Abstract
Mobilized peripheral blood is the most common graft source for allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning. In assessing the effect of donor cell dose and graft composition on major transplant outcomes in the reduced-intensity setting, prior studies focused primarily on CD34+ cell dose and reported conflicting results, especially in relation to survival end-points. While the impact of total nucleated cell dose has been less frequently evaluated, available studies suggest higher total nucleated cell dose is associated with improved survival outcomes in the reduced-intensity setting. In order to further explore the relationship between CD34+ cell dose and total nucleated cell dose on reduced-intensity transplant outcomes, we analyzed the effect of donor graft dose and composition on outcomes of 705 patients with hematologic malignancies who underwent reduced-intensity peripheral blood stem cell transplantation at the Dana Farber Cancer Institute from 2000 to 2010. By multivariable analysis we found that higher total nucleated cell dose (top quartile; ≥10.8 × 1010 cells) was associated with improved overall survival [HR 0.69 (0.54–0.88), P=0.0028] and progression-free survival [HR 0.68 (0.54–0.85), P=0.0006]. Higher total nucleated cell dose was independently associated with decreased relapse [HR 0.66 (0.51–0.85), P=0.0012] and increased incidence of chronic graft-versus-host disease [HR 1.4 (1.12–1.77), P=0.0032]. In contrast, higher doses of CD34+ cells (top quartile; ≥10.9 × 106/kg) had no significant effect on graft-versus-host disease or survival outcomes. These data suggest total nucleated cell dose is a more relevant prognostic variable for reduced-intensity transplant outcomes than the more commonly studied CD34+ cell dose.
Introduction
The impact of graft composition and donor cell dose on allogeneic hematopoietic stem cell transplantation outcomes has been extensively studied in myeloablative conditioning transplantation, and CD34+ cell dose is often utilized as the product-related variable of choice for outcomes analysis. Given that a cell-based graft-versus-tumor effect is the central therapeutic mechanism in reduced-intensity conditioning (RIC) transplantation, product-related variables may have an even more significant impact on transplant outcomes in the RIC setting. However, relatively few studies have reported on product-related outcome data in RIC transplants. These studies have primarily focused on CD34+ cell dose, most reporting that higher CD34+ cell doses are associated with more rapid engraftment with a variable effect on the incidence of graft-versus-host disease (GVHD) and survival outcomes.1–5 Gomez-Almaguer and colleagues reported on 138 recipients of RIC hematopoietic stem cell transplants, showing that higher CD34+ doses correlated with improved overall survival.6 A CIBMTR registry analysis of 1054 RIC hematopoietic stem cell transplant recipients found that lower CD34+ doses correlated with increased transplant-related mortality and decreased overall survival.7 In contrast, Remberger and colleagues reported that higher CD34+ doses were actually associated with higher relapse rates and lower overall survival.8 Relative to CD34+ cell dose, total nucleated cell (TNC) dose has been less frequently studied. However, in the limited number of studies that have examined TNC, a trend toward improved survival outcomes with higher TNC dose, independently of CD34+ dose, has been found.9,10 The current study examines the impact of both TNC and CD34+ cell dose on major transplant outcomes in a cohort of 705 consecutive patients undergoing RIC allogeneic peripheral blood hematopoietic stem cell transplantation.
Methods
We analyzed data from 705 consecutive patients with hematologic malignancies undergoing RIC utilizing granulocyte colony-stimulating factor-mobilized donor peripheral blood mononuclear cells as a graft source. Patients who underwent either in vivo T-cell depletion with antithymocyte globulin or ex-vivo T-cell depletion were excluded from the study. All patients had at least 3 years of follow-up after their transplant. All patients provided consent to the use of protected health data for research, as approved by a common institutional review board of the Dana-Farber/Harvard Cancer Center.
Definitions and end-points
Neutrophil engraftment was defined as the first day of an absolute neutrophil count >500/μL on three consecutive measurements. Platelet recovery was defined as the first day of two consecutive measurements of >20,000/μL (unsupported). Acute and chronic GVHD were graded by consensus grading criteria and their cumulative incidence was calculated through day +200 after hematopoietic stem cell transplantation. Chronic GVHD was defined clinically by treating physicians utilizing standard criteria ultimately supplanted by NIH Consensus Conference recommendations.11 Relapse was defined as disease recurrence documented by morphological, histological or radiographic means. Treatment-related mortality was defined as death while in continuous remission. Progression-free survival was defined as the time from transplantation to relapse or death from any cause, and overall survival was defined as the time from transplantation to death from any cause.
Statistical methods
For each cell type of interest, cell dose level was classified into groups using quartile cutoffs (Table 1). The Spearman coefficient for TNC versus CD34+ cells was 0.25 (0.18–0.32) (Figure 1). The effects of cell doses on categorical or continuous outcomes were tested using the Fisher exact test or Wilcoxon rank-sum test. Progression-free survival and overall survival were estimated using the Kaplan-Meier method. Patients alive without a relapse were censored at the date of last contact. Log-rank tests were used to compare progression-free survival and overall survival between groups and Cox proportional hazards regression models were used to study the effects of cell doses on the survival outcomes while adjusting for other risk factors. Time to GVHD onset was analyzed using early relapse and death without GVHD as competing risks. Time to relapse and time to non-relapse mortality were analyzed using each other as competing risks. In competing risk settings, Gray methods were used to compare between groups and competing risk regressions were used to test the significance of cell dose effects adjusting for other covariates. Other factors adjusted for in the regression setting included patients’ age, donors’ age, GVHD prophylaxis, patient-donor sex-match status, and the disease risk index.12 Analyses were conducted on total numbers of CD34+, nucleated (TNC), CD3+, CD4+, and CD8+ donor cells infused as well as cell number per kg of recipient body weight. Outcomes are reported with regard to CD34+ cell dose per kg of actual body weight or total TNC dose, as these have been routine reporting metrics in prior analyses for these graft-associated variables. An exploratory analysis was also performed for total CD34+ dose as well as TNC/kg.
Table 1.
Quartile summaries.
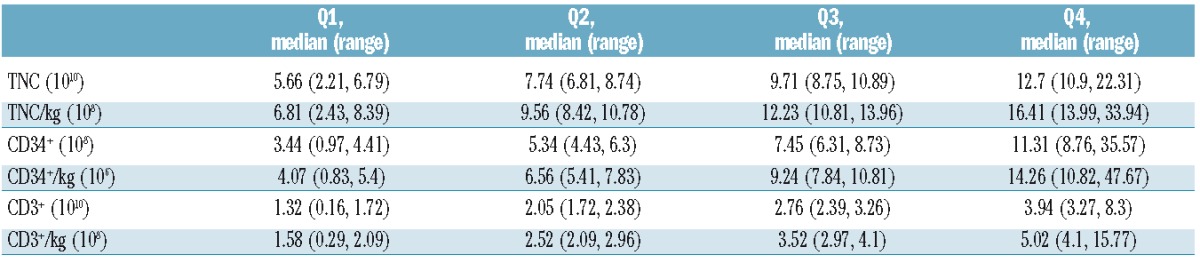
Figure 1.
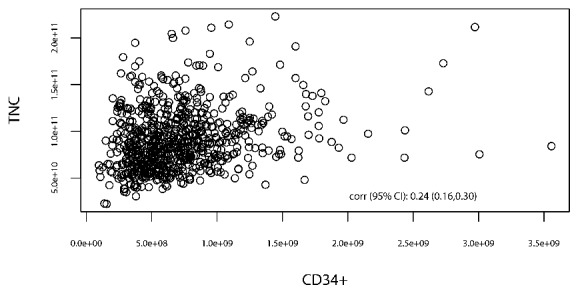
Scatter plot of TNC vs. CD34+ cell dose with Spearman coefficient of 0.25 (0.18–0.32).
Results
Patients
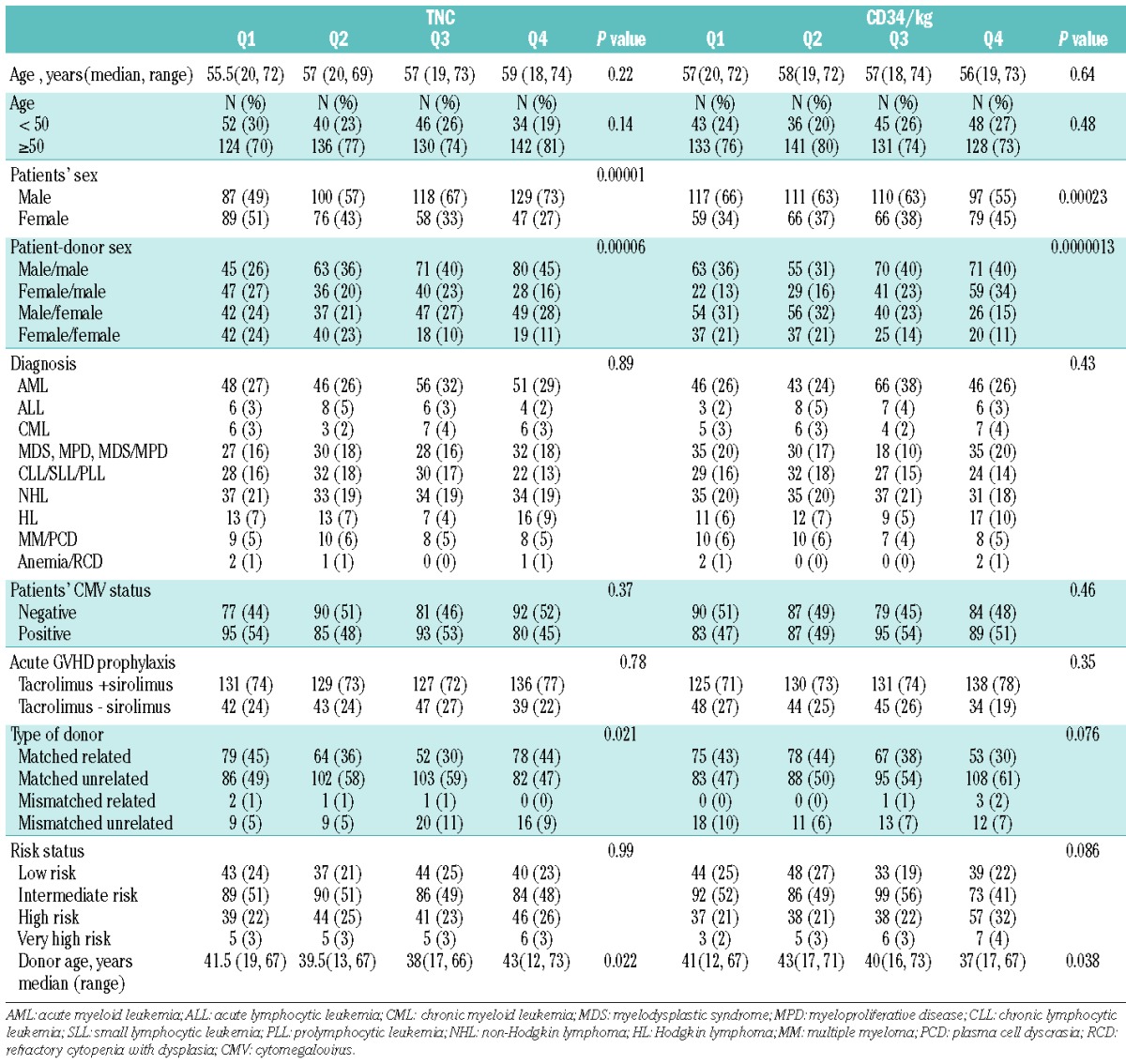
The patients’ characteristics are listed in Table 2. The vast majority of patients (99%) received RIC with busulfan and fludarabine (IV busulfan total dose of 3.2 or 6.4 mg/kg; IV fludarabine total dose of 120 mg/m2). GVHD prophylaxis was mostly tacrolimus based, with or without sirolimus (74% versus 24%, respectively). The median age of the patients was 57 years (range, 18–74 years). Sixty-two percent of the patients were male. Acute myeloid leukemia was the most common diagnosis (29%). The patients’ disease risk status was categorized as low (23%), intermediate (50%), high (24%) or very high (3%) based on the disease risk index described by Armand et al.12 Transplants were categorized as matched (8/8 high-resolution HLA-A, B, C and DRB1-matched) with their respective recipients; matched related donor (39%), matched unrelated donor (53%), or mismatched (7/8 high-resolution HLA-A, B, C and DRB1-matched); mismatched related donor (1%), mismatched unrelated donor (8%).
Table 2.
Patients’ characteristics by quartiles of TNC and CD34/kg.
During the first year, all patients received acyclovir as prophylaxis against herpes simplex virus/varicella zoster virus infections, and sulfamethoxazole/trimethoprim or atovaquone as prophylaxis against Pneumocystis jirovecii pneumonia. Patients were monitored for cytomegalovirus reactivation during the first 100 days after transplantation, and pre-emptive therapy with valganciclovir was given if viral reactivation occurred. Granulocyte colony-stimulating factor was routinely used in most patients to accelerate engraftment.
Engraftment
TNC dose did not influence the onset or durability of engraftment. In contrast, as expected, time to neutrophil engraftment was significantly shorter for the highest quartile of CD34+ cells infused/kg compared with the lower three quartiles combined in a competing risk regression (P=0.0004). This analysis censored subjects who did not have a nadir absolute neutrophil count below 500/μL.
Graft-versus-host disease
Neither TNC nor CD34+ cell dose affected grades II–IV acute GVHD. However, there was a significant association between TNC dose and the development of chronic GVHD. The 1-year cumulative incidence of chronic GVHD across the four TNC dose quartiles (Q1-Q4) were 37%, 44%, 47%, and 52%, respectively (P=0.017). In a regression model evaluating the quartiles, Q1 was associated with decreased risk [hazard ratio (HR)=0.80, P=0.0004] versus Q4. Dichotomized regression analysis was performed by collapsing the top three quartiles and comparing them to Q1. In this dichotomized analysis, Q1 was associated with a decreased risk of chronic GVHD versus Q2+Q3+Q4 (HR=0.71, P=0.0063) (Figure 2). There was no association between CD34+ cell dose and chronic GVHD.
Figure 2.
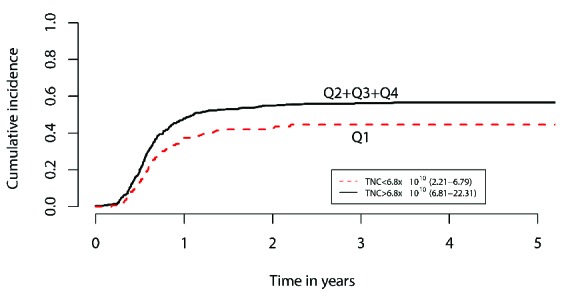
Regression analyses of TNC dose. Dichotomized regression analysis of the top three quartiles (collapsed) vs. quartile one, showing increased cumulative risk of chronic GVHD at 1 year with higher TNC dose (HR=0.71, P=0.0063).
Relapse
The 3-year cumulative incidence of relapse across the four TNC dose groups (Q1-Q4) was 54%, 60%, 54%, and 42%, respectively (P=0.017). In a regression model evaluating the quartiles, each of the first three quartiles was associated with increased risk of relapse compared to Q4 (HR=1.41, 1.63, and 1.53; P=0.029, 0.0013, and 0.0068, respectively). In a dichotomized regression analysis performed by collapsing Q1, Q2 and Q3, Q4 was associated with decreased risk of relapse when compared with Q1+Q2+Q3 (HR=0.66, P=0.0012) (Figure 3A). After adjusting for chronic GVHD as a time-dependent covariate in a Cox model, high TNC dose (Q4 versus Q1+Q2+Q3) was still significantly associated with better progression-free survival (P<0.0001) and lower relapse rate (P<0.0001).
Figure 3.
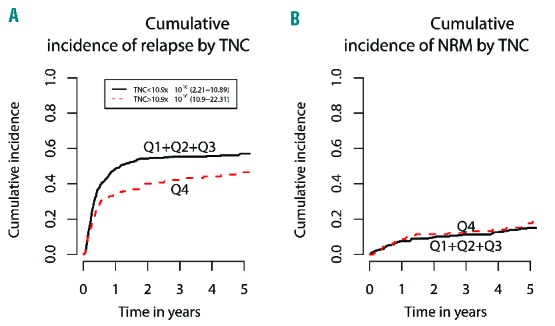
Regression analyses of TNC dose. (A) Dichotomized regression analysis of the first three quartiles (collapsed) vs. quartile four, showing decreased risk of 3-year cumulative relapse with higher TNC dose (HR=0.66, P=0.0012). (B) Dichotomized regression analysis of the first three quartiles (collapsed) vs. quartile four, showing no significant effect of TNC dose on 3-year cumulative incidence of non-relapse mortality (NRM) (P=0.43).
CD34+ cell dose did not affect relapse rates. The 3-year cumulative incidence of relapse across the four CD34+/kg dose groups (Q1-Q4) was 48%, 51%, 51%, and 59%, respectively (P=0.20). In dichotomized regression analysis of CD34+ cell dose, no significant effect was found by collapsing Q1, Q2 and Q3 and comparing with Q4 (P=0.15), or by collapsing Q2+Q3+Q4 and comparing with Q1 (P=0.13).
Non-relapse mortality
There was no association between TNC dose (Figure 3B) or CD34+ cell infusion numbers and the 3-year cumulative incidence of non-relapse mortality.
Progression-free and overall survival
The 3-year progression-free survival across the four TNC dose groups (Q1-Q4) was 37%, 32%, 32%, and 46%, respectively (P=0.029), and the 3-year overall survival was 48%, 49%, 40%, and 58% (P=0.050). In a regression model evaluating the quartiles, each of the first three quartiles was associated with worse progression-free survival compared with Q4 (HR=1.32, 1.48, and 1.64; P=0.046, 0.0034, and 0.0002, respectively), as well as worse overall survival (HR=1.42, 1.31, and 1.62; P=0.020, 0.069, and 0.0009, respectively). In a dichotomized analysis by collapsing Q1, Q2 and Q3, Q4 was associated with better overall survival and progression-free survival (HR=0.69 and 0.68; P=0.0028 and 0.00067, respectively) (Figure 4A,B). CD34+ cell counts bore no association with 3-year progression-free or overall survival.
Figure 4.
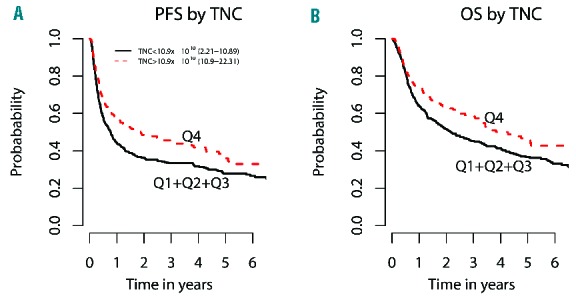
Dichotomized regression analyses of TNC dose. (A) Dichotomized regression analysis of first three quartiles (collapsed) vs. quartile four, showing improved 3-year progression-free survival (PFS) with higher TNC dose (HR=0.68, P=0.00067). (B) Dichotomized regression analysis of first three quartiles (collapsed) vs. quartile four, showing improved 3-year overall survival (OS) with higher TNC dose (HR=0.69, P=0.0028).
Graft T-lymphocyte composition
In an effort to elucidate which components of the graft could account for the significant impact of TNC on chronic GVHD, relapse, progression-free survival, and overall survival, we performed an exploratory analysis on a subset of patients in whom the CD3+ (n=445), CD4+ (n=443) and CD8+ (n=444) fractions of the graft were reliably enumerated. No association between CD3+, CD4+ or CD8+ cell count and these outcomes was identified, either because the analysis was underpowered to demonstrate a significant difference or because other specific cellular components rather than total CD3+, CD4+ or CD8+ cell count influence these outcomes.
Total CD34+ cell dose and total nucleated cells per kilogram
Exploratory analysis was performed for total CD34+ dose as well as TNC/kg, demonstrating no significant association between either total CD34+ dose or TNC/kg and the aforementioned transplant outcomes.
Discussion
The majority of studies analyzing the impact of graft composition on major transplant outcomes in RIC HSCT have focused on the influence of CD34+ cell dose, reporting mixed results with regard to survival outcomes, engraftment and incidence of GVHD. In their study of 86 patients who underwent RIC allogeneic HSCT, Perez-Simon and colleagues reported an increased incidence of chronic GVHD and decreased relapse rates with increased CD34+/kg (depending on disease state at transplant), but no significant effect on overall survival or event-free survival.1 Pulsipher and colleagues reported on 160 patients undergoing RIC allogeneic HSCT, describing faster engraftment, reduced transplant-related mortality and better 3-year overall survival in the cohort that received a high CD34+ cell dose (>4.5×106 cells/kg) without there being any associated increase in the incidence of acute or chronic GVHD.4 In an analysis of 1054 RIC transplant recipients reported to the CIBMTR from 2002 to 2011, Torlen and colleagues determined that low CD34+ cell dose (<4×106 CD34+ cells/kg) was associated with increased non-relapse mortality and inferior overall survival.7 In a recent analysis, Remberger and colleagues reported inferior outcomes for patients receiving fewer than 2.5×106 CD34+ cells/kg, but also poorer survival due to increased relapse rates in patients receiving over 11×106 CD34+ cells/kg, albeit in pooled analyses of both myeloablative conditioning and RIC transplant patients.8 These examples typify the myriad of published analyses that have, thus far, failed to demonstrate reproducible, consensus data regarding the effect of CD34+ cell dose on common RIC transplant outcomes.1–5,13–18
In contrast to CD34+ cell dose, there have been only a limited number of studies that report on the effect of TNC dose on RIC transplant outcomes. Baron and colleagues analyzed graft composition data from 125 RIC patients, showing a non-significant, but appreciable trend (HR=0.08, P=0.06) toward improved overall survival, as well as progression-free survival, with higher TNC dose.9 In their study of 253 patients with acute myeloid leukemia undergoing RIC transplantation, Gorin and colleagues found that CD34+/kg cell dose had no effect on either GVHD or overall survival, but that increased TNC dose was independently associated with more chronic GVHD as well as improved overall survival.10 In the aforementioned study by Remberger and colleagues, TNC dose showed no correlation with survival outcomes, relapse rates, engraftment or GVHD. As discussed for the CD34+ cell dose, albeit with the caveat of far fewer published data, the impact of TNC dose on RIC transplant outcomes remains undefined.
To our knowledge, our current single institution study of 705 RIC patients is the largest to date to anlayze the effects product-related variables on transplant outcomes in the RIC setting. We found improved survival data (overall survival and progression-free survival) with higher TNC dose, whereas CD34+ cell dose (total and per kg) did not have a significant effect on these critical outcomes. Based on recent studies demonstrating an association between RIC transplant survival outcomes and absolute lymphocyte count recovery19 as well as donor chimerism,20 we analyzed the effect of TNC dose on these additional prognostic indicators, but failed to demonstrate any significant association. Nevertheless, our findings support prior reports9,10 indicating that TNC dose is potentially a more relevant product-related variable than CD34+ cell dose with regard to RIC transplant outcomes. It is unlikely that the top quartile of TNC enumerated in our cohort (>10.9×1010 cells) represents the ideal cell target across transplant center collection facilities despite its association with lower relapses rates and improved survival. Rather, our experience may suggest that “more is better” when it comes to TNC. An improved survival benefit with higher TNC doses should influence those current RIC transplant protocols that cap the TNC infusion dose. Importantly, our analysis showed no significant association between TNC and CD34+ cell dose (Spearman correlation coefficient of 0.24), further demonstrating the mutual exclusivity of these product-related parameters.
It remains unclear whether there is an identifiable cell subset within the TNC population that drives this observed survival benefit (e.g. dendritic cells, antigen-presenting cells, natural killer cells, T-regulatory cells, T-effector cells), or if such benefit is engendered by a pleiotropic effect of the immunomodulatory milieu that accompanies larger TNC doses. Various studies analyzing graft T-cell composition have not convincingly demonstrated any particular cellular component as reproducibly having either deleterious or beneficial effects on transplant outcomes.21–24 In their study of 63 RIC transplant patients, Cao and colleagues reported improved freedom from progression, overall survival and attainment of full donor T-cell chimerism with higher CD8+ cell doses, with no correlation between CD8+ dose and GVHD and no significant effect of CD3+ or CD4+ subsets on any of these outcomes.21 In contrast, Mohty and colleagues22 reported increased acute GVHD with higher CD8+ doses in their analysis of 57 RIC transplant patients and Collins and colleagues23 reported no significant effect of CD3+, CD4+ or CD8+ lymphocyte subsets on overall survival in their review including 70 RIC transplant patients. Reshef and colleagues reported on T-cell subset data in 200 RIC transplant patients, describing improved survival and reduced relapse rates with increased CD8+ cells, with no significant effect of CD3+, CD4+ or CD34+ subsets on these outcomes (of note, TNC dose was not reported in this study).24 While our subset analysis of CD3+, CD4+ and CD8+ cell doses in approximately 445 RIC transplant patients failed to demonstrate any significant association between these T-lymphocyte subsets and our observed transplant outcomes, these studies both suggest that the CD34-negative fraction of the graft is most critical to transplant outcomes after RIC. These findings support more comprehensive analysis of graft subset populations as a compelling direction for future research. Such an analysis could potentially elucidate a cell subset (or synergistic groups of cell subsets) within the TNC population that most directly confers a survival benefit, allowing for the future possibility of improving RIC transplant outcomes through directed graft manipulation.
Acknowledgments
The authors would like to thank the Ted and Eileen Pasquarello Research Fund at DFCI and the National Institutes of Health (National Cancer Institute grant 5R01-CA183559) for their support of this work.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/4/499
References
- 1.Perez-Simon JA, Diez-Campelo M, Martino R, et al. Impact of CD34+ cell dose on the outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102(3):1108–1113. [DOI] [PubMed] [Google Scholar]
- 2.Panse JP, Heimfeld S, Guthrie KA, et al. Allogeneic peripheral blood stem cell graft composition affects early T-cell chimaerism and later clinical outcomes after non-myeloablative conditioning. Br J Haematol. 2005;128(5):659–667. [DOI] [PubMed] [Google Scholar]
- 3.Mehta J, Frankfurt O, Altman J, et al. Optimizing the CD34+ cell dose for reduced-intensity allogeneic hematopoietic stem cell transplantation. Leuk Lymphoma. 2009;50(9):1434–1441. [DOI] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;14(13):2606–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsirigotis P, Shapira MY, Or R, et al. The number of infused CD34+ cells does not influence the incidence of GVHD or the outcome of allogeneic PBSC transplantation, using reduced-intensity conditioning and antithymocyte globulin. Bone Marrow Transplant. 2010;45(7):1189–1196. [DOI] [PubMed] [Google Scholar]
- 6.Gómez-Almaguer D, Gómez-Peña Á, Jaime-Pérez JC, et al. Higher doses of CD34+ progenitors are associated with improved overall survival without increasing GVHD in reduced-intensity conditioning allogeneic transplant recipients with clinically advanced disease. J Clin Apher. 2013;28(5): 349–355. [DOI] [PubMed] [Google Scholar]
- 7.Törlén J, Ringdén O, Le Rademacher J, et al. Low CD34 dose is associated with poor survival after reduced-intensity conditioning allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2014;20(9): 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Remberger M, Törlén J, Ringdén O, et al. The effect of total nucleated and CD34+ cell dose on outcome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(5): 889–893. [DOI] [PubMed] [Google Scholar]
- 9.Baron F, Maris MB, Storer BE, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19(5):822–828. [DOI] [PubMed] [Google Scholar]
- 10.Gorin NC, Labopin M, Boiron JM, et al. Results of genoidentical hemopoietic stem cell transplantation with reduced-intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond – the Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24(24): 3959–3966. [DOI] [PubMed] [Google Scholar]
- 11.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12): 945–956. [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaucha JM, Gooley T, Bensinger WI, et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98(12):3221–3227. [DOI] [PubMed] [Google Scholar]
- 14.Mohty M, Bilger K, Jourdan E, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17(5):869–875. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O, Barrett AJ, Zhang MJ, et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell doses. Br J Haematol. 2003;121(6):874–885. [DOI] [PubMed] [Google Scholar]
- 16.Bittencourt H, Rocha V, Chevret S, et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;99(8):2726–2733. [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Savani BN, Albert PS, Barrett AJ. Efficacy of CD34+ stem cell dose in patients undergoing allogeneic peripheral blood stem cell transplantation after total body irradiation. Biol Blood Marrow Transplant. 2007;13(3):339–344. [DOI] [PubMed] [Google Scholar]
- 18.Gorin NC, Labopin M, Reiffers J, et al. Higher incidence of relapse in patients with acute myelocytic leukemia infused with higher doses of CD34+ cells from leukapheresis products autografted during the first remission. Blood. 2010;116(17):3157–3162. [DOI] [PubMed] [Google Scholar]
- 19.Kim HT, Armand P, Frederick D, et al. Absolute lymphocyte count recovery after allogeneic hematopoietic stem cell transplantation predicts clinical outcome. Biol Blood Marrow Transplant. 2015;21(5):873–880. [DOI] [PubMed] [Google Scholar]
- 20.Koreth J, Kim HT, Nikiforow S, et al. Donor chimerism early after reduced-intensity conditioning hematopoietic stem cell transplantation predicts relapse and survival. Biol Blood Marrow Transplant. 2014;20(10): 1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao TM, Shizuru JA, Wong RM, et al. Engraftment and survival following reduced-intensity allogeneic peripheral blood hematopoietic cell transplantation is affected by CD8+ T-cell dose. Blood. 2005;105(6):2300–2306. [DOI] [PubMed] [Google Scholar]
- 22.Mohty M, Bagattini S, Chabannon C, et al. CD8+ T cell dose affects development of acute graft-vs-host disease following reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Exp Hematol. 2004;32(11):1097–1102. [DOI] [PubMed] [Google Scholar]
- 23.Collins NH, Gee AP, Durett AG, et al. The effect of the composition of unrelated donor bone marrow and peripheral blood progenitor cell grafts on transplantation outcomes. Biol Blood Marrow Transplant. 2010;16(2): 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reshef R, Huffman AP, Gao A, et al. High graft CD8 cell dose predicts improved survival and enables better donor selection in allogeneic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2015;33(21):2392–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]


