Abstract
Acute lymphoblastic leukemia (ALL) is a heterogeneous disease at the genetic level. Chromosomal abnormalities are used as diagnostic, prognostic and predictive biomarkers to provide subtype, outcome and drug response information. t(12;21)/ETV6-RUNX1 and high hyper-diploidy are good-risk prognostic biomarkers whereas KMT2A (MLL) translocations, t(17;19)/TCF3-HLF, haploidy or low hypodiploidy are high-risk biomarkers. t(9;22)/BCR-ABL1 patients require targeted treatment (imatinib/dasatinib), whereas iAMP21 patients achieve better outcomes when treated intensively. High-risk genetic biomarkers are four times more prevalent in adults compared to children. The application of genomic technologies to cases without an established abnormality (B-other) reveals copy number alterations which can be used either individually or in combination as prognostic biomarkers. Transcriptome sequencing studies have identified a network of fusion genes involving kinase genes - ABL1, ABL2, PDGFRB, CSF1R, CRLF2, JAK2 and EPOR. In vitro and in vivo studies along with emerging clinical observations indicate that patients with a kinase-activating aberration may respond to treatment with small molecular inhibitors like imatinib/dasatinib and ruxolitinib. Further work is required to determine the true frequency of these abnormalities across the age spectrum and the optimal way to incorporate such inhibitors into protocols. In conclusion, genetic biomarkers are playing an increasingly important role in the management of patients with ALL.
Clinical epidemiology of acute lymphoblastic leukemia
Acute lymphoblastic leukemia (ALL) is a heterogeneous disease at the demographic, clinical and genetic levels. Although ALL can occur at any age, it is more prevalent among children, particularly those aged 3–6 years old. More than 50% of the 600 patients diagnosed annually in England and Wales will be aged 0–14 years old, and fewer than 20% will be over 60 years old (A Moorman, unpublished observations, 2016). Males are diagnosed with ALL more frequently than females, resulting in a sex ratio of 1.4:1, respectively. Survival rates from ALL have improved dramatically over the past four decades but vary significantly with age. Children treated on modern protocols have survival rates exceeding 90%.1,2 In contrast, survival from adult ALL is approximately 40% for those patients aged between 25 and 59 years old and is significantly lower (<20%) for older adults.3–5 Improvements in outcome have resulted from optimizing the use of a relatively small number of anti-leukemic drugs, better supportive care, and the introduction of treatment stratification based on risk factors. Traditional risk factors include sex, age, white cell count (WCC) and immunophenotype (B-cell/T-cell) with males, older patients and those with higher white cell count or T-cell ALL having a greater risk of relapse and death. More recently, treatment response (reduction in leukemic burden) has been used to direct treatment.1,2,6 Measuring treatment response or minimal residual disease is performed either by tracking the leukemic clone in serial samples by PCR or flow cytometry looking for specific Ig/TCR rearrangements or immunophenotypic profiles. Minimal residual disease (MRD) is a useful tool for treatment stratification and has been adopted by many clinical study groups in order to risk-stratify patients. One of the major advantages of MRD is that it is applicable to the majority of patients (>90%). However, as MRD measures treatment response, it is protocol dependent, and MRD time points and thresholds need to be carefully assessed for each type of protocol. There is ongoing debate about how to best integrate genetic risk factors and MRD into a cohesive clinical strategy for improving outcome in ALL and different models are emerging (see below). However, one important advantage of genetic risk factors is that they can also act as useful therapeutic targets; for example, the recently identified network of gene fusions which are sensitive to tyrosine kinase inhibitors.7
Genetic landscape of acute lymphoblastic leukemia
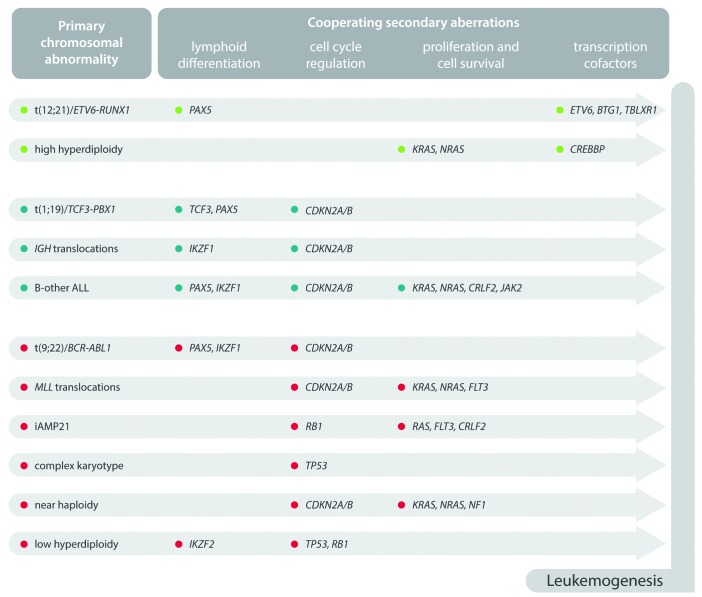
Like all cancers, ALL is characterized by the sequential acquisition of genetic aberrations which drive the initiation and maintenance of the leukemic clone.8,9 Broadly speaking, genetic abnormalities can be considered as primary or secondary events. Primary abnormalities are responsible for the initiation of a pre-leukemic clone which, upon the acquisition of additional secondary or cooperating genetic changes, converts into overt ALL. Elegant studies have demonstrated that the pre-leukemic clone can lie dormant for several years prior to activation.10 Primary abnormalities in ALL are often chromosomal translocations, resulting in chimeric fusion genes, or gross aneuploidy (gain or loss of multiple whole chromosomes); whereas secondary abnormalities are usually copy number alterations (CNA) (frequently micro-deletions) and point mutations. Primary abnormalities are, by definition, present in all the cells comprising the leukemic clone and define the key features of the leukemia. In contrast, secondary abnormalities are present only in a subset of the leukemic cells and give rise to a complex branching sub-clonal architecture.11 In ALL, there is a strong correlation between the primary chromosomal abnormality and the spectrum of secondary or co-operating mutations observed in that subtype (Figure 1).12 The vast majority of aberrations act either as primary or secondary abnormalities; however, a few have been reported as both types in different contexts. The comprehensive genetic testing of patients suspected of having ALL can confirm the diagnosis of ALL and identify important prognostic and predictive biomarkers which can be used to tailor therapy. Primary genetic abnormalities are more reliable prognostic markers than secondary aberrations, probably due to the fact that they define the key features of the clone and are ubiquitous. Therefore, the focus of most screening algorithms in ALL is on the reliable detection of key primary chromosomal abnormalities used to stratify patients into different risk groups. This can be achieved using a combination of cytogenetics, FISH (fluorescence in situ hybridization) and RT-PCR (reverse transcription polymerase chain reaction) but more modern techniques such as multiplex ligation-dependent probe amplification (MLPA), DNA copy number arrays and targeted gene re-sequencing are increasingly being used to screen for new and emerging genetic biomarkers. In this article, I will describe and discuss established, new, and emerging diagnostic, prognostic and predictive genetic biomarkers in the major subtype of B-cell precursor ALL.
Figure 1.
Overview of key co-operating mutations in relation to distinct genetic subtypes of B-cell precursor acute lympholastic leukemia.
Good-risk prognostic genetic biomarkers
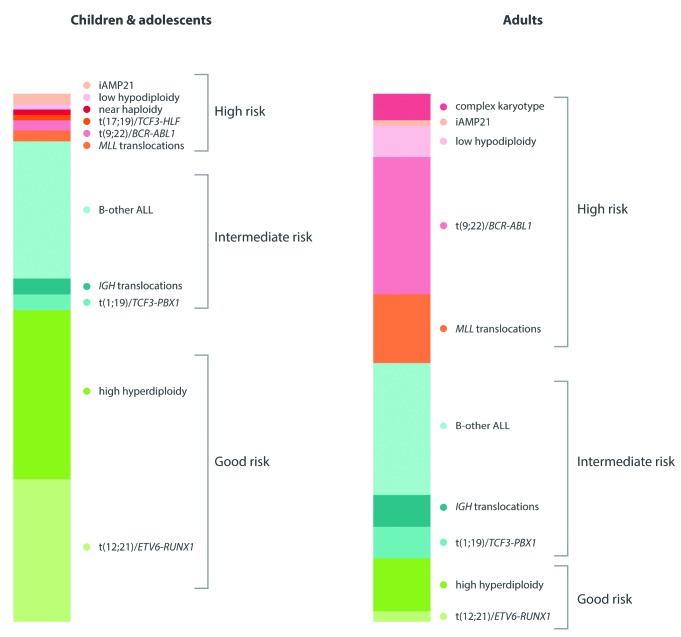
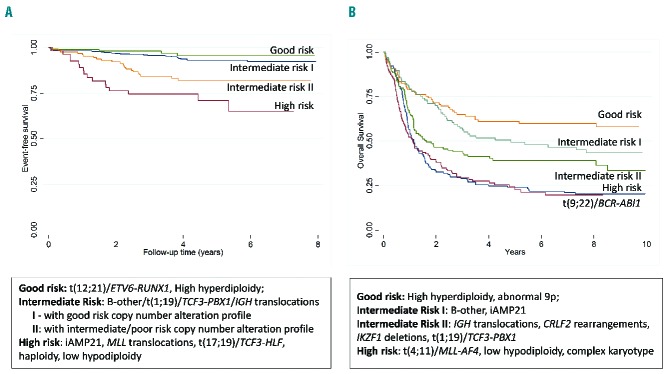
t(12;21)/ETV6-RUNX1 and high hyperdiploidy (51–65 chromosomes) are well recognized diagnostic and prognostic biomarkers in both pediatric and adult ALL. ETV6-RUNX1 results from the chromosomal translocation, t(12;21)(p13;q22) which is cytogenetically cryptic and therefore FISH or RT-PCR is required for its accurate detection. High hyperdiploidy (51–65 chromosomes) is readily detectable by cytogenetics but also by the application of locus specific and centromeric FISH probes as the pattern of chromosomal gain is non-random with eight chromosomes accounting for more than 75% gains; namely chromosomes X, 4, 6, 10, 14, 17, 18 and 21.13–17 These two biomarkers account for approximately 60% of pediatric and adolescent ALL but less than 15% of adult ALL (Figure 2) with ETV6-RUNX1 being virtually non-existent among adults over 30 years of age.18 Patients with either of these abnormalities have a very good outcome compared to their age-matched counterparts, and overall survival (OS) rates at five years is over 90% in pediatric ALL and 55% in adult ALL (Figure 3A).19,20 Although many studies have examined the prognostic relevance of secondary abnormalities (including IKZF1 deletion, ETV6 deletions, RAS pathway mutations) within these two subgroups, no reliable additional biomarkers have emerged.21–25 In addition, within high hyperdiploidy, many studies have assessed specific trisomies, modal chromosome number and structural abnormalities as additional prognostic markers. Although specific trisomies (+4, +10, +17 and +18) have emerged as clinically relevant biomarkers within particular treatment protocols, they have not proved to be universally applicable.13–17 Given the excellent outcome of patients with ETV6-RUNX1 and high hyperdiploidy in pediatric ALL, it is difficult to envisage further clinically actionable biomarkers emerging from within this risk group.
Figure 2.
Frequency of primary chromosomal abnormalities in children and adults with B-cell precursor acute lymphoblastic leukemia.
Figure 3.
Outcome of patients with acute lymphoblastic leukemia (ALL) by genetic risk group. (A) Event-free survival of children and adolescents with B-cell precursor ALL treated on ALL2003 and stratified by cytogenetics and copy number alterations profile. (B) Survival of adults treated on UKALLXII stratified by genetic risk group.
High-risk prognostic genetic biomarkers
Five chromosomal abnormalities [KMT2A (MLL) translocations, t(9;22)/BCR-ABL1, t(17;19)/TCF3-HLF, near haploidy and low hypodiploidy] are well recognized prognostic biomarkers of high-risk disease at all ages.8 The KMT2A (MLL) gene located at 11q23 undergoes rearrangements, usually translocations, with a plethora of partner genes; with AFF1 (AF4), MLLT1 (ENL), MLLT4 (AF6), MLLT3 (AF9) and MLLT10 (AF10) accounting for more than 85% ALL cases.26 Near haploidy and low hypodiploidy are defined by massive chromosomal loss resulting in a modal number of less than 30 chromosomes and 30–39 chromosomes, respectively.27 Recent studies have identified the RAS gene and TP53 mutations, respectively, as key additional drivers of these distinct ploidy subgroups.28 Both subgroups display a propensity to undergo chromosome doubling which can create a diagnostic dilemma if only the doubled-up subclone is dividing and hence masquerades as high hyperdiploidy.27 However, the pattern of chromosomal loss/gain is distinctive, and these two sub-groups are usually distinguishable from one another. All MLL translocations, as well as BCR-ABL1 and TCF3-HLF, are readily detectable by cytogenetics, FISH and RT-PCR. Given the promiscuity of the MLL gene, FISH with a dual-color break-apart directed to the 11q23 locus is the most convenient method of detection. The frequency of these five high-risk genetic aberrations is four times higher in adults compared to children and adolescents (Figure 2), explaining, in part, the strong correlation between age and outcome (Figure 3A and B). KMT2A (MLL) translocations are also highly prevalent in infant ALL (<1 year) where they account for approximately 80% of patients.29 Patients with one of these five abnormalities are classified as high risk in UK protocols and treated with the most intensive regimens.19 If treated as standard risk, patients with one of these aberrations have an approximately 3-fold increased risk of relapse and/or death compared to intermediate-risk patients.19 BCR-ABL1 is a predictive biomarker for targeted therapy with a tyrosine kinase inhibitor, such as imatinib or dasatinib.30 Tyrosine kinase inhibitors directly inhibit the leukemogenic effect of the BCR-ABL1 oncoprotein and, in combination with standard chemotherapy, produce significantly superior outcomes in patients of all ages.31,32 Recent studies of MLL-rearranged ALL have highlighted the importance of epigenetic dysregulation in this subgroup and, in particular, the requirement for the histone methyltransferase, DOT1L.33 These studies raise the possibility of developing a targeted therapy for these high-risk patients using DOT1L inhibitors such as EPZ004777.34
In adult ALL, patients without an established chromosomal translocation or ploidy sugroups are classified as having a complex karyotype if their karyotype harbors five or more chromosomal abnormalities.35 This definition identified approximately 5% patients which have a higher risk of relapse or death in some, but not all, treatment protocols (Figure 3B).35–37 Given the subjective nature of chromosomal analysis and the vagaries of cytogenetic nomenclature, complex karyotype is not an ideal biomarker for the reliable identification of high-risk patients. The intensive research described below aimed at unraveling the genetics of B-other ALL. This will, in time, provide a more reliable biomarker for this subset of patients.
t(1;19)(q23;p13)/TCF3-PBX1
Approximately 3% of children/adolescents and 6% of adults harbor the translocation t(1;19) which in more than 95% cases results in TCF3-PBX1 fusion.38 It is an intriguing biomarker in ALL: as a diagnostic biomarker it correlates tightly with a pre-B immunophenotype with the leukemic cells expressing cytoplasmic μ. It is readily and reliably identified by cytogenetics, FISH and RT-PCR, making it an ideal prognostic biomarker in practical terms. However, outcome studies have produced variable results and there is a stark contrast between pediatric and adult ALL regarding how these patients are viewed in terms of risk. In pediatric cohorts, early studies reported TCF3-PBX1 as a biomarker of poor prognosis, but most recent studies from protocols delivering more intensive chemotherapy have reported considerably improved outcome (OS >80%).19,39–41 Interestingly, some studies have reported an association with central nervous system (CNS) relapse and a poor outcome after first relapse, indicating substantial clinical heterogeneity, which may point to the presence of additional prognostic biomarkers in this subgroup.40 There is a similar story in adult ALL, with the larger and more recent studies showing TCF3-PBX1 to be associated with intermediate risk.35,42,43 However, some clinical study groups classify adult patients with TCF3-PBX1 as high risk and treat these patients more aggressively.44
Intrachromosomal amplification of chromosome 21 (iAMP21)
In the past few years, iAMP21 has become an important prognostic and predictive biomarker in childhood ALL. iAMP21 is a grossly abnormal chromosome generated via breakage-fusion-bridge cycles and chromothripsis.45 The result of these rearrangements is the amplification and loss of multiple regions along the length of chromosome 21. The consistent feature of all iAMP21 cases is the amplification of the RUNX1 locus located at 21q22.12, which provides the basis for a convenient and reliable detection assay: FISH using locus-specific probes.46 The internationally accepted definition of iAMP21 is three or more extra copies of the RUNX1 gene on a single abnormal chromosome 21, equating to more than 5 signals per cell.46 Patients with iAMP21 are older than other children with ALL (median age 9 years) but a lower median WCC.47,48 Studies by the UK and the Children’s Oncology Group (COG) have reported that iAMP21 patients treated as standard risk have a very high rate of relapse (>80%) but that this is significantly reduced (<20%) when the patients are treated intensively, notably on the most intensive arm of UKALL2003 (regimen C).49,50 Thus, iAMP21 can be considered both a prognostic and predictive biomarker in pediatric ALL. However, the Associazione Italiana di Ematologia ed Oncologia Pediatrica (AEIOP) and Berlin-Frankfurt-Munster (BFM) study groups have reported that MRD can also be used to identify iAMP21 patients at risk of relapse.51,52 As iAMP21 is extremely rare among adult patients (≥25 years), its prognostic effect in this age group is unclear.
Translocation involving the IGH locus
IGH translocations are well recognized in lymphoid malignancies where the juxtaposition of an oncogene to the IGH enhancer drives its overexpression.53 IGH translocations are frequent in lymphomas and mature leukemias. However, recent studies have revealed an extensive network of IGH translocations specific to BCP-ALL, which drive the expression of a variety of oncogenes.54 The most common IGH translocation involves CRLF2, accounting for approximately 25% of cases. Other recurrent translocation partners include five members of the CEBP gene family and ID4, accounting for approximately 10% and 7% of cases, respectively. Although numerous partner genes have yet to be identified, there does not appear to be any functional link between the partner genes of IGH translocations. Given the wide spectrum of these partners and the finding that several, including IGH-CRLF2, are cytogenetically cryptic, FISH using a break-apart probe directed to the IGH locus provides a reliable detection method. The most notable clinical feature of patients with IGH translocations is their age profile. Their frequency is low among children under ten years of age (<3%) but considerably higher (10%) among adolescents and young adults (15–24 years).54 Patients with IGH translocations have been shown to have an inferior outcome compared to other patients in both the adolescent and young adult setting.54
Dissecting B-other acute lymphoblastic leukemia
Approximately 70% of pediatric and 60% of adult patients with ALL harbor a genetic abnormality regarded as an established diagnostic and/or prognostic biomarker (Figure 2). Patients without one of these abnormalities are collectively referred to as B-other ALL. Unraveling the genetic landscape of B-other ALL has been the main focus of research efforts over the past ten years. A wide variety of techniques, ranging from cytogenetics to whole genome sequencing, have been applied in order to define and assess biomarkers in this subgroup. Two main approaches have shaped the current research strategy. Firstly, a plethora of micro-deletions affecting genes in key pathways, including lymphoid differentiation, cell-cycle differentiation and proliferation were discovered using SNP arrays in both childhood and adult ALL.55–58 Numerous studies have assessed the role of individual lesions and copy number alteration (CNA) profiles as prognostic biomarkers. Almost all of these CNA are secondary aberrations which are sub-clonal and can be acquired, lost or enriched for between diagnosis and relapse.59–61 Secondly, gene expression profiling has been used to define cytogenetic subgroups and at the same time identify novel subgroups of patients.62,63
IKZF1 and ERG deletions
IKZF1 deletions occur in 15% of pediatric and 30% of adult ALL, but are more prevalent among patients with BCR-ABL1 (>60%).14,64–66 In addition, they are associated with other high-risk features such as older age, high WCC, persistent MRD, and Down syndrome.12,67 Initial reports suggested that patients harboring an IKZF1 deletion had a significantly inferior outcome, implying that it was a reliable prognostic marker.68 However, more recent studies based on larger and more representative cohorts have suggested that its effect is pleiotropic.69–73 In particular, a COG study found that IKZF1 deletions were prognostic among National Cancer Institute (NCI) high-risk (age >10 years or WCC >50×109/L) patients but not NCI standard-risk patients.74 In addition, studies have found that the presence of an IKZF1 deletion does not abrogate the prognosis associated with other good risk genetic abnormalities such as ETV6-RUNX1 and ERG deletions (see below).21,72,73,75 These results correlate with those from studies that have examined the interaction of IKZF1 deletions and MRD. Several studies have now reported that IKZF1 deletions are not prognostic among patients who clear their disease rapidly. Instead the prognostic effect is restricted to, or at least strongest in, patients with higher levels of disease burden after initial chemotherapy.69,70,76 Furthermore, IKZF1 deletions were not associated with a greater risk of second relapse or death after a first marrow relapse.77 However, within the context of BCR-ABL1 ALL, it appears that IKZF1 deletions are strongly prognostic even when these patients are treated with imatinib-containing regimens.66 Therefore, it is likely that IKZF1 deletions, which are secondary abnormalities, are also a “secondary” marker of poor outcome rather than being a key independent prognostic biomarker.
Three studies have now identified a distinct subgroup of pediatric B-other ALL patients characterized by a monoallelic deletion of the ERG gene.63,72,73 The frequency is 10%–15% of B-other ALL which equates to 3%–5% of BCP-ALL overall. Interestingly, these patients have an excellent outcome of over 90% at five years despite having a very high incidence of IKZF1 deletions (approx. 40%).63,72,73 Even though the presence of an ERG deletion appears to define a distinct subgroup of B-other ALL, there is evidence that it is a sub-clonal event which can be lost or gained between diagnosis and relapse. Its role as a robust prognostic marker requires urgent further investigation, particularly within adult ALL.
CRLF2 deregulation
The interstitial deletion in the PAR1 region of chromosome X and Y gives rise to dysregulation of CRLF2 via juxtaposition of this gene to the P2RY8 promoter.78,79 Overexpression of CRLF2 can also arise from an IGH translocation and, more rarely, an activating mutation.80 In addition, high CRLF2 expression can occur in patients who lack a clear genetic alteration at this locus. CRLF2 rearrangements have been associated with activation of the JAK-STAT, ERK and mTOR/PI3K pathways and it is noteworthy that approximately 50% of cases also harbor a JAK2 mutation.7,78,79,81 The overall frequency of CRLF2 rearrangements in BCP-ALL is 5% but is higher in B-other (30%) and patients with Down syndrome (>50%).67,78 Studies investigating the prognostic relevance of CRLF2 have varied in the method used to identify cases. Some studies have focused on genetic rearrangements whilst others have measured CRLF2 mRNA expression. Perhaps unsurprisingly the results of these studies have been conflicting with some studies concluding that it was a prognostic marker of poor outcome82,83 while others concluded that it was not relevant in the context of other risk factors.52,67,75,84 Interestingly, three studies found discordant results depending on whether CRLF2 involvement was determined by genetics or expression,74,82,85 thereby emphasizing the requirement for standardized definitions. In addition, P2RY8-CRLF2 is a secondary abnormality and often present only within low level sub-clones which do not drive relapse.86 Although CRLF2 does not appear to be a robust prognostic marker, it is an attractive therapeutic target particularly within the context of Down syndrome patients who are prone to the toxic side-effects of chemotherapy. Therefore, inhibition of the JAK and PI3K pathways represent potential therapeutic strategies in these cases.7,87
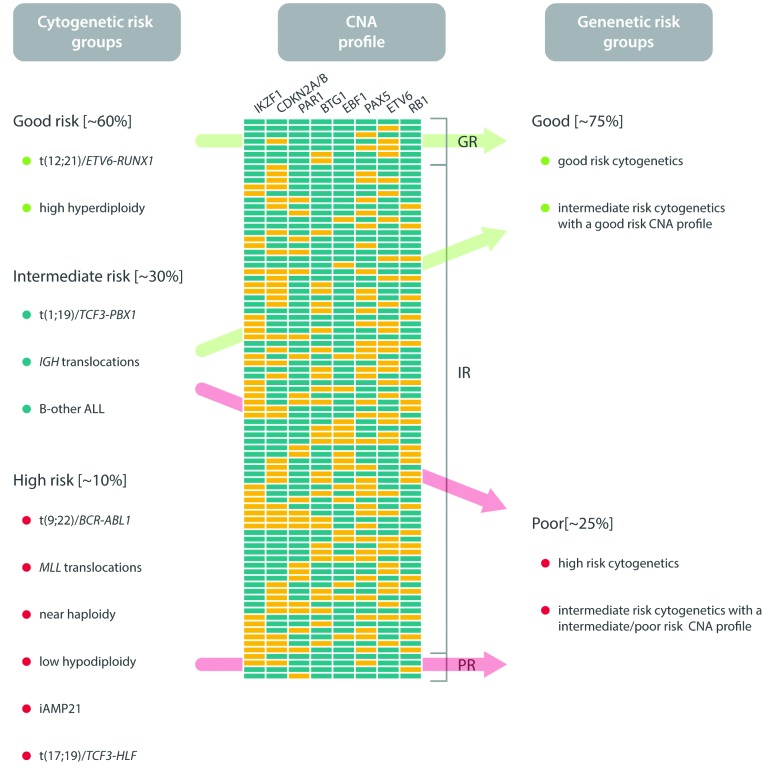
Copy number alteration profiling
The major limitation to assessing the prognostic relevance of individual CNAs is that it does not take into account the fact that many cases will harbor more than one deletion while others may have none. An alternative approach has been to integrate a CNA profile into the existing established cytogenetic risk-group classification. Here a CNA profile based on the presence or absence of the eight most frequently deleted genes segregates patients with intermediate-risk cytogenetics (mostly B-other) into two new genetic risk groups (Figure 4).88 The prognosis of patients with good- or high-risk cytogenetics was unaffected by their CNA profile. However, intermediate cytogenetic risk patients, separated into two subgroups (good risk vs. intermediate-/high-risk CNA profile) with differential OS rates (98% vs. 87%).88 Thus, this approach has identified a group of B-other ALL patients with a good-risk CNA profile and a very low risk of relapse who potentially could be considered for treatment deintensification. The validity of such an approach is supported by observations that the prognostic effect of IKZF1 deletions depends on the presence/absence of other deletions (e.g. ERG and CDKN2A/B deletions) and MRD levels.63,72,73,76
Figure 4.
Proposal for a new integrated risk classification of childhood B-cell precursor acute lymphoblastic leukemia based on primary chromosomal abnormalities and copy number alteration (CNA) profile (yellow indicates deleted; blue indicates not deleted).
BCR-ABL1-like
Four independent studies reported a subgroup of B-other ALL patients with a gene expression profile similar to BCR-ABL1 positive ALL.68,89–91 Although these patients lacked the fusion gene they shared the same poor outcome. The subgroups were termed BCR-ABL1-like or Ph-like and both accounted for approximately 50% of B-other ALL cases. There were significant differences in the genetic make-up of these subgroups; especially with respect to the prevalence of IKZF1 deletions, CRLF2 rearrangements and JAK2 mutations.68,89 Crucially, the gene expression signatures were not transferable, and when applied to the same cohort of patients, the signatures classified different patients as BCR-ABL1-like/Ph-like.92 Therefore, gene expression signatures do not provide an ideal prognostic biomarker for use between different clinical trials. However, the common features of these subgroups are poor outcome and enrichment of CRLF2 rearrangements and IKZF1 deletion, albeit to varying extents. Although it is unlikely that any gene signature expression will become a robust prognostic biomarker within B-other ALL, there is clearly a subset of patients with B-other ALL who have a poor outcome, and it is now emerging that members of this group can be identified from their distinctive genomic abnormalities.
Kinase activating gene fusions
In 2012, Roberts and colleagues performed RNA sequencing of a small cohort of BCR-ABL1-like cases and discovered chimeric fusions involving PDGFRB, ABL1 and JAK2.87 Since this initial study, a complex network of kinase-activating aberrations has been revealed, with many occurring in single patients.7,93,94 The common feature of these chimeric genes is the fusion of the 3′ end of a kinase gene with the 5′ portion of so-called activating gene, of which over 30 have now been reported. Theoretically, all these kinase-activating lesions can be targeted with appropriate small molecule inhibitors, and hence become useful predictive biomarkers. For example, in vitro and in vivo studies have demonstrated that ABL1, ABL2, PDGFRB and CSF1R fusions are sensitive to imatinib and dasatinib, and that CRLF2, JAK2 and EPOR are sensitive to JAK inhibitors (e.g. ruxolitinib).7 Moreover, a small number of children harboring ABL1, ABL2, PDGFRB or CSF1R fusions with refractory disease achieved a complete remission following treatment with imatinib or dasatinib.7,95–98 However, it should be noted that these patients were highly selected and that follow up was extremely limited. Nonetheless, these laboratory and clinical observations provide encouraging evidence that targeted therapies could be offered routinely to patients with one of these predictive biomarkers in the near future. Their prevalence has yet to be firmly established in large representative cohorts. Initial screening of patients in the UK has indicated that ABL1, ABL2, PDGFRB or CSF1R fusions collectively occur in 1%–2% of B-cell precursor ALL independent of age; whereas CRLF2, JAK2 and EPOR fusions together occur in 3%–5% of childhood ALL rising to approximately 15% in adolescent and young adult ALL (A Moorman, unpublished observations, 2016). Current published studies have suggested that IGH-CRLF2, PR2Y8-CRLF2 and EBF1-PDGFRB are the most prevalent of these gene fusions. The optimal detection method is challenging given the number of genes involved and the complex nature of some of the chromosomal rearrangements which give rise to these fusion genes. FISH using probes to target the kinase gene provides a simple and efficient strategy for detection of many of the fusions, especially ABL1, PDGFRB, CSF1R, JAK2 and CRLF2, and can readily be incorporated into current screening algorithms. However, assays based on next generation sequencing technology are on the horizon and are likely to provide a more comprehensive approach.
Conclusion and future perspectives
The extensive genetic heterogeneity found within ALL provides a wealth of potential genetic biomarkers that could be used to assist patient management. Prognostic biomarkers such as ETV6-RUNX1 and high hyperdiploidy can be used to define a cohort of patients with a low risk of relapse on standard therapy whereas patients with high-risk cytogenetics require more intensive or targeted therapy. In the past ten years, genomic analysis has revolutionized the way researchers and clinicians think about the biology of ALL, and new therapeutic strategies and options are beginning to emerge. Additional research is required to assess the clinical utility of some of these discoveries, as a number of questions remain unanswered. For example: 1) what is the optimal way to use copy number alterations as prognostic biomarkers in B-other ALL and within the context of MRD-driven protocols¿ 2) Which kinase-activating abnormalities are predictive biomarkers for treatment with an appropriate inhibitor¿ 3) What is the role of these new genetic biomarkers in directing therapy after first relapse¿ In addition to addressing these translational questions, the large-scale application of whole genome and exome sequencing will undoubtedly identify new genetic biomarkers which may add to or replace our current repertoire of prognostic and predictive biomarkers.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/4/407
References
- 1.Vora A, Goulden N, Mitchell C, et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2014;15(8):809–818. [DOI] [PubMed] [Google Scholar]
- 2.Vora A, Goulden N, Wade R, et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. [DOI] [PubMed] [Google Scholar]
- 3.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827–1833. [DOI] [PubMed] [Google Scholar]
- 4.Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115(2):206–214. [DOI] [PubMed] [Google Scholar]
- 5.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol. 2012;157(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. [DOI] [PubMed] [Google Scholar]
- 7.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moorman AV. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26(3):123–135. [DOI] [PubMed] [Google Scholar]
- 9.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6(3):193–203. [DOI] [PubMed] [Google Scholar]
- 11.Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356–361. [DOI] [PubMed] [Google Scholar]
- 12.Schwab CJ, Chilton L, Morrison H, et al. Genes commonly deleted in childhood B-cell precursor acute lymphoblastic leukemia: association with cytogenetics and clinical features. Haematologica. 2013;98(7):1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorman AV, Richards SM, Martineau M, et al. Outcome heterogeneity in childhood high-hyperdiploid acute lymphoblastic leukemia. Blood. 2003;102(8):2756–2762. [DOI] [PubMed] [Google Scholar]
- 14.Heerema NA, Raimondi SC, Anderson JR, et al. Specific extra chromosomes occur in a modal number dependent pattern in pediatric acute lymphoblastic leukemia. Genes Chromosomes and Cancer. 2007;46(7):684–693. [DOI] [PubMed] [Google Scholar]
- 15.Sutcliffe MJ, Shuster JJ, Sather HN, et al. High concordance from independent studies by the Children’s Cancer Group (CCG) and Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies 4, 10, and 17 in children with NCI Standard-Risk B-precursor Acute Lymphoblastic Leukemia: a Children’s Oncology Group (COG) initiative. Leukemia. 2005;19(5):734–740. [DOI] [PubMed] [Google Scholar]
- 16.Dastugue N, Suciu S, Plat G, et al. Hyperdiploidy with 58–66 chromosomes in childhood B-acute lymphoblastic leukemia is highly curable: 58951 CLG-EORTC results. Blood. 2013;121(13):2415–2423. [DOI] [PubMed] [Google Scholar]
- 17.Paulsson K, Forestier E, Andersen MK, et al. High modal number and triple trisomies are highly correlated favorable factors in childhood B-cell precursor high hyperdiploid acute lymphoblastic leukemia treated according to the NOPHO ALL 1992/2000 protocols. Haematologica. 2013;98(9):1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jabbar Al Obaidi MS, Martineau M, Bennett CF, et al. ETV6/AML1 fusion by FISH in adult acute lymphoblastic leukemia. Leukemia. 2002;16(4):669–674. [DOI] [PubMed] [Google Scholar]
- 19.Moorman AV, Ensor HM, Richards SM, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010;11(5):429–438. [DOI] [PubMed] [Google Scholar]
- 20.Chilton L, Buck G, Harrison CJ, et al. High hyperdiploidy among adolescents and adults with acute lymphoblastic leukaemia (ALL): cytogenetic features, clinical characteristics and outcome. Leukemia. 2014;28(7):1511–1518. [DOI] [PubMed] [Google Scholar]
- 21.Enshaei A, Schwab CJ, Konn ZJ, et al. Long-term follow-up of ETV6-RUNX1 ALL reveals that NCI risk, rather than secondary genetic abnormalities, is the key risk factor. Leukemia. 2013;27(11):2256–2259. [DOI] [PubMed] [Google Scholar]
- 22.Bhojwani D, Pei D, Sandlund JT, et al. ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia. 2012;26(2):265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barbany G, Andersen MK, Autio K, et al. Additional aberrations of the ETV6 and RUNX1 genes have no prognostic impact in 229 t(12;21)(p13;q22)-positive B-cell precursor acute lymphoblastic leukaemias treated according to the NOPHO-ALL-2000 protocol. Leuk Res. 2012;36(7):936–938. [DOI] [PubMed] [Google Scholar]
- 24.Paulsson K, Johansson B. High hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2009;48(8): 637–660. [DOI] [PubMed] [Google Scholar]
- 25.Boer JM, Veer Avd, Rizopoulos D, et al. Prognostic Value of Rare IKZF1 deletions in Childhood B-Cell Precursor Acute Lymphoblastic Leukemia: An International Collaborative Study. Blood. 2014;124(21): 368. [DOI] [PubMed] [Google Scholar]
- 26.Meyer C, Hofmann J, Burmeister T, et al. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27(11):2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison CJ, Moorman AV, Broadfield ZJ, et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol. 2004;125(5):552–559. [DOI] [PubMed] [Google Scholar]
- 28.Holmfeldt L, Wei L, Diaz-Flores E, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet. 2013;45(3):242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pieters R, Schrappe M, De LP, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240–250. [DOI] [PubMed] [Google Scholar]
- 30.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112(13):4808–4817. [DOI] [PubMed] [Google Scholar]
- 31.Fielding AK, Rowe JM, Buck G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13(9):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernt KM, Zhu N, Sinha AU, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernt KM, Armstrong SA. Targeting epigenetic programs in MLL-rearranged leukemias. Hematology Am Soc Hematol Educ Program. 2011;2011:354–360. [DOI] [PubMed] [Google Scholar]
- 35.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): Analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII / Eastern Cooperative Oncology Group (ECOG) 2993 Trial. Blood. 2007;109(8):3189–3197. [DOI] [PubMed] [Google Scholar]
- 36.Motllo C, Ribera JM, Morgades M, et al. Prognostic significance of complex karyotype and monosomal karyotype in adult patients with acute lymphoblastic leukemia treated with risk-adapted protocols. Cancer. 2014;120(24):3958–3964. [DOI] [PubMed] [Google Scholar]
- 37.Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood. 2008;111(5):2563–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barber KE, Harrison CJ, Broadfield ZJ, et al. Molecular Cytogenetic Characterisation of TCF3 (E2A) / 19p13.3 Rearrangements in B-Cell Precursor Acute Lymphoblastic Leukaemia. Genes Chromosomes Cancer. 2007;46(5):478–486. [DOI] [PubMed] [Google Scholar]
- 39.Raimondi S, Behm F, Robertson PK, et al. Cytogenetics of pre-B-cell acute lymphoblastic leukemia with emphasis on prognostic implications on the t(1;19). J Clin Oncol. 1990;8:1380–1388. [DOI] [PubMed] [Google Scholar]
- 40.Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kager L, Lion T, Attarbaschi A, et al. Incidence and outcome of TCF3-PBX1-positive acute lymphoblastic leukemia in Austrian children. Haematologica. 2007;92(11):1561–1564. [DOI] [PubMed] [Google Scholar]
- 42.GFCH. Cytogenetic Abnormalities in Adult Acute Lymphoblastic Leukemia: Correlations with Hematologic Findings and Outcome. A Collaborative Study of the Groupe Francais de Cytogenetique Hematologique. Blood. 1996;87(8):3135–3142. [PubMed] [Google Scholar]
- 43.Garg R, Kantarjian H, Thomas D, et al. Adults with acute lymphoblastic leukemia and translocation (1;19) abnormality have a favorable outcome with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and high-dose cytarabine chemotherapy. Cancer. 2009;115(10):2147–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beldjord K, Chevret S, Asnafi V, et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739–3749. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Schwab C, Ryan SL, et al. Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature. 2014;508(7494):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison CJ, Haas O, Harbott J, et al. Detection of prognostically relevant genetic abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: recommendations from the Biology and Diagnosis Committee of the International Berlin-Frankfurt-Munster study group. Br J Haematol. 2010;151(2):132–142. [DOI] [PubMed] [Google Scholar]
- 47.Moorman AV, Richards SM, Robinson HM, et al. Prognosis of children with acute lymphoblastic leukaemia (ALL) and intrachromosomal amplification of chromosome 21 (iAMP21). Blood. 2007;109:2327–2330. [DOI] [PubMed] [Google Scholar]
- 48.Harrison CJ, Moorman AV, Schwab C, et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia. 2014;28(5):1015–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moorman AV, Robinson H, Schwab C, et al. Risk-directed treatment intensification significantly reduces the risk of relapse among children and adolescents with acute lymphoblastic leukemia and intrachromosomal amplification of chromosome 21: a comparison of the MRC ALL97/99 and UKALL2003 trials. J Clin Oncol. 2013;31(27):3389–3396. [DOI] [PubMed] [Google Scholar]
- 50.Heerema NA, Carroll AJ, Devidas M, et al. Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk children’s oncology group studies: a report from the children’s oncology group. J Clin Oncol. 2013;31(27):3397–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Attarbaschi A, Panzer-Grumayer R, Mann G, et al. Minimal residual disease-based treatment is adequate for relapse-prone childhood acute lymphoblastic leukemia with an intrachromosomal amplification of chromosome 21: the experience of the ALL-BFM 2000 trial. Klin Padiatr. 2014;226(6–7):338–343. [DOI] [PubMed] [Google Scholar]
- 52.Attarbaschi A, Morak M, Cario G, et al. Treatment outcome of CRLF2-rearranged childhood acute lymphoblastic leukaemia: a comparative analysis of the AIEOP-BFM and UK NCRI-CCLG study groups. Br J Haematol. 2012;158(6):772–777. [DOI] [PubMed] [Google Scholar]
- 53.Dyer MJ, Akasaka T, Capasso M, et al. Immunoglobulin heavy chain locus chromosomal translocations in B-cell precursor acute lymphoblastic leukemia: rare clinical curios or potent genetic drivers¿ Blood. 2010;115(8):1490–1499. [DOI] [PubMed] [Google Scholar]
- 54.Russell LJ, Enshaei A, Jones L, et al. IGH@ translocations are prevalent in teenagers and young adults with acute lymphoblastic leukemia and are associated with a poor outcome. J Clin Oncol. 2014;32(14):1453–1462. [DOI] [PubMed] [Google Scholar]
- 55.Kawamata N, Ogawa S, Zimmermann M, et al. Molecular allelokaryotyping of pediatric acute lymphoblastic leukemias by high-resolution single nucleotide polymorphism oligonucleotide genomic microarray. Blood. 2008;111(2):776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paulsson K, Cazier JB, Macdougall F, et al. Microdeletions are a general feature of adult and adolescent acute lymphoblastic leukemia: Unexpected similarities with pediatric disease. Proc Natl Acad Sci USA. 2008;105(18):6708–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strefford JC, Worley H, Barber K, et al. Genome complexity in acute lymphoblastic leukemia is revealed by array-based comparative genomic hybridization. Oncogene. 2007;26(29):4306–4318. [DOI] [PubMed] [Google Scholar]
- 58.Mullighan CG, Goorha S, Radtke I, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446(7137):758–764. [DOI] [PubMed] [Google Scholar]
- 59.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hogan LE, Meyer JA, Yang J, et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011;118(19):5218–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang JJ, Bhojwani D, Yang W, et al. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008;112(10):4178–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeoh EJ, Ross ME, Shurtleff SA, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1(2):133–143. [DOI] [PubMed] [Google Scholar]
- 63.Harvey RC, Mullighan CG, Wang X, et al. Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood. 2010;116(23):4874–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mullighan CG, Miller CB, Radtke I, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453(7191):110–114. [DOI] [PubMed] [Google Scholar]
- 65.Martinelli G, Iacobucci I, Storlazzi CT, et al. IKZF1 (Ikaros) deletions in BCR-ABL1-positive acute lymphoblastic leukemia are associated with short disease-free survival and high rate of cumulative incidence of relapse: a GIMEMA AL WP report. J Clin Oncol. 2009;27(31):5202–5207. [DOI] [PubMed] [Google Scholar]
- 66.van der Veer A, Zaliova M, Mottadelli F, et al. IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood. 2014;123(11):1691–1698. [DOI] [PubMed] [Google Scholar]
- 67.Buitenkamp TD, Pieters R, Gallimore NE, et al. Outcome in children with Down’s syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia. 2012;26(10):2204–2211. [DOI] [PubMed] [Google Scholar]
- 68.Mullighan CG, Su X, Zhang J, et al. Deletion of IKZF1 and Prognosis in Acute Lymphoblastic Leukemia. N Engl J Med. 2009;360(5):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmi C, Valsecchi MG, Longinotti G, et al. What is the relevance of Ikaros gene deletions as prognostic marker in pediatric Philadelphia negative B-cell precursor acute lymphoblastic leukemia¿ Haematologica. 2013;98(8):1226–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waanders E, van der Velden VH, van der Schoot CE, et al. Integrated use of minimal residual disease classification and IKZF1 alteration status accurately predicts 79% of relapses in pediatric acute lymphoblastic leukemia. Leukemia. 2011;25(2):254–258. [DOI] [PubMed] [Google Scholar]
- 71.Moorman AV, Schwab C, Ensor HM, et al. IGH@ translocations, CRLF2 deregulation, and microdeletions in adolescents and adults with acute lymphoblastic leukemia. J Clin Oncol. 2012;30(25):3100–3108. [DOI] [PubMed] [Google Scholar]
- 72.Clappier E, Auclerc MF, Rapion J, et al. An intragenic ERG deletion is a marker of an oncogenic subtype of B-cell precursor acute lymphoblastic leukemia with a favorable outcome despite frequent IKZF1 deletions. Leukemia. 2014;28(1):70–77. [DOI] [PubMed] [Google Scholar]
- 73.Zaliova M, Zimmermannova O, Dorge P, et al. ERG deletion is associated with CD2 and attenuates the negative impact of IKZF1 deletion in childhood acute lymphoblastic leukemia. Leukemia. 2014;28(1):182–185. [DOI] [PubMed] [Google Scholar]
- 74.Chen IM, Harvey RC, Mullighan CG, et al. Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2012;119(15):3512–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Veer A, Waanders E, Pieters R, et al. Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood. 2013;122(15): 2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dagdan E, Zaliova M, Dörge P, et al. The Strong Prognostic Effect of Concurrent Deletions of IKZF1 and PAX5, CDKN2A, CDKN2B or PAR1 in the Absence of ERG Deletions (IKZF1plus) in Pediatric Acute Lymphoblastic Leukemia Strongly Depends on Minimal Residual Disease Burden after Induction Treatment Blood. 2014;124(21): 131. [Google Scholar]
- 77.Moorman AV, Erhorn A, Parker C, et al. The Clinical Relevance Of Genetics In Predicting Outcome After a First Relapse In Children With B-Cell Precursor Acute Lymphoblastic Leukaemia. Blood. 2013;122(21):2566. [Google Scholar]
- 78.Russell LJ, Capasso M, Vater I, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114(13): 2688–2698. [DOI] [PubMed] [Google Scholar]
- 79.Mullighan CG, Collins-Underwood JR, Phillips LA, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41(11):1243–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chapiro E, Russell L, Lainey E, et al. Activating mutation in the TSLPR gene in B-cell precursor lymphoblastic leukemia. Leukemia. 2010;24(3):642–645. [DOI] [PubMed] [Google Scholar]
- 81.Tasian SK, Doral MY, Borowitz MJ, et al. Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood. 2012;120(4):833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cario G, Zimmermann M, Romey R, et al. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115(26):5393–5397. [DOI] [PubMed] [Google Scholar]
- 83.Harvey RC, Mullighan CG, Chen IM, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ensor HM, Schwab C, Russell LJ, et al. Demographic, clinical, and outcome features of children with acute lymphoblastic leukemia and CRLF2 deregulation: results from the MRC ALL97 clinical trial. Blood. 2011;117(7):2129–2136. [DOI] [PubMed] [Google Scholar]
- 85.Palmi C, Vendramini E, Silvestri D, et al. Poor Prognosis for P2RY8-CRLF2 Fusion but not for CRLF2 Over-expression in Children with Intermediate Risk B-Cell Precursor Acute Lymphoblastic Leukemia. Leukemia. 2012;26(10):2245–2253. [DOI] [PubMed] [Google Scholar]
- 86.Morak M, Attarbaschi A, Fischer S, et al. Small sizes and indolent evolutionary dynamics challenge the potential role of P2RY8-CRLF2-harboring clones as main relapse-driving force in childhood ALL. Blood. 2012;120(26):5134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22(2):153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moorman AV, Enshaei A, Schwab C, et al. A novel integrated cytogenetic and genomic classification refines risk stratification in pediatric acute lymphoblastic leukemia. Blood. 2014;124(9):1434–1444. [DOI] [PubMed] [Google Scholar]
- 89.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kiyokawa N, Iijima K, Yoshihara H, et al. An Analysis Of Ph-Like ALL In Japanese Patients. Blood. 2013;122(21):352. [Google Scholar]
- 91.Kronnie Gt, Silvestri D, Vendramini E, et al. Philadelphia-Like Signature In Childhood Acute Lymphoblastic Leukemia: The AIEOP Experience. Blood. 2013;122(21):353. [Google Scholar]
- 92.Boer JM, Marchante JRM, Horstmann MA, et al. BCR-ABL1-Like Cases In Pediatric Acute Lymphoblastic Leukemia: A Comparison Between COG/St. Jude and Dutch DCOG Signatures. Blood. 2013;122(21):2633. [Google Scholar]
- 93.Kobayashi K, Mitsui K, Ichikawa H, et al. ATF7IP as a novel PDGFRB fusion partner in acute lymphoblastic leukaemia in children. Br J Haematol. 2014;165(6):836–841. [DOI] [PubMed] [Google Scholar]
- 94.Lilljebjorn H, Agerstam H, Orsmark-Pietras C, et al. RNA-seq identifies clinically relevant fusion genes in leukemia including a novel MEF2D/CSF1R fusion responsive to imatinib. Leukemia. 2014;28(4):977–979. [DOI] [PubMed] [Google Scholar]
- 95.Lengline E, Beldjord K, Dombret H, Soulier J, Boissel N, Clappier E. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica. 2013;98(11):e146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weston BW, Hayden MA, Roberts KG, et al. Tyrosine kinase inhibitor therapy induces remission in a patient with refractory EBF1-PDGFRB-positive acute lymphoblastic leukemia. J Clin Oncol. 2013;31(25):e413–416. [DOI] [PubMed] [Google Scholar]
- 97.Schwab C, Andrews R, Chilton L, et al. EBF1-PDGFR Fusion in Paediatric Acute Lymphoblastic Leukaemia (ALL): Genetic Profile and Clinical Implications. Blood. 2014;124(21):1068. [Google Scholar]
- 98.Schwab C, Andrews R, Chilton L, et al. SSBP2-CSF1R Is a Recurrent Fusion in BOther Acute Lymphoblastic Leukaemia with Variable Clinical Outcome. Blood. 2014;124(21):3773. [Google Scholar]