The use of the proteasome inhibitor bortezomib and immunomodulatory drugs such as thalidomide and lenalidomide have markedly improved outcome in patients with multiple myeloma (MM).1 However, the majority of patients will eventually relapse, necessitating salvage therapy.
HOVON 86 is a dose escalating phase I–II study, evaluating the feasibility (phase I) and efficacy (phase II) of once weekly escalated doses of bortezomib and lenalidomide in combination with fixed dose dexamethasone followed by lenalidomide maintenance in patients with MM in first relapse or refractory after first-line therapy. In previous trials bortezomib was explored predominantly in a twice weekly schedule. We wished to explore once weekly bortezomib with the possibility to increase the dose of bortezomib in combination with an escalated dose of lenalidomide and dexamethasone.
The maximum tolerated dose (MTD) and recommended dose level (RDL) were determined according to a ′3+3′ dose escalation scheme at bortezomib 1.6mg/m2 intravenously (IV); lenalidomide 10mg; dexamethasone 20mg, followed by lenalidomide maintenance.
44 out of 70 (63%) eligible patients started maintenance. ORR on protocol was 89%, with 71% ≥VGPR, including 27% CR. The median number of cycles to achieve ≥PR was 1. The median progression-free survival (PFS) and overall survival (OS) rates were 19 and 42 months, respectively. Hematologic toxicity CTC grade 3–4 occurred in 30% of patients and 17% of patients developed polyneuropathy (PN) grade 3–4.
This study was approved by the ethics committees of the Erasmus MC Cancer Institute and the participating sites. All patients gave written informed consent, and the trial was conducted according to the European Clinical Trials Directive 2005 and the Declaration of Helsinki, trial EudraCT number: 2007-002533-37.
Patients were eligible for this study if they were aged ≥18 years with primary refractory MM or first relapse after prior achievement of an objective response (≥PR) on first-line treatment. Main exclusion criteria included prior therapy with bortezomib or lenalidomide, the presence of PN or neuropathic pain grade ≥2 as defined by NCI CTCAE version 3.0.
Bortezomib was administered intravenously at a starting dose of 1.3 mg/m2 on days 1, 8 and 15 of a 28-day cycle. During the phase I part, the bortezomib dose was escalated to 1.6 mg/m2, while lenalidomide was administered orally starting at 10 mg/day, days 1–21. The dose of lenalidomide was planned to increase by 5 mg starting at dose level 3 and 4. Dexamethasone fixed dose was 20 mg on days 1, 2, 8, 9, 15 and 16. During the phase I part, the MTD and the RDL for phase II of bortezomib and lenalidomide were determined using a slightly modified ′3+3′ dose escalation scheme. When 3 or 6 patients were entered, inclusion was discontinued until the dose-limiting toxicity (DLT) had been determined at day 22 of cycle I.
The primary objective of the phase I study was to determine the DLT, MTD and RDL. The secondary objective was to evaluate toxicity. For the phase II part the (s)CR+VGPR rate was considered as the primary endpoint. Secondary endpoints included ORR defined as sCR, CR, VGPR and PR, DoR, PFS, OS, TTNT, and toxicity. Furthermore, PFS and OS from the start of maintenance were also estimated.
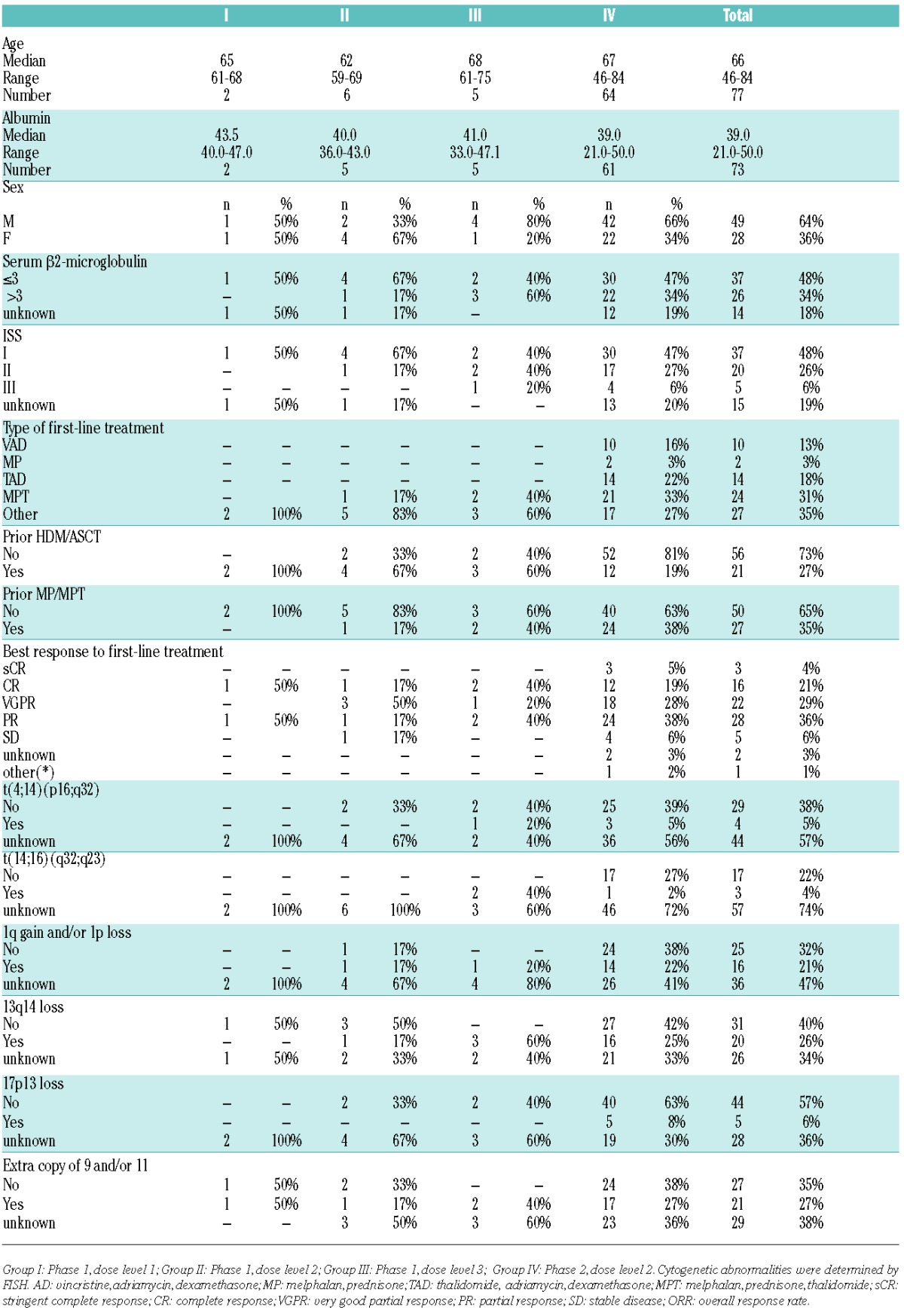
81 relapsed MM patients were enrolled from September 2008 to January 2012. Of these 81 patients, 4 were non-eligible based on not having measurable disease. Baseline characteristics at registration of the 77 eligible patients are documented (see Table 1).
Table 1.
Patients’ characteristics.
13 patients participated in the phase I part of the study. The DLT was reached at the third dose level with two grade 3 non-hematological adverse events; there were no DLTs in dose level 1 and 2. Therefore, the RDL for phase II was established at dose level 2: bortezomib 1.6 mg/m2, lenalidomide 10 mg, dexamethasone 20 mg.
In patients who had achieved at least VGPR after a maximum of 6 cycles, treatment was continued with 2 cycles as consolidation. All patients from phase I and phase II who had achieved at least a PR after induction, received maintenance with lenalidomide at a dose of 10 mg on days 1–21, which started at day 29 of the last cycle. Maintenance cycles were repeated at 28-day intervals until relapse, progression or the occurrence of a medical condition that required stopping the treatment.
44 out of 70 (63%) patients treated at the RDL in phase I and II completed 6 induction and 2 consolidation cycles and started with lenalidomide maintenance, as shown in Figure 1. The median number of induction treatment cycles is 3 (range 1–6) in phase I and 4 (range 1–8) in phase II. The median duration of maintenance is 12 months (range 11 to 45 months) in phase I and 19 months (range 1–37) in phase II.
Figure 1.
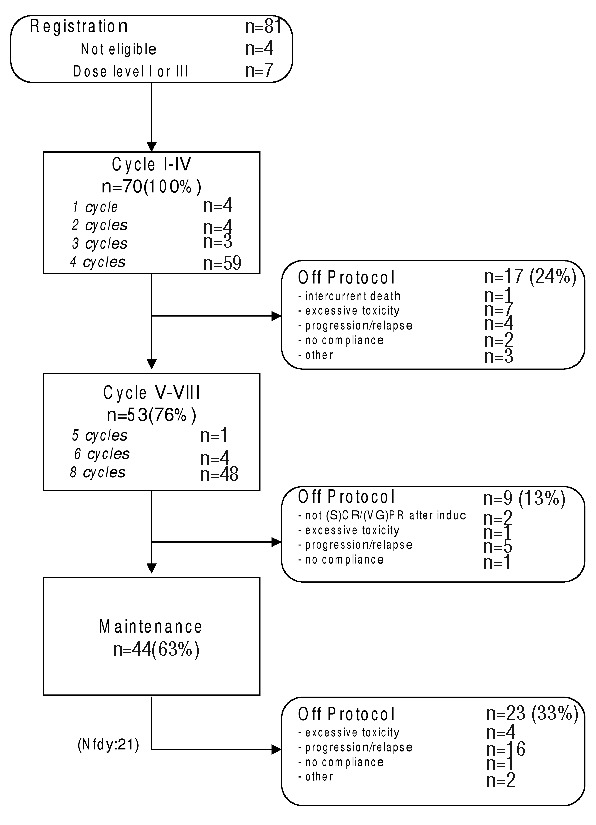
Flow diagram HOVON 86. The treatment data is summarized by the flow diagram. It shows the number and proportion of patients in a specific treatment cycle, and the number of off protocol patients along with the reason for going out of protocol treatment. The diagram is generated for all included patients in both phases together.
49 out of 70 patients (70%) went off protocol, while the remaining 21 (30%) patients were still receiving maintenance treatment at the time of data analysis. The main reasons for going off protocol were progression or relapse (n=25/70) (36%) and/or toxicity (n=12/70) (17%).
Of the 70 patients treated at the RDL in phase I (n=6) and in phase II (n=64), the (s)CR +VGPR rate after induction was 64% (95%CI: 53%–76%), including 20% CR (95%CI:10%–30%). The ORR was 89% (95%CI: 81%–96%). The median number of cycles to reach at least a PR was 1 (range 1–6). The median number of cycles to best response during induction was 2 (range 1–8).
The analysis of PFS and OS measured from study entry was available for all patients. At the time of final analysis 45 out of 70 patients were still alive with a median follow-up time of 25 months, range 14–49 months. There were 25 (36%) deaths of which 17 (24%) patients died from progression. The median PFS and OS were 19 months (range 1–49 months) and 42 months (range 1–49 months), respectively. The median DoR was 28 months (range 2–48 months). The median TTNT was 19 months (range 1–49 months), (Figure 2A–D).
Figure 2.
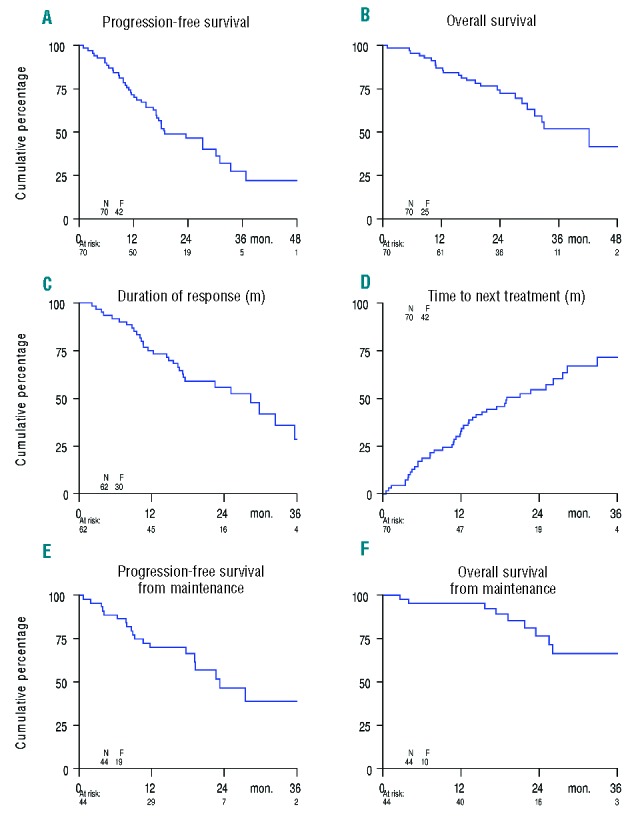
Kaplan-Meier survival curves among patients with multiple myeloma. (A) Progression-Free Survival; (B) Overall Survival; (C) Duration of response; (D) Time to next treatment; (E) Progression-Free Survival from start of maintenance; (F) Overall Survival from start of maintenance.
The median duration of maintenance was 19 months (range 1–45 months). Median PFS and OS from the start of maintenance were 23 months (range 1–45 months), and 36 months (range 3–45 months), respectively (Figure 2 E,F).
Prognostic markers considered in a logistic regression analysis of response and the Cox regression analyses of PFS and OS included sex, age (≥65 vs. <65 years), albumin, serum β2-microglobulin (>3 vs. ≤3mg/l), ISS (II+III vs. I), as well as FISH markers t(4;14), t(14;16), gain of 1q21 and/or loss of 1p, del(17p13), and trisomy 9 and/or 11. Other factors included: prior thalidomide use, prior HDM/ASCT, and response to first-line treatment. The analyses were based on all available data. FISH analysis was done at inclusion in HOVON 86.
It was observed that an extra copy of chromosome 9 and/or 11 was associated with reaching a CR (OR, 0.11; 95%CI, 0.01–0.98; P=0.02). Furthermore, prior thalidomide treatment had a significant negative impact on achievement of VGPR or CR (OR, 0.15; 95%CI, 0.04–0.54; P=0.003). No significant association of any other risk factor with PFS or OS was observed.
Hematologic and non-hematologic adverse events (AEs) NCI CTCAE grade 1–4 occurred in 69 out of 70 patients. 17% had to stop treatment early due to AEs.
Hematologic AEs of grade 1–4 occurred in 47% of patients, with 30% being grade 3–4 events. Dose reductions were performed in 11 out of 17 (65%) patients.
41 out of 70 (59%) patients developed PN grade ≥1. 41% patients had grade 1–2, and 17% patients had grade 3–4 PN. The median time to develop PN grade ≥1 was 123 days, and to develop a maximum grade PN was 168 days. In patients who experienced bortezomib related PN and neuropathic pain, dose interruptions/ adjustments were effectuated according to protocol based on the guidelines developed for bortezomib.2–4
There were 6 incidences of second primary malignancies (SPM) in 5 patients. SPMs included 3 invasive solid tumors, 1 MDS-RAEB-2 and 2 skin cancers, i.e. basal cell carcinoma and squamous cell carcinoma. The median time to first incidence of SPM was 17 months (range 8–35 months).
Previous trials that tested the efficacy of combinations of novel drugs have shown an ORR of 63–74%.5–9 The bortezomib, lenalidomide and dexamethasone regimen tested in the current trial compares favorably to these results. In the study done by Richardson et al., 64 patients with relapsed or relapsed and refractory MM received up to eight 21-day cycles of bortezomib 1.0 mg/m2, lenalidomide15 mg/day and dexamethasone followed by maintenance therapy. ORR was 64% including patients who had been exposed to either agent alone. The median PFS and OS at a median follow-up of 44 months were 9.5 and 30 months, respectively.10 The comparisons across studies should be interpreted with caution because of potential confounding factors such as differences in patient characteristics and prior therapies, as mentioned.
We observed that the higher dose bortezomib (1.6 mg/m2 weekly) was associated with 17% grade 3–4 PN, higher than that observed in the study by Richardson et al. who reported an incidence of 3% grade 3 PN using a bortezomib dose of 1.0 mg/m2.10,11 Of note, in our study bortezomib was administered intravenously, which is different from the current standard, i.e. 1.3 mg/m2 subcutaneously.
A weekly escalated dose of bortezomib 1.6 mg/m2 plus lenalidomide 10 mg/day, combined with low-dose dexamethasone is a feasible, effective treatment for relapsed, refractory MM patients. Future applications of this schedule may benefit from subcutaneous dosing in order to reduce the incidence of PN.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12(6):664–689. [DOI] [PubMed] [Google Scholar]
- 2.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–172. [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348(26):2609–2617. [DOI] [PubMed] [Google Scholar]
- 4.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. [DOI] [PubMed] [Google Scholar]
- 5.Ciolli S, Leoni F, Casini C, Breschi C, Santini V, Bosi A. The addition of liposomal doxorubicin to bortezomib, thalidomide and dexamethasone significantly improves clinical outcome of advanced multiple myeloma. Br J Haematol. 2008;141(6):814–819. [DOI] [PubMed] [Google Scholar]
- 6.Terpos E, Kastritis E, Roussou M, et al. The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia. 2008;22(12):2247–2256. [DOI] [PubMed] [Google Scholar]
- 7.Kim YK, Sohn SK, Lee JH, et al. Clinical efficacy of a bortezomib, cyclophosphamide, thalidomide, and dexamethasone (Vel-CTD) regimen in patients with relapsed or refractory multiple myeloma: a phase II study. Ann Hematol. 2010;89(5):475–482. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo A, Ambrosini MT, Benevolo G, et al. Bortezomib, melphalan, prednisone, and thalidomide for relapsed multiple myeloma. Blood. 2007;109(7):2767–2772. [DOI] [PubMed] [Google Scholar]
- 9.Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22(7):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson PG, Xie W, Jagannath S, et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123(10):1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–440. [DOI] [PubMed] [Google Scholar]


