centers were included in this retrospective analysis. No data were available for the remaining patients due to small centers having included only a few patients. The study was approved by competent authorities, namely the Comité Consultatif sur le Traitement de l’Information en matière de Recherche dans le domaine de la Santé (CCTIRS) and the Commission Nationale de l’Informatique et des Libertés (CNIL). All CRFs were included in a LYSARC (The Lymphoma Academic Research Organization) specific database.
Study assessments
Response was assessed by computerized tomography (CT) or FDG positron emission tomography (FDG-PET/CT) scans according to the revised response criteria for malignant lymphoma.10,11 It was specified in the Temporary Recommendations for Use of the NPP to perform an evaluation of the disease after 4 cycles of BV. Safety and tolerance were evaluated by recording the frequencies and severities of all adverse events according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE) v4.0. BV dose reduction to 1.2 mg/kg was recommended for grade 3 toxicity.
Statistical analyses
The primary objective was the best response to BV. Response at the end of BV treatment, its duration, disease-free survival (DFS), PFS, OS and toxicity profile were secondary objectives.
Response duration was measured from the date of the first response evaluated as CR/unconfirmed CR (uCR) or partial response (PR) to the date of the first documented disease progression, relapse or death from any cause, whichever occurred first. DFS was measured from the date of the first response evaluated as CR/CRu to the date of the first documented disease progression or relapse or death from any cause, whichever occurred first. PFS was measured from the date of the first BV cycle to the date of the first documented disease progression, relapse or death from any cause, whichever occurred first. OS was defined as the time from BV onset to death from any cause and was censored at the date of the last update. OS and PFS were estimated with the Kaplan-Meier method, using exact 95% confidence intervals (95% CIs), and with P<0.05 defining statistical significance. Analyses were computed using SAS software version 9.2.
Results
Patient characteristics
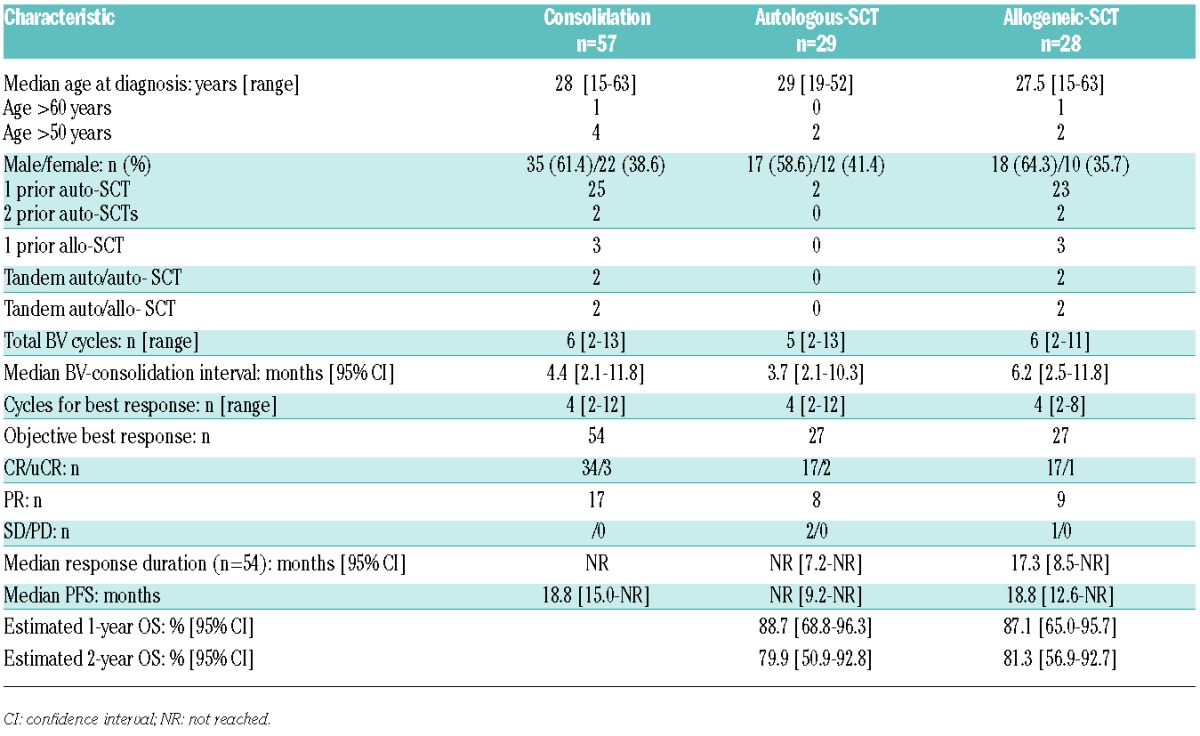
Table 1 summarizes the characteristics of the 240 patients (156 (65%) males and 84 (35%) females), whose median age at HL diagnosis was of 30 (range: 14–78) years. At diagnosis, performance status was 0/1 for 146 patients, almost 60% had stage III/IV HL, 46.2% had bulky mediastinum and about 40% had extranodal disease. Eighteen patients had previous malignancies, including six non-HL and six patients with human immunodeficiency virus seropositive. In this large cohort, patients were young (<60 years) and first-line chemotherapy was based on an ABVD regimen for 82.8% of them; only around 24% of patients had previously received radiotherapy.
Table 1.
Demographics and baseline clinical characteristics of 240 HL patients.
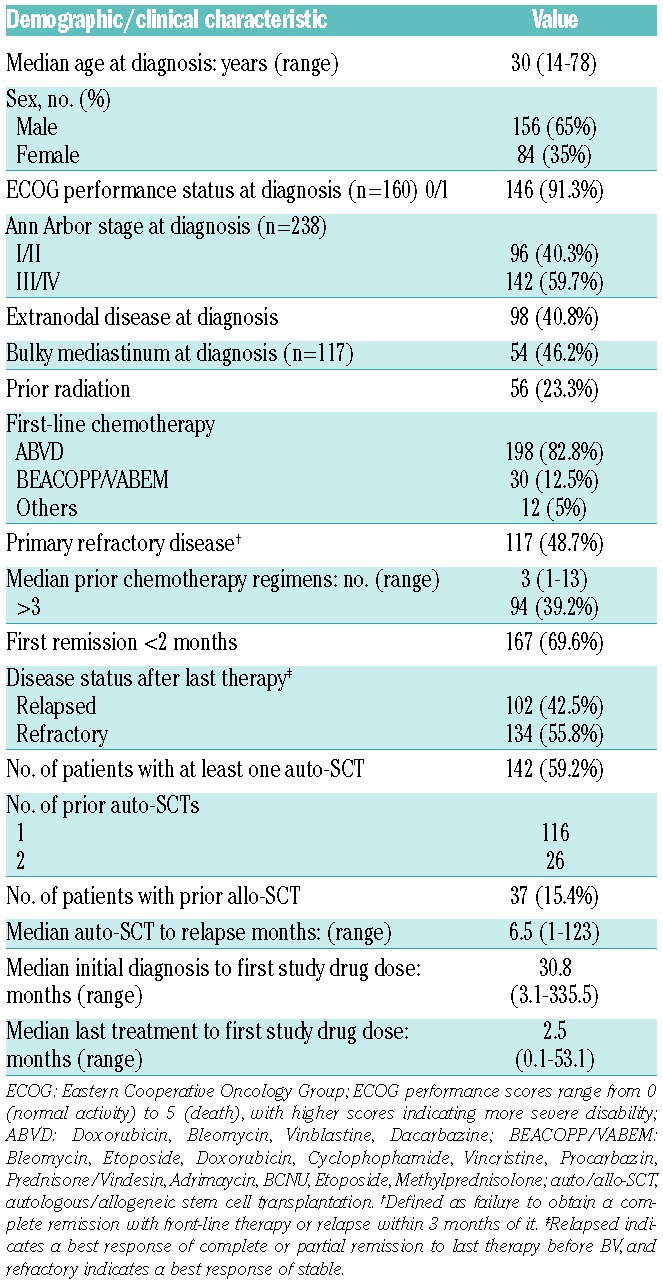
Thirty-seven patients (24 men and 13 women) had undergone allo-SCT before BV. Their median age at HL diagnosis was 27 (range: 14–56), and 32 (range: 18–60) years at BV initiation. Patients had received a median of four (range: 1–13) prior chemotherapy regimens and 75% of them had already undergone radiotherapy. The median remission duration was 4.6 months (range: 0.3–27.6) and 32 out of 37 (86.5%) of them underwent auto-SCT before allo-SCT. Conditioning regimens were mostly reduced-intensity conditioning (RIC), mainly fludarabine-based RIC for 25 out of 37 patients
Safety
All patients enrolled in this retrospective analysis received at least one BV infusion with a median of six (range: 1–16) cycles; only 10 patients received all 16 cycles. Dose intensity was >75% for 91.6% of the patients. Only 40 patients had at least one dose reduction from 1.8 to 1.2 mg/kg (because of neuropathy for 11 patients and hepatic cytolysis for 2 patients).
The most common treatment-related adverse events (≥10%) were anemia (39%), peripheral neuropathy (29.3%), thrombocytopenia (28%), neutropenia (23%), diarrhea (14%) and infections (10%) (Figure 1).
Figure 1.
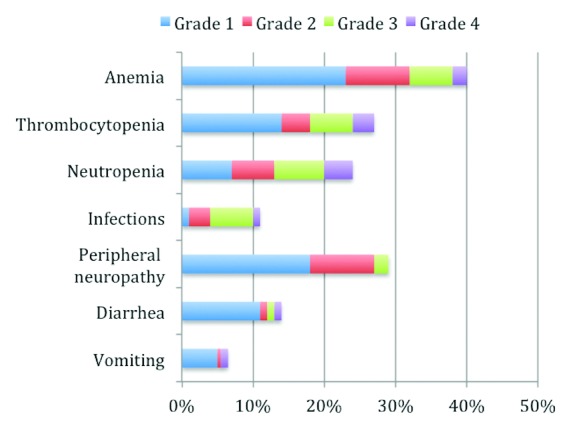
Frequencies of BV-related adverse events.
Peripheral neuropathy was present before BV treatment in 5% of the patients and increased with the number of cycles, peaking at cycle 7. Other observed grade 3 or 4 toxicities concerned 8 patients: 2 with hepatic cytolysis, 1 hypersensitivity requiring a desensitization procedure, 4 patients with bone pain and 1 with viral meningoencephalitis.
BV was discontinued for 229 patients, but for only 17 (7.4%) due to toxicity. Other causes were HL progression in 126 (55%) patients, planned transplant for 58 (25.3%), donor lymphocyte infusion (DLI) for 6 patients, radiotherapy for 11, 7 deaths and 4 patients lost to follow-up.
Safety was highly acceptable for the 37 patients with prior allo-SCT. Anemia, thrombocytopenia and neutropenia were reported in 27.6%, 28.6% and 36.7% of them, respectively. Infections occurred less frequently (6.9%) probably because of the frequent use of anti-infectious agents in the post-transplant context. Vomiting and diarrhea (both grade <3) occurred in 14.3% and 17.2% of them, respectively. Peripheral neuropathy, no grade 3 or 4 neuropathy, was reported in 46.9% of patients.
Efficacy
Responses and outcomes (DFS, PFS, OS) are summarized in Table 2 and Figure 2, respectively. The median follow-up was 16.1 (95% CI [0.2–32.6]) months.
Table 2.
Key responses in all patients and those relapsing after allo-SCT.
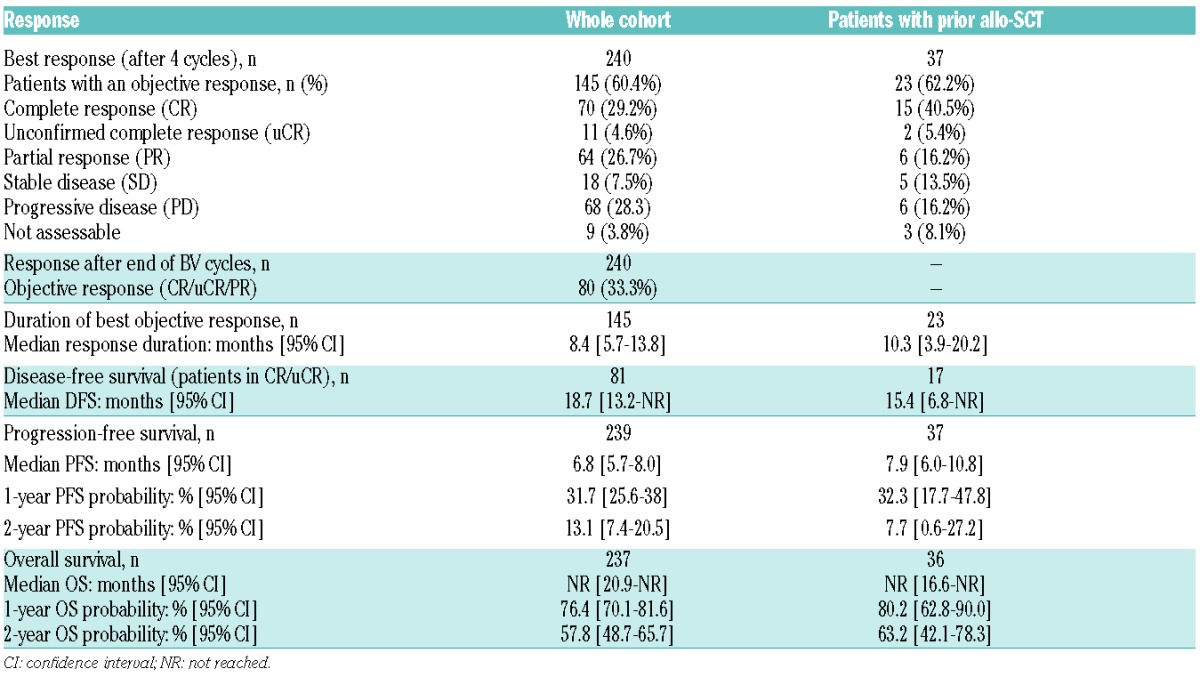
Figure 2.
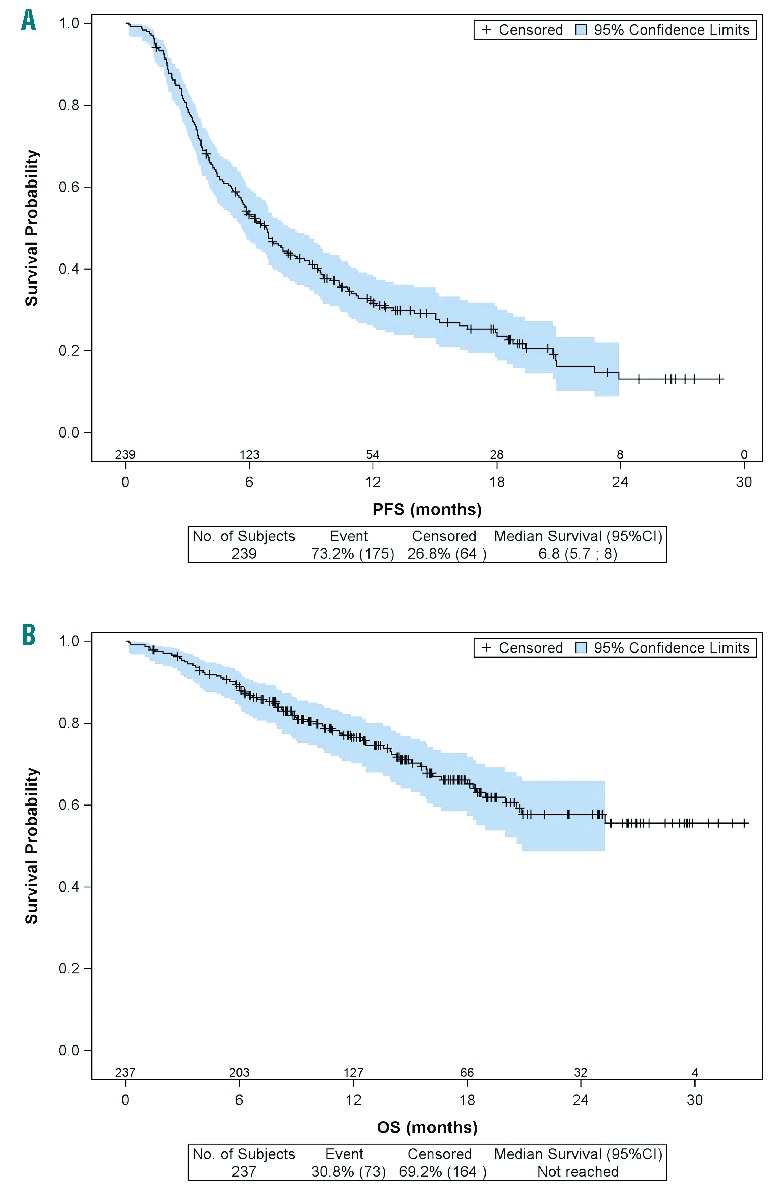
Kaplan-Meier plots of probabilities of PFS and OS for the whole cohort.
Primary endpoint: best response to BV treatment
Response was assessed using CT and the 1999 Cheson criteria for 19.9% of the patients, with PET-CT and the 2007 Cheson criteria in 78.7% and the others were evaluated clinically. The best response to BV treatment was observed after a median of four cycles of BV (range: 1–16). It was a CR for 70 (29.2%) patients, uCR for 11 (4.6%), PR for 64 (26.7%), stable disease (SD) for 18 (7.5%) and progressive disease (PD) for 68 (28.3%), for an objective response rate of 60.4%.
The best response rate was not worse for the 37 patients with prior allo-SCT, with a CR at 39.5%, uCR at 5.3% and PR at 18.4%, corresponding to an objective response rate of 60.5%.
Best responses did not differ significantly for several subgroups of patients, with 51.2% objective responses for patients with more than three treatment lines of therapy before BV, 64.3% for patients with more than one auto- or allo-SCT before BV, 62.9% for patients relapsing <6 months after a first transplant and 58.2% for patients with HL refractory to first-line therapy (or with a first median response duration <12 months). Notably, older patients had poorer response rates: objective responses were observed for only 39.3% of the 28 patients >60 years and 40.8% of the 49 >50 year old patients, with CR rates of 21.4% and 22.4%, respectively.
Response at the end of BV treatment
Patients received a median of six BV cycles (range: 1–16). Responses to BV treatment were assessed using the 1999 Cheson criteria for 22.3% and the 2007 Cheson criteria for 77.2% of patients.
Response rates at the end of BV treatment cycles were dramatically lower than the best response rates after a median of four cycles, because of relapses or progression. For the whole cohort, the objective response rate after a median of six BV cycles was 33.5% (versus 60.4% best response after four cycles), with CR for 52 (21.8%), uCR for 5 (2.1%), PR for 23 (9.6%), SD for 8 (3.3%) and PD for 129 (54%) patients.
Response duration
The median response duration was 8.4 [95% CI, 5.7–13.8] months for the 145 patients in CR/uCR/PR. Focusing on the 23 responders who had relapsed after allo-SCT before BV, the median response duration was slightly longer at 10.3 [95% CI, 3.9–20.2] months.
DFS
For the whole cohort, 81 patients achieved CR/uCR as best responses after BV. The median DFS for these 81 patients was 18.7 months. For the 37 patients with prior allo-SCT before BV, median DFS was 15.4 (95% CI [6.8-not reached (NR)]) months.
PFS
The median PFS was 6.8 months for 239 assessable patients and 11.3 months for 145 patients with objective responses (CR/uCR/PR). The quality of the best response after BV was associated with a longer median PFS: 20.9 (95% CI [16.2-NR]) months for patients in CR versus 6.9 [95% CI, 5.8–8.3] months (P<0.0001).
The median PFS did not differ significantly for patients who had received more than three treatment lines before BV (6.3 months), patients with more than one auto- or allo-SCT before BV (7.6 months), patients relapsing <6 months after a first SCT (6.3 months) and patients with HL refractory to first-line therapy or with a first median response duration <12 months (6.3 months). For the 37 patients given BV for relapse after allo-SCT, the median PFS was 7.9 [95% CI 6.0–10.8] months.
In contrast, age was a prognostic factor for PFS after BV: the median PFS was significantly shorter for patients >60 years: at 4.4 versus 7.1 months (P<0.021).
Progression/relapse after BV
Disease progressed or relapsed after BV in 156 (65.8%) patients: 123 (80.4% of them) received chemotherapy, 39 (25.7%) underwent transplantation (auto-SCT for 18 patients, with 3 having received tandem auto-/allo-SCT, and allo-SCT for 21 patients) and 17 (11.3%) were given radiotherapy.
OS
After a median follow-up of 16.1 months, median OS was not achieved; estimated 1-year OS was 76.4% [95% CI 70.1–81.6] and estimated 2-year OS was 57.8% [95% CI 48.7–65.7]. The most common causes among the 76 deaths were HL progression (68%), concurrent illness (9.3%) and consolidation toxicity (5.3%).
OS did not differ significantly for patient subsets according to the number of prior treatment lines received or transplants before BV, for patients with lasting first transplant to relapse interval <6 months or for those with HL refractory to first-line therapy or with remission lasting <12 months. For the 37 patients given BV for relapse after allo-SCT, their respective estimated 1-year and 2-year OS rates were, interestingly, slightly longer than for the entire cohort: 80.2% [62.8–90.0] and 63.2% [42.1–78.3], respectively.
Impact of consolidation post-BV
Eleven patients underwent consolidation radiotherapy after obtaining objective best responses to BV (5 CR and 6 PR); 2 patients in PR achieved CR after radiotherapy. Fifty-seven (18.2%) patients received post-BV SCT consolidation, after obtaining objective responses for 54 patients and in a setting of SD for 3. Response was assessed using the 1999 Cheson criteria for 12.5% and the 2007 Cheson criteria for 87.5% of patients.
Consolidation was auto-SCT for 29 patients and allo-SCT for 28 patients, whose median age was 28 years, with only 4 patients >50 years, and the majority were men (61.8%). Before BV, 30 out of 57 patients had undergone auto- or allo-SCT(s), predominantly auto-SCT. Twenty-four SCT-consolidated patients achieved CR, with no difference between auto-SCT and allo-SCT (Table 3). Both transplant groups were comparable with minor differences, especially in terms of the median number of BV cycles before transplantation (five cycles for the auto-SCT group versus six for the allo-SCT group, not significant). No significant difference was found for the median durations of responses, neither median PFS (at 18.8 [95% CI 15.0 NR] months) nor median OS (not reached). Probabilities of 1-year and 2-year OS were, respectively, 88.7% and 79.9% after auto-SCT consolidation and 87.1% and 81.3% after allo-SCT (not significant).
Table 3.
Baseline characteristics and outcomes of 57 patients who received consolidation after BV.
Patients who underwent auto- or allo-SCT as consolidation after BV had better outcomes than those not prescribed a consolidation. Among the 145 objective responders to BV, 54 patients transplanted as consolidation had a median PFS of 18.8 versus 8.7 months for the 91 patients without transplant (P<0.0001) (Figure 3). These results confirmed the importance of considering BV as a bridge to transplant. Consolidation feasibility was also demonstrated for the 57 patients with allo-SCT before BV: 15 of them underwent consolidation after BV, either an auto-SCT (n=5), a second allo-SCT (n=4), or one or more DLIs (n=6).
Figure 3.
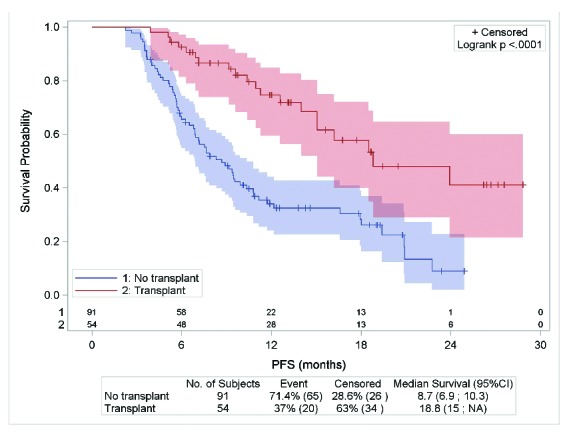
PFS according to auto- or allo-SCT consolidation for 145 patients with objective responses to BV.
For 41 very high-risk patients with refractory HL (refractory to first-line therapy or remission lasting <12 months) who received BV followed by transplant as consolidation, the median PFS was a very promising 18.5 [95% CI, 3.9–28.8] months.
Discussion
After a median of four BV cycles, the ORR for refractory/relapsed HL patients was 60.4%, including 33.8% CR/uCR. However, at the end of BV treatment (a median of six cycles), the response rate declined to 23.9% CR/uCR and 9.6% PR. For the 145 responders, the median response duration was 8.4 months. After a median followup of 16 months, the median PFS slightly exceeded 6 months, while median OS had not been reached, with the estimated 1-year and 2-year OS of 76.4% and 57.8%, respectively. BV had an acceptable toxicity profile, with no treatment-related deaths, while grade 1/2 sensory neuropathy occurred in 27% of the patients. These results for a heavily pretreated patient cohort are comparable to those obtained in the pivotal phase II trial (75% ORR, 34% CR, median response duration 6.7 months and median PFS 5.6 months).5
In our cohort, 48.7% of the patients had primary refractory HL and did not undergo auto-SCT prior to BV, which indicates a more resistant disease than that of patients in the phase II trial.5 BV achieved CR or stable PR that served as a bridge to SCT. Among the 57 patients who received a consolidation transplant after BV, 30 had never been transplanted before BV: 27 auto-SCT and 3 allo-SCT. The median PFS for this subgroup was about 18 months. The results of other studies have suggested this bridge to transplant concept, with a respective median PFS at 5.1 months, 1-year PFS at 92.3% and PFS at 50% with a 13 month follow-up for primary refractory HL.7,12,13 The findings of a comparative study indicated that BV before allo-SCT improved 2-year PFS.14 As a targeting agent, BV is an ideal treatment before transplant, that enabled some patients to proceed to transplantation with a CR and no organ toxicity.
The results of the pivotal phase II trial were updated with a median follow-up of 3 years.15 Among their 34 patients who obtained CR, 47% remained progression-free after a median of 53.3 months. A third of 18 patients remaining in CR or PR underwent allo-SCT consolidation. Even though durable remissions post-BV without additional treatment are possible, our results for our large cohort demonstrated the strong impact of auto- or allo-SCT consolidation on the outcomes of high-risk HL patients responding to BV: median PFS was markedly prolonged from 8.7 to 18.8 months after transplant. Consolidation radiotherapy could be an alternative option for patients not eligible for transplant or in PR after BV: in our experience, 2 patients in PR achieved CR after additional radiotherapy, as supported by a case report.16
In the pivotal phase II trial, patients had not undergone allo-SCT before BV.5 BV efficacy and safety after allo-SCT were evaluated in 24 and 16 HL patients17,18 with ORRs of between 50% and 69%. Thirty-seven patients received BV in the French NPP, with an ORR of 60.5% (including 39.5% CR) and a median PFS of 7.9 months; there was no difference compared to patients without previous allo-SCT.
The possibility of combining BV with DLI had previously been shown for 4 patients,19 wherein the authors hypothesized that concomitant BV and DLI could potentially further enhance the graft-versus-lymphoma effect, perhaps through the release of cytokines such as interleukin-6, chemokine ligand 17 and/or tumor necrosis factor-α. In our experience with 57 patients relapsing after allo-SCT, BV was followed by one or several DLIs for 6 patients and by a second allo-SCT for 5; all of the DLI recipients and 4 of the 5 second allo-SCT patients were alive at the last follow-up.
Although BV showed a remarkable efficacy against high-risk relapsed, refractory HL, 7.5% and 28.3% of our cohort patients achieved only SD and PD, respectively. The use of BV as first-line salvage therapy seems to have improved the response rates of 37 phase II trial patients (ORR 75%, CR 34%).20 Promising associations are BV-bendamustine or a sequential combination of gemcitabine, vinorelbine, pegylated liposomal doxorubicin and BV: a phase I-II trial enrolling 45 patients and a retrospective analysis of 11 patients obtained, respectively, 94% ORR and 82% CR, or 100% ORR and 72.5% CR, with both regimens having manageable adverse events.21,22 Combining BV with PD-1 blockade therapies could also warrant being assessed in the future.
In conclusion, the results of our large retrospective analysis of 240 patients with refractory/relapsed HL from 89 treatment centers (including 37 patients with prior allo-SCT) in a real-life setting support the previously reported BV efficacy in heavily pretreated CD30+ HL patients with manageable toxicity. Due to the short response duration of about 8.4 months, and the improved outcomes with consolidation, we recommend early auto- or allo-SCT or even radiotherapy to consolidate responses and to cure HL. Resorting to DLI or a second allo-SCT after BV achieves durable remissions in this difficult-to-treat setting of post-allograft relapses.
Acknowledgments
The authors would like to thank Sophie Pallardy and Sami Boussetta for statistical analysis, the Lymphoma Study Academic Research Organisation (LYSARC) for study management and the clinicians of the LYSA who contributed to the collection of clinical data, especially M. Larmarque, A. Contejean & C. Haioun (Créteil), A. Quinquenel (Reims), A. Chauchet (Besançon), C. Tomowiak (Poitiers), S. François (Angers), L. Fornecker (Strasbourg), S. Garciaz (Marseille), M. Lenoble (Montfermeil), G. Salmeron (Versailles), L. Terriou (Lille).
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/4/466
References
- 1.Van Den Neste E, Casasnovas O, André M, et al. Classical Hodgkin’s lymphoma: the Lymphoma Study Association guidelines for relapsed and refractory adult patients eligible for transplant. Haematologica. 2013;98(8):1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9(1):15–27. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Janik JE, Younes A. Brentuximab vedotin (SGN-35). Clin Cancer Res. 2011;17(20):6428–6436. [DOI] [PubMed] [Google Scholar]
- 4.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–1821. [DOI] [PubMed] [Google Scholar]
- 5.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothe A, Sasse S, Goergen H, et al. Brentuximab vedotin for relapsed or refractory CD30+ hematologic malignancies: the German Hodgkin Study Group experience. Blood. 2012;120(7):1470–1472. [DOI] [PubMed] [Google Scholar]
- 7.Gibb A, Jones C, Bloor A, et al. Brentuximab vedotin in refractory CD30+ lymphomas: a bridge to allogeneic transplantation in approximately one quarter of patients treated on a Named Patient Programme at a single UK center. Haematologica. 2013;98(4): 611–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinzani PL, Viviani S, Anastasia A, et al. Brentuximab vedotin in relapsed/refractory Hodgkin’s lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials. Haematologica. 2013;98(8):1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monjanel H, Deville L, Ram-Wolff C, et al. Brentuximab vedotin in heavily treated Hodgkin and anaplastic large-cell lymphoma, a single centre study on 45 patients. Br J Haematol. 2014;166(2):306–308. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4): 1244. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Palmer JM, Thomas SH, et al. Brentuximab vedotin enables successful reduced-intensity allogeneic hematopoietic cell transplantation in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2012;119(26):6379–6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garciaz S, Coso D, Peyrade F, et al. Brentuximab vedotin followed by allogeneic transplantation as salvage regimen in patients with relapsed and/or refractory Hodgkin’s lymphoma. Hematol Oncol. 2014;32(4):187–191. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Palmer JM, Tsai N-C, et al. Brentuximab vedotin is associated with improved progression-free survival after allogeneic transplantation for Hodgkin lymphoma. Biol Blood Marrow Transplant. 2014;20(11):1864–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dozzo M, Zaja F, Volpetti S, Sperotto A, Magli A, Fanin R. Brentuximab vedotin in combination with extended field radiotherapy as salvage treatment for primary refractory Hodgkin lymphoma. Am J Hematol. 2015;90(4):E73. [DOI] [PubMed] [Google Scholar]
- 17.Gopal AK, Ramchandren R, O’Connor OA, et al. Safety and efficacy of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic stem cell transplantation. Blood. 2012;120(3):560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlo-Stella C, Ricci F, Dalto S, et al. Brentuximab vedotin in patients with Hodgkin lymphoma and a failed allogeneic stem cell transplantation: results from a named patient program at four Italian centers. Oncologist. 2015;20(3):323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theurich S, Malcher J, Wennhold K, et al. Brentuximab vedotin combined with donor lymphocyte infusions for early relapse of Hodgkin lymphoma after allogeneic stem-cell transplantation induces tumor-specific immunity and sustained clinical remission. J Clin Oncol. 2013;31(5):e59–63. [DOI] [PubMed] [Google Scholar]
- 20.Chen RW, Palmer J, Martin P, et al. Results of a phase II trial of brentuximab vedotin as first line salvage therapy in relapsed/refractory HL prior to AHCT. Blood. 2014;24(21):abst 501. [Google Scholar]
- 21.LaCasce A, Bociek RG, Matous J, et al. Brentuximab vedotin in combination with bendamustine for patients with Hodgkin lymphoma who are relapsed or refractory after frontline therapy. Blood. 2014;124(21):abst 293. [Google Scholar]
- 22.Michallet A-S, Guillermin Y, Deau B, et al. Sequential combination of gemcitabine, vinorelbine, pegylated liposomal doxorubicin and brentuximab as a bridge regimen to transplant in relapsed or refractory Hodgkin lymphoma. Haematologica. 2015;100(7):e269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]


