Abstract
In the field of hematopoietic stem cell transplantation, the common approach is to focus outcome analyses on time to relapse and death, without assessing the impact of post-transplant interventions. We investigated whether a multi-state model would give insight into the events after transplantation in a cohort of patients who were transplanted using a strategy including scheduled donor lymphocyte infusions. Seventy-eight consecutive patients who underwent myeloablative T-cell depleted allogeneic stem cell transplantation for acute myeloid leukemia or myelodysplastic syndrome were studied. We constructed a multi-state model to analyze the impact of donor lymphocyte infusion and graft-versus-host disease on the probabilities of relapse and non-relapse mortality over time. Based on this model we introduced a new measure for outcome after transplantation which we called ‘treatment success’: being alive without relapse and immunosuppression for graft-versus-host disease. All relevant clinical events were implemented into the multi-state model and were denoted treatment success or failure (either transient or permanent). Both relapse and non-relapse mortality were causes of failure of comparable magnitude. Whereas relapse was the dominant cause of failure from the transplantation state, its rate was reduced after graft-versus-host disease, and especially after donor lymphocyte infusion. The long-term probability of treatment success was approximately 40%. This probability was increased after donor lymphocyte infusion. Our multi-state model helps to interpret the impact of post-transplantation interventions and clinical events on failure and treatment success, thus extracting more information from observational data.
Introduction
In the field of allogeneic stem cell transplantation (SCT), outcome analyses are mainly focused on time to relapse and death, measured from the moment of transplantation.1 This approach, however, does not take into account the increasing availability and potential of post-SCT interventions. These interventions, in particular donor lymphocyte infusions (DLI), are of utmost interest as part of a treatment strategy in which T-cell depleted (TCD-)SCT is followed by T-cell based therapeutic interventions. T-cell depletion of the graft efficiently prevents the development of severe graft-versus-host disease (GvHD) directly after transplantation, but also adversely affects post-transplant anti-tumor and anti-infectious immunity.2–4 Early intervention with DLI after TCD-SCT may prevent the relapse of the malignancy and improve immune reconstitution against pathogens, but is associated with the reintroduction of the risk of GvHD.5,6 However, a long-term delay of DLI in patients without clinical signs of GvHD may increase relapse risk in these patients. At present, the optimal timing of DLI has not been established.
There is a growing interest in the analysis of treatment outcome measures other than overall survival and relapse-free survival. Although these are crucial endpoints, they do not concentrate on highly relevant intermediate events or interventions essential to evaluate causes of failure or success after transplantation. To assess the dynamic impact of post-transplant clinical events and interventions, the methodology of multi-state models has been developed.7–9 Multi-state models have several advantages with respect to more widely used standard survival analysis methods.10 The primary advantage is that sequences of events -such as SCT, DLI, GvHD, death - and competing events - such as relapse and death before relapse - can be modeled simultaneously. Thus, the models can deal with different post-transplant sequences and timing of events. In contrast to composite survival outcomes such as failure-free survival11 or GvHD-free survival12 where a patient’s subsequent events after the first failure are not considered anymore, multi-state models can be used to assess the role of temporary states such as GvHD in relation to additional interventions after transplantation. These models are essential to evaluate more complex treatment strategies like TCD-SCT with differentially scheduled post-transplant cellular immune interventions.
Even though this methodology has been available for over a decade thanks to the work of Klein and colleagues, the number of clinical questions in the field of hematology addressed by means of these models has been very limited thus far.8,13–17 This has partly been caused by a lack of familiarity with the method or its potential, and partly by insufficient data quality: a multi-state model requires reliable follow-up information for the events in the model. Another major reason was the lack of easily accessible software. This has changed in the last years, among others by the development of the package ‘mstate’ in R that enables users to analyze general multi-state models.7,9
In the current study we investigated how multi-state models can be applied to complex phenomena including cellular interventions after transplant. We focused on two topics: (1) the impact of DLI and development of GvHD requiring immuno-suppressive treatment (IS) on the failure probabilities of relapse and non-relapse mortality (NRM) over time, and; (2) the probability of treatment success, which is defined as the absence of disease and GvHD requiring IS over time. We demonstrate how a multi-state model helps to interpret the consequences of the therapeutic interventions after SCT in a cohort of patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) who were transplanted in our center using a TCD-SCT strategy including scheduled post-transplant DLI.
Methods
Study protocol
Seventy-nine patients who underwent myeloablative allogeneic SCT for AML or MDS in complete remission (CR) in our center between January 2002 and June 2011 were included in this analysis. Two other patients who received CD4+-DLI in the context of an experimental CD4+-DLI study during this time period were not included in the analysis. Until June 2007 only patients with mixedchimerism but without early relapse of AML or MDS were eligible for prophylactic DLI at 6 months after SCT. If severe GvHD (overall grade II or higher) was present, DLI infusion was postponed. From June 2007 onwards, patients with poor or very poor-risk AML were eligible for low dose prophylactic DLI at 3 months after SCT (see Table 1 for definition of risk groups). For details on transplantation protocol, engraftment, dosing of DLI and assessment of GvHD and mixed-chimerism, see the Online Supplementary Appendix. The study was approved by Leiden University Medical Center Research Ethics Committee. Informed consent was obtained prior to data collection. Data were analyzed as of December 2012.
Table 1.
Patient characteristics.

Definitions of events after SCT
For an accurate and objective determination of the duration of severe GvHD, the time interval between the start date of IS (indicated for GvHD) until cessation of IS (stop IS) was taken. In patients with an unrelated donor receiving prophylactic cyclosporine, the date of the start of additional IS, e.g. prednisone, was taken as the starting point of severe GvHD.
Relapse after SCT was defined as an increase of blasts in the bone marrow (BM) to ≥5% by morphology; and/or by the presence of >1% blasts in peripheral blood (PB); and/or by the reappearance of molecular and/or cytogenetic AML markers. NRM was defined as death in continuous complete remission after SCT.
Statistical analysis
For all analyses, time was measured from the date of SCT. Probabilities of overall survival with associated 95% confidence intervals (95% CI) were calculated by the Kaplan-Meier method. Overall survival was estimated with SPSS/PASW Statistics 20, release 20.0.0 (2011). The cumulative incidence of prophylactic DLI was estimated in a competing risks framework, considering relapse and NRM before prophylactic DLI as competing events. The cumulative incidence of DLI was estimated by means of the ‘cmprsk’ library in R.
All other outcomes were analyzed by means of a multi-state model (see the Online Supplementary Appendix for an explanation of the methodology). The quantities of interest in the multi-state model were estimated in R, version 3.0.1, with library ‘mstate’.7,9
Results
Patient cohort
Characteristics of patients and transplantation procedures are presented in Table 1. A rejection was observed in one patient (day 43). The median follow-up of surviving patients of the entire cohort was 63 months (range 20–128 months). The cumulative incidence of DLI was 21% at 6 months and 29% at 9 months (see Online Supplementary Figure S2). At the end of follow-up, 37 patients (47%) had received prophylactic DLI (median 2 prophylactic DLIs per patient; range 1–4). Although very poor-risk patients were scheduled for early DLI, in practice the timing of DLI was not very different for those patients compared to the other patients (median time to DLI for the 5 very poor-risk patients: 6.9 months (range 3.2–9.9 months) for the other patients: 7.7 months (3.0–27.0 months)). Forty-two patients (53% of 79 patients) did not receive DLI (very poor-risk AML n=8, poor-risk AML n=24, intermediate-risk AML n=7, MDS n=3). Reasons for not receiving DLI were rejection (n=1), NRM (n=13), early relapse (n=9), severe GvHD (n=8), full-donor hematopoiesis (n=9, including one patient with poor-risk AML who relapsed at day 270), and logistical problems (n=2; these patients with very poor-risk AML who were transplanted before June 2007 showed mixed-chimerism at 6 months after SCT but relapsed before DLI was initiated (at days 216 and 275, respectively)).
The estimated 2-year overall survival calculated from the start day of SCT of the entire cohort of 79 patients was 58% (95% CI 48–68%). The two-year overall survival of patients with intermediate-risk AML, poor-/very poor-risk AML, and MDS calculated from the start day of SCT were 67% (95% CI 40%–94%), 55% (95% CI 42–68%), and 67% (95% CI 35%–98%), respectively (see Figure 2).
Figure 2.
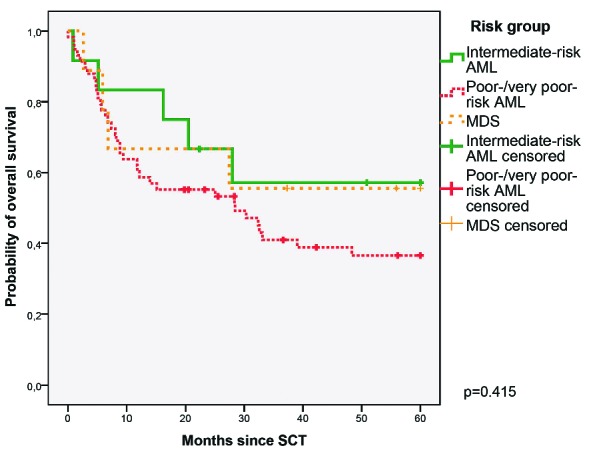
Overall survival curves. Kaplan Meier curves illustrating probabilities of overall survival of 12 patients with intermediate-risk AML (solid green line), 58 patients with poor-/very poor-risk AML (dashed red line), and 9 patients with MDS (dotted orange line).
The multi-state model
A multi-state model was constructed to model the occurrence and impact of relevant events after SCT (see Figure 1). Since no patient received prophylactic DLI before IS had been tapered, the transition between ‘start IS’ and ‘DLI’ was omitted from the model. Only one patient in the entire cohort experienced a rejection (at day 43), which excluded transition to the state DLI, and therefore both this patient and the state ‘Rejection’ were not included in the model. Outcomes of the other 78 patients were analyzed in the multi-state model. None of them were lost to follow-up. The number of events in the dataset and the number of patients in each state at the end of their follow-up time are presented in the Online Supplementary Figure S1.
Figure 1.
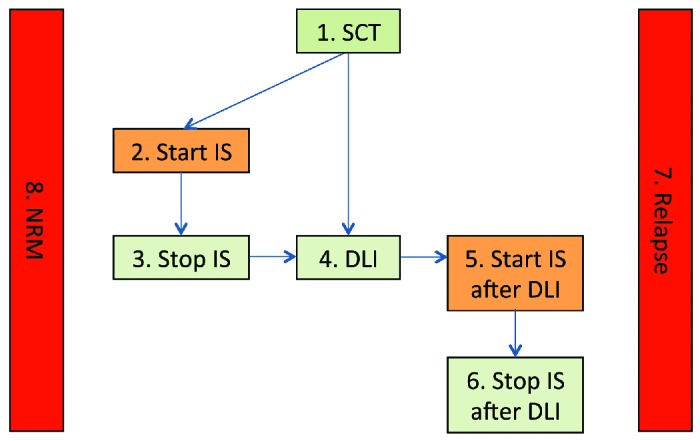
Multi-state model. The name of each state reflects the event the occurrence of which makes the patient enter the state; he/she remains in this state until a next event occurs or until the end of follow-up. Patients entered a new state on the day when such an event took place and could not visit the same state twice. The starting state of all patients is 1. SCT. The intermediate states are depicted by numbers 2 to 6. Each arrow indicates a possible transition to an intermediate state in the model. Transition between severe GvHD (Start IS) and initiation of DLI was only possible after cessation of systemic immunosuppression. The absorbing states (in red) are Relapse and NRM. States 1, 3, 4, 6 (in green) indicate treatment success; states 2, 5, 7, 8 (in orange and red) indicate treatment failure. Transition to the absorbing states was possible from any other state in the model; for simplicity these transitions are omitted from the figure.
Outcomes of the multi-state model
All transition probabilities from SCT during the first 60 months of the follow-up are presented in the Online Supplementary Figure S3. The proportions of patients suffering relapse and NRM are comparable in size. From 11 months after SCT, a substantial proportion of patients (ca. 30%) remained in the ‘DLI’ state, implying they had received DLI, were still alive without relapse and did not require IS for GvHD after DLI. Correspondingly, we observed that only a minority of patients suffered from severe GvHD after DLI (see ‘Start IS after DLI’, Online Supplementary Figure S3) and that the probability to be in the ‘Start IS’ or ‘Start IS after DLI’ state was zero after 33 months, indicating that IS could be tapered successfully in all patients with an episode of severe GvHD.
It cannot be excluded that patients likely to develop GvHD post-DLI failed before DLI could have been administered. However, several factors are likely to contribute to the reduced development of GvHD after DLI. The timing of DLI is one factor. DLI was not given to patients with active GvHD, but postponed in these patients (n=9). After DLI, new episodes of IS-requiring GvHD were hardly observed (1 out of 9 patients (11%) with previous IS-requiring GvHD, 5 out of 28 (18%) without previous IS-requiring GvHD). In conclusion, postponing DLI appears to alleviate the induction of severe GvHD, even in patients with GvHD prior to infusion of donor lymphocytes. Thus, the selection of patients who were unlikely to develop GvHD does not appear to have played a major role.
Finally, the small probability of remaining in the state SCT in the long run indicates that follow-up without experiencing a further event was highly uncommon, which is in line with the strategy of scheduled prophylactic post-transplant DLI. The wide confidence intervals reflect the small size of the dataset and show that all estimates should be interpreted with caution.
Figure 3 gives an overview of the outcomes of the multi-state model, by combining all individual transition probabilities as represented in the Online Supplementary Figure S3 in one figure. The area below the relapse curve of this figure represents relapse-free survival and indicates how the patients alive and relapse-free were distributed over the different states at different moments during follow-up.
Figure 3.
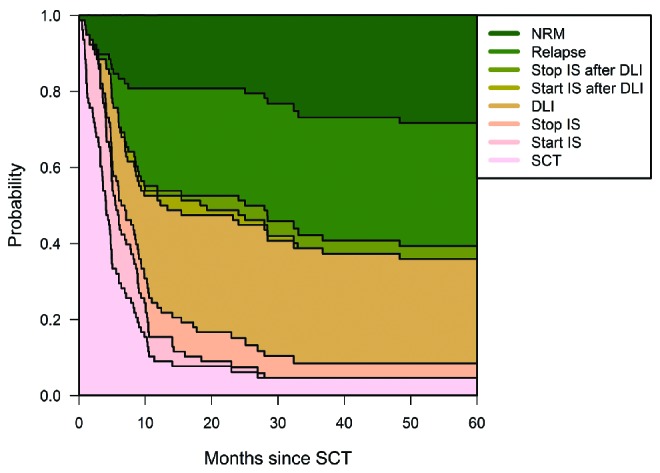
Transition probabilities to all states from SCT. Transition probabilities derived from the multi-state model of Figure 1. At each point in time, the distance between 2 adjacent curves represents the probability of being in the corresponding state, given that the patient was in the ‘SCT’ state at time 0. Online Supplementary Figure S3 shows these same transition probabilities in separate panels. As before, names of states represent the events with which patients enter them. Since all patients started in the ‘SCT’ state, the probability of being in that state was 1 at time 0 and decreased afterward, because patients could only leave this state. For all other states, the probability to have entered this state at time 0 was 0. For the ‘Relapse’ and ‘NRM’ states, which are absorbing states, the probabilities can only increase over time since patients cannot leave these states anymore. For the 5 intermediate states, probabilities increase and decrease over time. The figure shows that already at 1 year post-SCT only a very small minority of patients are still in the SCT state. The probability to receive IS is always low, both before and after DLI, and transient in all cases. For relapse-free patients, from ca. 1 year onward, the dominant state is being alive after DLI.
Predictions from 7.5 months after SCT
The multi-state model enables the update of predictions of subsequent failures when information about post-SCT events accumulates over time. This approach can be considered as an extension of landmark modeling in which both start and end time, and starting and end state are variable. Figure 4 shows transition probabilities for patients in different starting states (1 to 4) at 7.5 months after SCT. The time-point 7.5 months after SCT was chosen as the start time of these analyses because prophylactic DLI was scheduled at 6 months after SCT, after which an effective immune response is generally seen 5–6 weeks later, adding up to 7.5 months.18 According to the model, patients who had not experienced severe GvHD and had not yet received prophylactic DLI within 7.5 months after SCT (in state ‘SCT’ at 7.5 months) had a predicted probability of relapse of 0.26 (95% CI: 0.07–0.45) within the first 2 years after transplantation. In contrast, patients in the state ‘DLI’ at the same time point had a predicted probability of relapse of 0.07 (95% CI: 0.00–0.21) within 2 years after transplantation. These results suggest that the risk of relapse, which is the major reason for failure from the ‘SCT’ state, is reduced after GvHD, and especially after DLI administration. In patients without any event, and also in patients in the ‘DLI’ state at 7.5 months after SCT, NRM did not play a prominent role during later follow-up.
Figure 4.
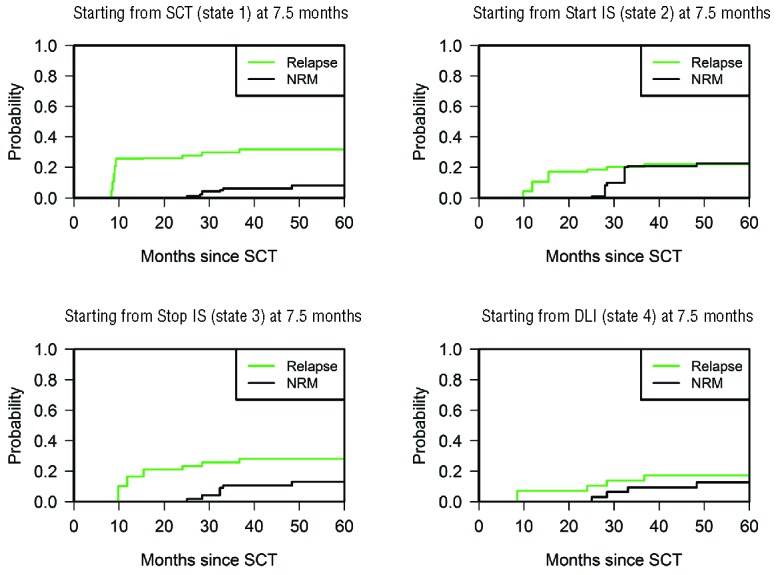
Predictions from 7.5 months after SCT. Estimated probabilities of relapse (green line) and NRM (black line) over time for patients present at 7.5 months since transplantation in states ‘SCT’, ‘Start IS’, ‘Stop IS’, and ‘DLI’, respectively. Results from ‘Start IS after DLI’ and ‘Stop IS after DLI’ are not shown because of the small sample size.
These differences in outcomes cannot be explained by relevant differences in the baseline risk characteristics of the patients in the respective states at 7.5 months, since (very) poor-risk patients were not underrepresented in the ‘DLI’ state compared to the other states (‘SCT’ state: 55% poor AML, 15% very poor AML; ‘Start IS’ state: 45% poor, 9% very poor; ‘DLI’ state: 58% poor, 17% very poor).
Treatment success
The same multi-state model can be used to summarize the outcomes of the transplantation procedure by focusing on the probability of treatment success at different time points after transplantation (see Figure 5). In general, the goal of our treatment strategy was to administer DLI safely to all patients at risk for relapse. Therefore, at each time point, treatment success included patients who experienced relapse-free survival, and did not suffer from severe GvHD at that time point, i.e. patients remaining in the state ‘SCT’, or present in the states ‘Stop IS’, ‘DLI’, or ‘Stop IS after DLI’. The probability of treatment success over time was calculated by adding up the estimated time-dependent probabilities of being in these states. The long-term estimated probability of treatment success from start was approximately 0.43 (Figure 5A). If a patient survived the first 7.5 months after SCT without subsequent events, this success probability substantially improved to approximately 0.60. Patients who required IS for GvHD at 7.5 months after SCT (Figure 5B) showed a similar success probability of approximately 0.55 at 60 months after SCT. By definition, these patients were not considered to experience treatment success at 7.5 months after SCT, but a part of them acquired treatment success after cessation of IS possibly followed by DLI, corresponding to an increasing curve. Patients who required IS after DLI or failing from NRM or relapse led the curve to decline again.
Figure 5.
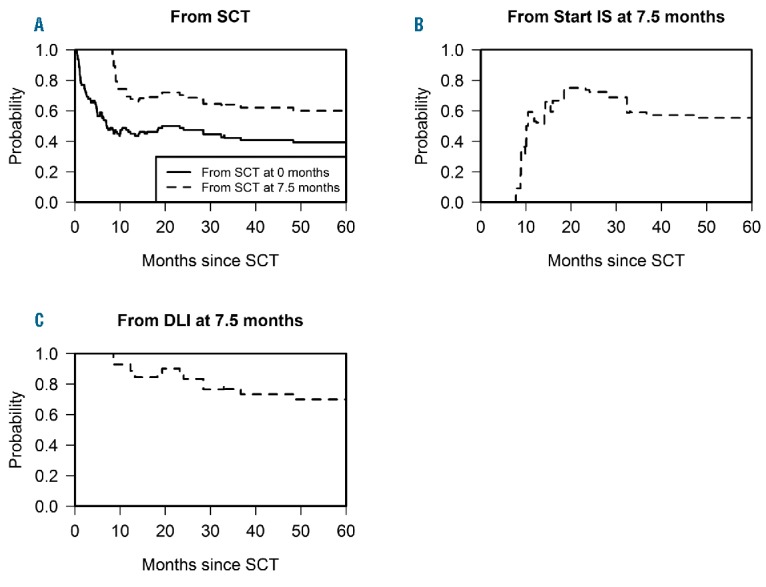
Probability of treatment success over time. Treatment success as calculated in the multi-state model of Figure 1 is defined as the probability of being alive without disease or severe GvHD, i.e., the sum of the probabilities of being in either of the states 1, 3, 4 and 6. (A) probability of treatment success for a patient in the ‘SCT’ state at 0 and 7.5 months after SCT, respectively. (B) probability of treatment success for a patient in the ‘Start IS’ state at 7.5 months after SCT. (C) probability of treatment success for a patient in the ‘DLI’ state at 7.5 months after SCT. Outcomes from ‘Start IS’ and ‘DLI’ at 0 months have been omitted since these do not represent a clinically meaningful situation. The curves from ‘SCT’ and ‘DLI’ start at probability 1 since a patient in one of these states by definition experiences treatment success at the moment when the clock starts; on the contrary, the curve from ‘Start IS’ begins at 0 because a patient in that state first has to make a transition to a beneficial state before being considered a treatment success. The steep rise in this curve can be interpreted as a sign of successful quick cessation of IS in the majority of patients who require IS for the treatment of early GvHD. The treatment success probabilities increase and decrease over time depending on the proportions of patients entering and leaving the beneficial states.
Finally, a comparison with the prospects for patients in the ‘DLI’ state at the same point in time (Figure 5C) suggests that their probability of treatment success was higher: 0.70 at 60 months after SCT.
Discussion
This detailed study of a group of AML/MDS patients with a long, complex follow-up illustrates the potential of a multi-state model to assess the risk of relapse and NRM for patients in a TCD-SCT population for which an infusion of donor lymphocytes was an essential intervention within the strategy. The model separates fatal and nonfatal events, thus enabling the summing up of several favorable episodes to estimate the probability of treatment success. The model also enables one to give long-term prognoses adjusted during follow-up which is relevant for clinical decision making.
We previously reported the feasibility of a TCD-SCT with sequential DLI strategy in a population of patients with acute lymphoblastic leukemia.19 In the present study, the majority of patients had poor or very poor-risk AML prior to SCT, and thus the estimated 2-year overall survival of 58% of the entire cohort appears favorable. A substantial proportion of patients receiving DLI did not experience an adverse event during later follow-up. This implies that few patients relapsed after DLI, and also that the development of severe GvHD after DLI was rare. These results cannot be explained by a selection for DLI of patients with favorable-risk characteristics at SCT as a comparison of the patients in different states at 7.5 months shows. Predictions from 7.5 months after SCT show that patients who had not experienced any event (i.e., no severe GvHD, DLI or relapse) so far had the highest probability of relapse, whereas NRM did not play a prominent role in these patients. According to these predictions, patients in the ‘DLI’ state at 7.5 months after SCT had a relatively low relapse risk and NRM, leading to the highest probability of treatment success. Finally, most patients never needed IS, and IS was stopped during later follow-up in the majority of patients who required IS for GvHD at 7.5 months after SCT. The results show the favorable outcomes of a strategy of TCD-SCT with DLI. The vast majority of patients alive and relapse-free were also free from IS. After 1 year, by far the largest part of this group consisted of patients who had received a DLI.
Although the decision to administer DLI may be driven by many clinical events, and although the variable timing of the DLI itself is associated with different disease histories hampering causal interpretation, the results show that ultimately being in the DLI state is beneficial. From these different observations it can be interpreted that the prevention of severe GvHD by T-cell depletion contributes to the reduction of NRM, and that the absence of significant GvHD leads to an increased relapse rate that may be decreased by DLI. Apparently, the low probability of relapse after DLI was not outbalanced by the induction of severe GvHD, which is supported by several other studies.20–22 In conclusion, treatment of patients with absent or limited GvHD after TCD-SCT can safely be consolidated by DLI, which appears to reduce the relapse rate.
The observation that the relapse risk was decreased after DLI without a high probability of severe GvHD can be explained by a sufficiently long time interval between the myeloablative conditioning regimen and infusion of DLI, allowing the recipient to recover from conditioning-induced inflammation, thus reducing pro-inflammatory conditions at the time of DLI, without fully extinguishing the beneficial effect of the anti-recipient hematopoietic cell directed T-cells.6,21,23–25 Timely infusion of donor lymphocytes will remain an important factor, because the delay of DLI will increase the time to a favorable immune response and is therefore associated with an increased relapse risk.26–28
Our treatment strategy was not part of a randomized trial, but we aimed to administer DLI to all patients with a high-risk of relapse, e.g. patients with high-risk AML or patients with persisting mixedchimerism, and to all high-risk patients from June 2007 onwards, whose risk may be modulated by DLI.5,22,23,29 The different DLI strategies for different risk groups make the role of timing of DLI even more difficult to assess and complicates the comparison of outcomes of patients with or without DLI. These issues can only be addressed properly in prospective randomized trials.30,31 However, in current transplant practice, randomized trials are performed infrequently due to both the relatively small numbers of patients affected and to patient and physician treatment preferences.32 This lack of randomized trials necessitates alternative analysis methods, in which as many insights as possible are extracted from observational data.17 For instance, a comparison of treatment strategies could be performed by analyzing the data from different centers by the same model.
Multi-state models such as the example presented herein offer several advantages over traditional statistical approaches. The models are more flexible than Cox models with time-dependent covariates since there is no proportional hazards assumption considering hazard ratios as constant over time. They are more comprehensive than landmark models where the particular choice of the landmark time point leads to restrictions. Moreover, landmark analyses do not take into account the probability of arriving in the state from where the prediction is made and are not suitable for analyzing sequences of events after the landmark time point. In addition, multi-state models do not only offer insight into the incidence of certain events as yielded by competing risks methods, but also into the probability of remaining in a certain condition over time, thus offering the option to consider new treatment outcomes. With our study, we illustrated the feasibility of assessing outcomes of a treatment strategy by means of a multi-state model.33 Multi-state models have been advocated before as a means to model alternative outcomes after SCT, e.g. current leukemia-free survival, yet these models focused on fewer events and on outcomes since SCT, not using the full potential of updated prediction.8,14,15,34 Other similar models can be constructed to assess several post-SCT interventions or events, such as the timing of CMV reactivation in relationship to the development of GvHD, also in the context of T-cell replete transplantations. Multi-state models can also help to extend and refine new outcomes such as GvHD-free, relapse-free survival after transplant, or failure-free survival after the initial systemic treatment of chronic graft-versus-host disease.11,35
In conclusion, our treatment strategy to infuse donor lymphocytes in patients without severe GvHD after myeloablative TCD-SCT did not lead to an increase of severe GvHD after DLI, and appeared to decrease relapse risk. Therefore, the probability of treatment success of these patients was relatively high. In the absence of randomized trials, large cohorts with detailed follow-up are required to further specify the role of prophylactic DLI in patients at risk for relapse. In general, multi-state models can increase insight into complex clinical situations by predicting outcomes of patients with different disease states which may change over time. The current study shows the potential of multi-state models in the analysis of new clinically meaningful outcomes.
Acknowledgments
The authors would like to thank Illyea Hawke (DKMS, German Bone Marrow Donor Center, Dresden, Germany) for correcting the language of the manuscript.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/4/506
Funding
This work was supported by research grant 2008–4263 from the Dutch Cancer Society, Amsterdam, the Netherlands.
References
- 1.Iacobelli S, Committee ES. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48 Suppl 1:S1–37. [DOI] [PubMed] [Google Scholar]
- 2.Barge RM, Starrenburg CW, Falkenburg JH, Fibbe WE, Marijt EW, Willemze R. Long-term follow-up of myeloablative allogeneic stem cell transplantation using Campath "in the bag" as T-cell depletion: the Leiden experience. Bone Marrow Transplant. 2006;37(12):1129–1134. [DOI] [PubMed] [Google Scholar]
- 3.Marmont AM, Horowitz MM, Gale RP, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78(8):2120–2130. [PubMed] [Google Scholar]
- 4.Chakrabarti S, Mackinnon S, Chopra R, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99(12):4357–4363. [DOI] [PubMed] [Google Scholar]
- 5.Peggs KS, Thomson K, Hart DP, et al. Doseescalated donor lymphocyte infusions following reduced intensity transplantation: toxicity, chimerism, and disease responses. Blood. 2004;103(4):1548–1556. [DOI] [PubMed] [Google Scholar]
- 6.Stevanovic S, van Bergen CA, van Luxemburg-Heijs SA, et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood. 2013;122(11):1963–1973. [DOI] [PubMed] [Google Scholar]
- 7.de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Comput Methods Programs Biomed. 2010;99(3):261–274. [DOI] [PubMed] [Google Scholar]
- 8.Klein JP, Szydlo RM, Craddock C, Goldman JM. Estimation of current leukaemia-free survival following donor lymphocyte infusion therapy for patients with leukaemia who relapse after allografting: application of a multistate model. Stat Med. 2000;19(21):3005–3016. [DOI] [PubMed] [Google Scholar]
- 9.de Wreede LC, Fiocco M, Putter H. mstate: An R Package for the Analysis of Competing Risks and Multi-State Models. J Stat Softw. 2011;38(7):1–30. [Google Scholar]
- 10.Putter H, Fiocco M, Geskus R. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. [DOI] [PubMed] [Google Scholar]
- 11.Inamoto Y, Flowers ME, Sandmaier BM, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124(8):1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolanos-Meade J, Logan BR, Alousi AM, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124(22):3221–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan BR. Review of multistate models in hematopoietic cell transplantation studies. Biol Blood Marrow Transplant. 2013;19(1 Suppl):S84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmoor C, Schumacher M, Finke J, Beyersmann J. Competing risks and multi-state models. Clin Cancer Res. 2013;19(1):12–21. [DOI] [PubMed] [Google Scholar]
- 15.Klein JP, Keiding N, Shu Y, Szydlo RM, Goldman JM. Summary curves for patients transplanted for chronic myeloid leukaemia salvaged by a donor lymphocyte infusion: the current leukaemia-free survival curve. Br J Haematol. 2000;109(1):148–152. [DOI] [PubMed] [Google Scholar]
- 16.Craddock C, Szydlo RM, Klein JP, et al. Estimating leukemia-free survival after allografting for chronic myeloid leukemia: a new method that takes into account patients who relapse and are restored to complete remission. Blood. 2000;96(1):86–90. [PubMed] [Google Scholar]
- 17.Brand R, Putter H, van Biezen A, et al. Comparison of allogeneic stem cell transplantation and non-transplant approaches in elderly patients with advanced myelodysplastic syndrome: optimal statistical approaches and a critical appraisal of clinical results using non-randomized data. PLoS One. 2013;8(10):e74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 19.Eefting M, Halkes CJ, de Wreede LC, et al. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. 2014;49(2):287–291. [DOI] [PubMed] [Google Scholar]
- 20.El-Cheikh J, Crocchiolo R, Furst S, et al. Donor CD3(+) lymphocyte infusion after reduced intensity conditioning allogeneic stem cell transplantation: single-center experience. Exp Hematol. 2013;41(1):17–27. [DOI] [PubMed] [Google Scholar]
- 21.Mackinnon S. Donor leukocyte infusions. Baillieres Clin Haematol. 1997;10(2):357–367. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamurthy P, Potter VT, Barber LD, et al. Outcome of donor lymphocyte infusion after T cell-depleted allogeneic hematopoietic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2013;19(4):562–568. [DOI] [PubMed] [Google Scholar]
- 23.Mapara MY, Kim YM, Wang SP, Bronson R, Sachs DH, Sykes M. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100(5):1903–1909. [DOI] [PubMed] [Google Scholar]
- 24.Peggs KS, Mackinnon S. Exploiting graft-versus-tumour responses using donor leukocyte infusions. Best Pract Res Clin Haematol. 2001;14(4):723–739. [DOI] [PubMed] [Google Scholar]
- 25.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. [DOI] [PubMed] [Google Scholar]
- 26.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23(9):1969–1978. [DOI] [PubMed] [Google Scholar]
- 27.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31): 4938–4945. [DOI] [PubMed] [Google Scholar]
- 28.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6): 1599–1606. [DOI] [PubMed] [Google Scholar]
- 29.Shaw BE, Byrne JL, Das-Gupta E, Carter GI, Russell NH. The impact of chimerism patterns and predonor leukocyte infusion lymphopenia on survival following T cell-depleted reduced intensity conditioned transplants. Biol Blood Marrow Transplant. 2007;13(5):550–559. [DOI] [PubMed] [Google Scholar]
- 30.Gordon D, Taddei-Peters W, Mascette A, Antman M, Kaufmann PG, Lauer MS. Publication of trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2013;369(20):1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Devereaux PJ, Yusuf S. When it comes to trials, do we get what we pay for¿ N Engl J Med. 2013;369(20):1962–1963. [DOI] [PubMed] [Google Scholar]
- 32.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–585. [DOI] [PubMed] [Google Scholar]
- 33.Klein JP, Shu Y. Multi-state models for bone marrow transplantation studies. Stat Methods Med Res. 2002;11(2):117–139. [DOI] [PubMed] [Google Scholar]
- 34.Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011;117(23):6375–6382. [DOI] [PubMed] [Google Scholar]
- 35.Holtan SG, DeFor TE, Lazaryan A, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125(8):1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]


