Abstract
We assessed the long-term results of autologous stem-cell transplantation for patients with first-relapsed or refractory Hodgkin lymphoma included in the prospective Lymphoma Study Association/Société Française de Greffe de Moelle H96 trial. This large multicenter phase II trial evaluated a risk-adapted strategy with single or tandem autologous stem-cell transplantation for 245 Hodgkin lymphoma patients. Poor-risk patients (n=150) had primary refractory Hodgkin lymphoma (n=77) or ≥2 risk factors at first relapse (n=73) and were eligible for tandem autologous stem-cell transplantation. Intermediate-risk patients (n=95) had one risk factor at first relapse and were eligible for single autologous stem-cell transplantation. With a median follow-up of 10.3 years, 10-year freedom from second failure and overall survival rates were, respectively: 64% (95% CI, 54% to 74%) and 70% (95% CI, 61% to 80%) for the intermediate-risk group, and 41% (95% CI, 33% to 49%) and 47% (95% CI, 39% to 55%) for the poor-risk group. Considering only patients who did not relapse after completing autologous stem-cell transplantation, the 15-year cumulative incidences of second primary malignancies were 24% for the 70 intermediate-risk patients and 2% for the 75 poor-risk ones. With long-term follow-up, the risk-adapted strategy remains appropriate. Tandem autologous stem-cell transplantation can still be considered an option for poor-risk patients, but integration of positron-emission tomography findings and new drugs may help to refine the need for a second autologous stem-cell transplant and possibly improve outcomes of patients with first-relapsed or refractory Hodgkin lymphoma.
Introduction
The true efficacy of a given treatment is only evident after prolonged follow-up. To determine it, analyses of long-term prospective rather than retrospective data are needed.
Two randomized studies established the advantage of autologous stem-cell transplantation (ASCT) over standard-dose salvage treatment for patients with relapsed Hodgkin lymphoma (HL) sensitive to chemotherapy.1,2 However, long-term prospective data on the efficacy and late effects of ASCT are lacking. Moreover, the long-term benefit of ASCT for patients with primary refractory HL has not been studied prospectively.
In 2008, our group published a prospective analysis, the H96 trial, whose primary end-point was to evaluate freedom from second failure (FF2F) for poor- and intermediate-risk HL groups.3 The results of this trial showed the interest of a risk-adapted strategy with single or tandem ASCT. The aim of the present study was to assess prospectively the long-term results and late effects of ASCT for first-relapsed or refractory HL in H96 trial patients.
Methods
Information on the methods has already been published in detail3 and is briefly summarized below. The study protocol was approved by the Ethics Committee of Saint-Louis Hospital (Paris, France).
Patients
Eligibility criteria were as follows: biopsy-proven HL (World Health Organization, classic type); either primary refractory or first-relapsed HL; age less than 60 years (age ≤50 years for patients scheduled to receive tandem ASCT). Written informed consent was required before enrollment.
Stratification and treatment
In the H96 trial, the intensity of high-dose therapy (single or tandem ASCT) was adapted to risk assessed at the onset of salvage treatment, based on the primary refractory status or the number of risk factors at first relapse, which included relapse less than 12 months after the end of first-line treatment, stage III or IV at relapse, and/or relapse in a previously irradiated site (>30 Gy) after combined-modality therapy. Patients were stratified as follows: the poor-risk group included patients with primary refractory HL or two or more risk factors at relapse; and the intermediate-risk group comprised patients with only one risk factor at relapse.
In the poor-risk group, salvage treatment was followed by tandem ASCT. Salvage treatment consisted of two cycles of ifosfamide, etoposide, and doxorubicin (IVA75) or mitoguazone, ifosfamide, vinorelbine, and etoposide (MINE). The first conditioning regimen consisted of cyclophosphamide, carmustine, etoposide, and mitoxantrone (CBVM) or carmustine, etoposide, cytarabine, and melphalan (BEAM). A second conditioning regimen was reserved for patients with no evidence of disease progression at that time. For previously unirradiated patients, it consisted of total-body irradiation (12 Gy in 6 × 2 Gy twice-daily fractions), cytarabine, and melphalan (TAM). For patients who had received prior dose-limiting radiation, the second conditioning regimen was BAM (the same as TAM except that busulfan replaced total body irradiation). After the second ASCT, radiotherapy was optional.
In the intermediate-risk group, salvage treatment was followed by single ASCT. Salvage treatment consisted of three IVA50 or MINE cycles and the conditioning regimen was BEAM. After ASCT, radiotherapy was optional.
Follow-up
Computed-tomography scans were performed 3 months after the last ASCT, every 6 months until 3 years after the ASCT and yearly thereafter. Response to treatment – complete response (CR), unconfirmed CR (CRu), partial response (PR), stable disease (SD) or progressive disease (PD) – was defined according to the 1999 international response criteria.4 Left ventricular ejection fraction was assessed at baseline, after salvage treatment and was recommended yearly thereafter.
Statistical analysis
Analyses were performed on an intent-to-treat basis (inclusion of every patient who received at least one cycle of salvage treatment) and per-protocol (restricted to the patients who completed ASCT). The primary end-point was FF2F, measured from the date of inclusion until progression, relapse or death from any cause, or the date of last contact for those who were failure-free. Overall survival (OS) was measured from the date of inclusion until death from any cause, or the date of last contact for those who were alive. FF2F and OS were estimated with 95% confidence intervals (95% CI). Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test, with P≤0.05 defining statistical significance. Cumulative incidences were compared using the Gray test.
Results
Between 1995 and 2002, 245 patients were enrolled. Their characteristics at diagnosis and at the time of treatment failure/relapse were reported previously.3 Briefly, the median age at inclusion was 32 (range, 11–60) years. The poor-risk group included 150 patients (77 with primary refractory HL and 73 with unfavorable relapse) and the intermediate-risk group comprised 95 patients. The median follow-up is now 10.3 years (10.4 years for the poor-risk group and 10.1 years for the intermediate-risk group).
Outcomes according to risk group
Outcomes based on intent-to-treat analysis
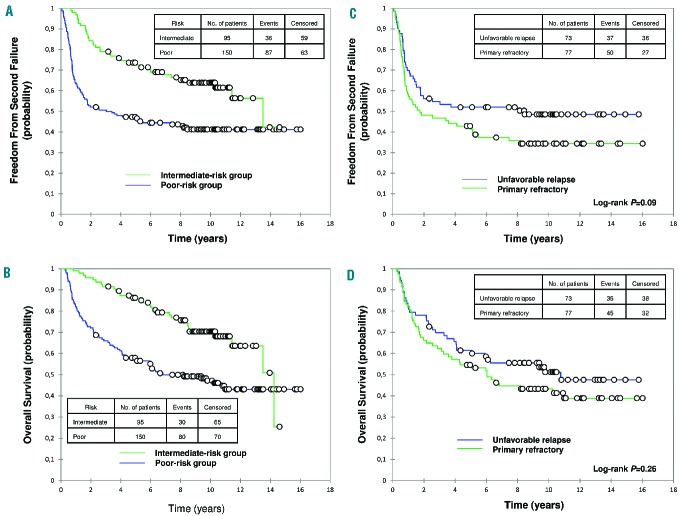
For the poor-risk group, the 10-year FF2F and OS were 41% (95% CI, 33% to 49%) and 47% (95% CI, 39% to 55%), respectively (Figure 1). Within this group, no significant difference was observed, respectively, between primary refractory HL and unfavorable relapse for the 10-year FF2F (34% and 49%; P=0.09) and OS (43% and 51%; P=0.26) (Figure 1). For the intermediate-risk group, the 10-year FF2F and OS were 64% (95% CI, 54% to 74%) and 70% (95% CI, 61% to 80%), respectively (Figure 1).
Figure 1.
Outcomes based on intent-to-treat analysis. (A) Freedom from second failure (FF2F) and (B) overall survival (OS) of patients with first-relapsed or primary refractory Hodgkin lymphoma according to risk group. (C) FF2F and (D) OS of patients with primary refractory or unfavorable relapse (≥2 of the following risk factors at first relapse: relapse <12 months, stage III or IV at relapse, and/or relapse within previously irradiated sites). Median follow-up: 10.3 years.
Outcomes of patients completing ASCT
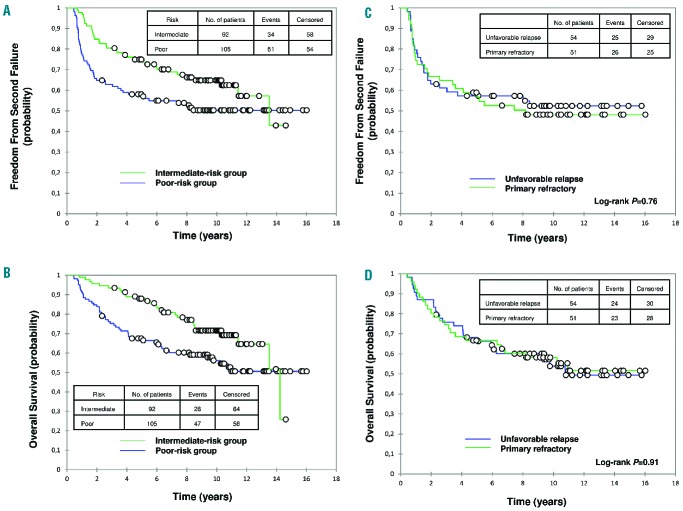
In the poor-risk group, for patients completing tandem ASCT (n=105), the 10-year FF2F and OS were 50% (95% CI, 41% to 60%) and 56% (95% CI, 46% to 66%), respectively (Figure 2). Within this group, no significant difference was observed, respectively, between primary refractory HL and unfavorable relapse for the 10-year FF2F (48% and 52%; P=0.76) and OS (58% and 54%; P=0.91) (Figure 2). In the intermediate-risk group, for patients completing ASCT (n=92), the 10-year FF2F and OS were 65% (95% CI, 55% to 75%) and 72% (95% CI, 62% to 81%), respectively (Figure 2).
Figure 2.
Outcomes of patients completing autologous stem-cell transplantation. (A) Freedom from second failure (FF2F) and (B) overall survival (OS) of patients with first-relapsed or primary refractory Hodgkin lymphoma according to risk group. (C) FF2F and (D) OS of patients with primary refractory or unfavorable relapse.
Outcomes according to the response to salvage treatment
The response to salvage treatment was assessable for 243 patients (76 with primary refractory HL, 73 with unfavorable relapse and 94 with intermediate risk).
For the poor-risk group, the 10-year FF2F according to each response category was 65% (95% CI, 49% to 80%) for CR/CRu (n=39), 47% (95% CI, 34% to 60%) for PR (n=55), unassessable (follow-up <10 years) for SD (n=24), and 23% (95% CI, 8% to 37%) for PD (n=31) (Online Supplementary Figure S1). Significant differences in FF2F were found between the CR/CRu and PR groups (P=0.03), PR and SD groups (P=0.002), and PR and PD groups (P=0.001). The 10-year OS was 68% (95% CI, 51% to 84%) for patients with CR/CRu, 58% (95% CI, 45% to 71%) for those with PR, 16% (95% CI, 0 to 31%) for patients with SD, and 26% (95% CI, 10% to 41%) for those with PD (Online Supplementary Figure S1). Concerning OS, no significant differences were observed between the CR/CRu and PR groups (P=0.1), whereas there were statistically significant differences between the CR/CRu and SD groups (P<0.001); the CR/CRu and PD groups (P<0.001); the PR and SD groups (P<0.001); and the PR and PD groups (P<0.001).
For the intermediate-risk group, the 10-year FF2F was 67% (95% CI, 55% to 79%) for patients with CR/CRu (n=65) and 64% (95% CI, 44% to 83%) for those with PR (n=26), with no significant difference between the two (P=0.95) (Figure 3). No patients had SD and three had PD. The 10-year OS was 72% (95% CI, 61% to 84%) for patients with CR/CRu and 66% (95% CI, 47% to 85%) for those with PR (P=0.97) (Online Supplementary Figure S1).
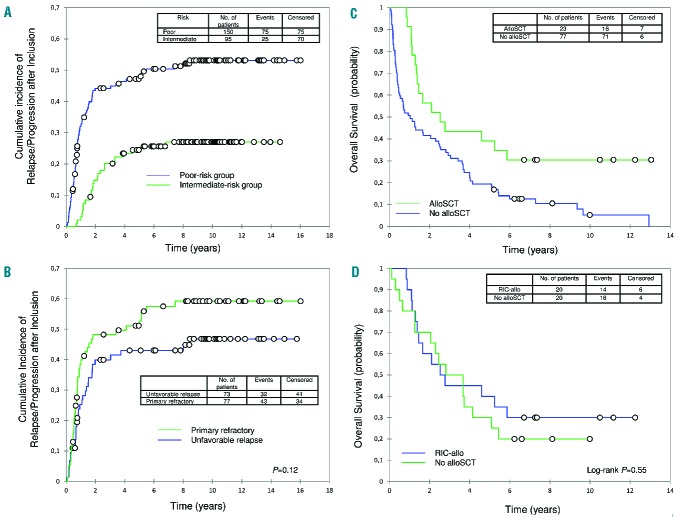
Figure 3.
Cumulative incidences of relapse/progression and overall survival probabilities. Cumulative incidence of relapse/progression after study inclusion (A) according to risk group and (B) for patients with primary refractory or unfavorable relapse. (C) From post-inclusion relapse/progression, overall survival (OS) of patients who underwent allogeneic stem-cell transplantation (alloSCT) or not. (D). From post-autologous stem-cell transplantation relapse, OS after matched analysis between patients who underwent alloSCT using a reduced-intensity conditioning regimen (RIC-allo) and patients who did not undergo alloSCT.
Relapse/progression after study inclusion
One-hundred patients relapsed/progressed after study inclusion (75 poor-risk and 25 intermediate-risk patients). For the poor-risk group, the 5- and 10-year cumulative incidences of relapse/progression were 48% and 53%, respectively (Figure 3). Within this group, the 5- and 10-year cumulative incidences of relapse/progression were 53% and 59% for the 43 primary refractory patients, and 43% and 47% for the 32 patients with unfavorable relapse, respectively (P=0.12) (Figure 3). Among the 43 poor-risk patients who relapsed after completing tandem ASCT, the median interval from second ASCT to relapse was 0.7 (0.2–8) years.
For the intermediate-risk group, the 5- and 10-year cumulative incidences of relapse were 25% and 27%, respectively (Figure 3). Among the 23 patients who relapsed after ASCT, the median ASCT-to-relapse interval was 1.5 (0.4–6.5) years.
After post-inclusion relapse/progression, 23 patients underwent allogeneic stem cell transplantation (alloSCT), 20 of them after a reduced-intensity conditioning (RIC-allo) regimen. Overall, from that relapse, the 10-year OS was 30% for the 23 patients or considering only the 20 RIC-allo patients (Figure 3). For the 77 relapsing/progressing patients who did not undergo alloSCT, the 10-year OS was only 5% (19% for the 20 intermediate-risk and 2% for the 57 poor-risk patients (Figure 3).
To assess the effect of RIC-allo in this setting better, we conducted a matched analysis using three relevant risk factors: age at relapse after ASCT (≥50 years),5 early relapse after ASCT (<6 months),5 and treatment arm (poor versus intermediate).
The 20 RIC-allo patients had undergone at least one previous ASCT, they were all younger than 50 years at relapse and four (all in the poor-risk group) had early relapse after ASCT. They were matched to 20 control patients who relapsed after ASCT, but did not undergo alloSCT. From the post-ASCT relapse, the median OS was 31.7 months for RIC-allo patients versus 38.9 months for matched controls, and the 10-year OS was 30% for RIC-allo patients versus 20% for matched controls; the survival curves did not differ significantly (P=0.55) (Figure 3).
Death
In all, 110 patients died [30 in the intermediate-risk group and 80 in the poor-risk group (35 unfavorable relapses and 45 primary refractory HL)]. Overall, HL was the main cause of death (n=83; 75% of causes of death). However, HL was the cause of 50% (n=15) of deaths in the intermediate-risk group whereas it represented 85% (n=68) of deaths in the poor-risk group. Other deaths resulted from infections (n=5), acute leukemia (n=5), post-alloSCT complications (n=5), solid tumors (n=3), non-Hodgkin lymphomas (n=2), cardiac toxicity (n=2), or other (n=5). The distribution of causes of death according to risk group is shown in Online Supplementary Figure S2.
Late effects
Second primary malignancies (SPM)
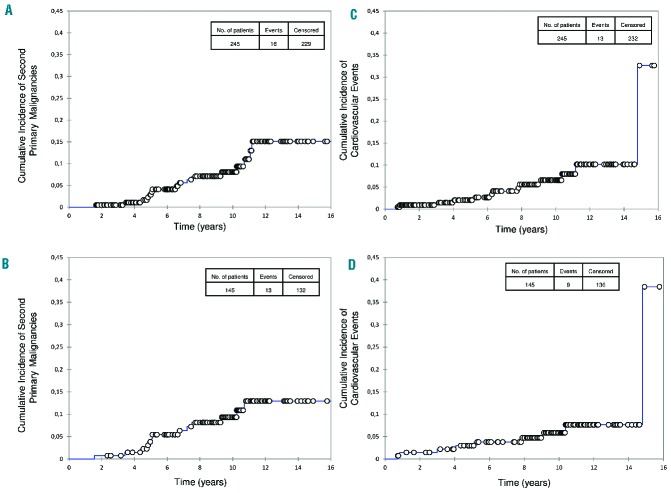
Sixteen SPM occurred (Table 1). Overall, from inclusion, the 10- and 15-year cumulative incidences of SPM were 8% and 15%, respectively (Figure 4). According to whether the patients were intermediate- or poor-risk, the 10-year cumulative incidences of SPM were 15% and 1.5%, respectively (Online Supplementary Figure S3). Considering only patients who did not relapse after completing ASCT, the 10- and 15- year cumulative incidences of SPM were 9% and 13%, respectively (Figure 4), with 10- and 15-year cumulative incidences of 16% and 24%, respectively, for the 70 intermediate-risk patients and 2% and 2% for the 75 poor-risk patients (Online Supplementary Figure S3). All acute leukemias were fatal, while some patients with non-Hodgkin lymphomas or solid tumors achieved long-term survival.
Table 1.
Characteristics of the 16 second primary malignancies.
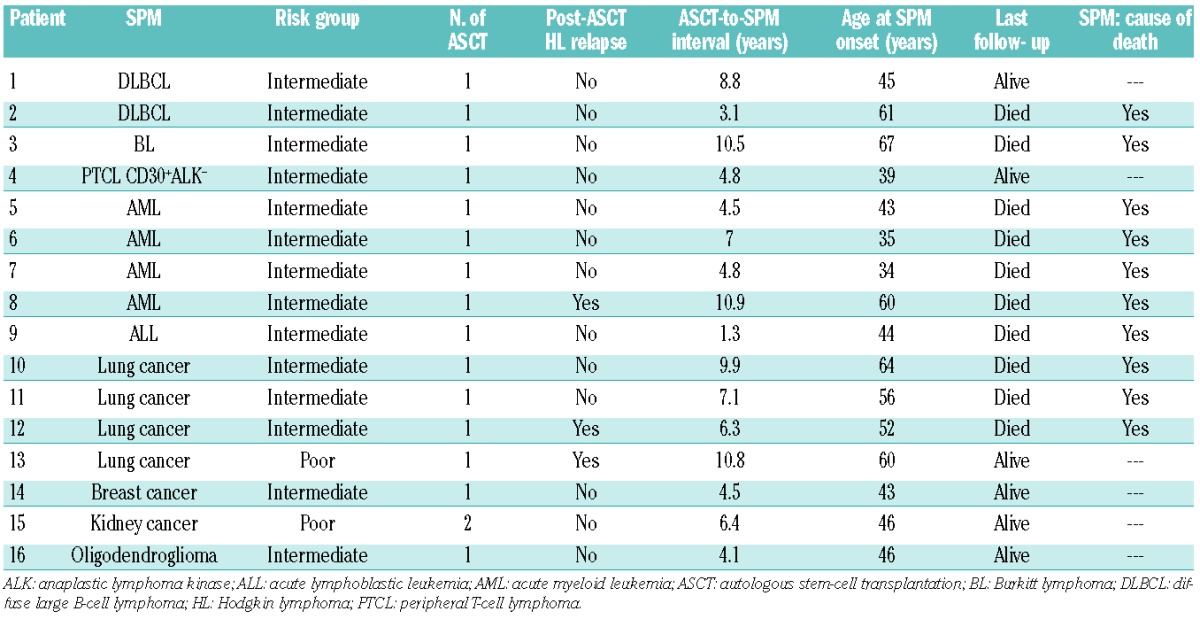
Figure 4.
Cumulative incidence of second primary malignancies (SPM) and cardiovascular events. Cumulative incidence of SPM (A) for the whole population and (B) for patients who did not relapse after completing autologous stem-cell transplantation (ASCT). Cumulative incidence of cardiovascular events (heart failure requiring treatment, coronary artery disease requiring treatment or death resulting from a cardiac or vascular cause) (C) for the whole population and (D) for patients who did not relapse after completing ASCT.
Cardiovascular events
These were defined as heart failure requiring treatment, coronary artery disease requiring treatment or death resulting from a cardiac or vascular cause. Thirteen cardiovascular events occurred. Overall, from inclusion, the 10-year cumulative incidence of cardiovascular events was 6.5% (Figure 4) (10% for the intermediate-risk group and 3% for the poor-risk group; Online Supplementary Figure S3). Considering only those patients not relapsing after completing ASCT, the 10-year cumulative incidence of cardiovascular events was 6% (Figure 4) (7% for the intermediate-risk group and 5% for the poor-risk group; Online Supplementary Figure S3).
Discussion
The long 10.3-year median follow-up of H96-trial patients gave us a unique opportunity to analyze long-term outcomes of a large series of adults with first-relapsed/refractory HL.
The 10-year OS was 70% for the intermediate-risk group and 47% for the poor-risk group. Several studies have shown that single ASCT can provide a cure in roughly 50% of unselected patients.6,7 Thus, the outcome of intermediate-risk patients treated with single ASCT in our trial was better than the outcome of these unselected patients treated with single ASCT. Moreover, the outcome of poor-risk patients treated with tandem ASCT in our trial was similar to the outcome of these unselected patients treated with single ASCT. The results of the SWOG 0410 phase II trial of tandem ASCT in 82 refractory/relapsed HL patients were recently reported: the 2-year progression-free survival (PFS) was 63%, reaching the predicted end-point of at least a 15% improvement based on the historical 2-year PFS of 45% in the previous SWOG 9011 study.8,9
Late effects, especially SPM and cardiovascular events, are well-known after first-line treatment of HL.10–16 In contrast, only a few retrospective studies have addressed long-term effects after ASCT for relapsed or refractory HL.17,18 Thus, long-term prospective data are lacking for first-relapsed or refractory HL treated with ASCT. Concerning the cumulative incidence of SPM, data from retrospective studies are difficult to interpret because of differences in median follow-up, selection of patients (all transplanted patients or only patients who survived ≥2 years after ASCT), period of inclusion, number of treatment lines before ASCT, heterogeneity of treatments, conditioning regimens and source of stem-cells.7,17–19 Given this, the cumulative incidences of SPM in retrospective studies ranged from 5.8% to 14.7% at 10 years, and from 8% to 15.3% at 15 years. In the present study, considering patients who did not relapse after completing single ASCT (intermediate-risk group), the 10- and 15-year cumulative incidences of SPM were 16% and 24%, respectively, which are higher than in retrospective studies. These results suggest that special attention to SPM should be recommended. The relatively low cumulative incidence of SPM in the poor-risk patients who did not relapse after completing ASCT is somewhat surprising. Data concerning SPM were collected in the same way in both risk groups without bias. We have no clear explanation for this low incidence, but we cannot rule out an effect due to chance.
Our study has some limitations. Since the start of this trial in 1995, changes have taken place in the standard treatment of patients with HL. Fluorodeoxyglucose positron-emission tomography (PET) scanning was not routinely done for disease assessment, and it is possible that PET scanning done before ASCT could have more accurately classified patients’ responses to salvage treatment. In the retrospective study by Devillier et al., for responders to salvage treatment according to the Cheson 2007 criteria, the PET response at the time of ASCT influenced outcome and identified patients requiring single or tandem ASCT.20 Additionally, because the H96 trial was not a randomized study comparing single versus tandem ASCT, tandem ASCT can only be considered an option for poor-risk patients. Various treatment strategies to improve outcomes after ASCT have been investigated, including PET-adapted approaches,21,22 dose-intensity of salvage treatment,23 radiation therapy after ASCT,24 tandem auto–alloSCT,25 or consolidation with brentuximab vedotin (BV).26 Recently, the AETHERA randomized trial showed that early consolidation with BV versus placebo after ASCT improved 2-year PFS in patients with HL with risk factors for relapse or progression after transplantation (63% versus 51%, respectively; hazard ratio 0,57; P=0.0013).26 Interim analysis of OS showed no significant difference between treatment groups, and, because the crossover of patients in the placebo group to the BV group confounds this survival analysis, whether early BV consolidation can provide a greater survival benefit than BV treatment after progression cannot yet be answered. A direct comparison between AETHERA and H96 is difficult given the differences in median follow-up (2.5 years for AETHERA versus 10.3 years for H96) and inclusion criteria: in AETHERA, patients were included after ASCT and had at least one of the following risk factors: primary refractory HL, relapsed HL with an initial remission duration of less than 12 months, or relapse after 12 or more months with extranodal involvement. In H96, patients were included at the time of relapse/progression, before salvage treatment, and were stratified into two risk groups. Furthermore, in AETHERA, patients had to have had CR, PR, or SD after salvage treatment, whereas in H96, all patients could undergo the first ASCT whatever their response, including PD. Because the definition of primary refractory HL is not equivocal, it is probably more adequate to analyze results of primary refractory patients between AETHERA and H96. In AETHERA, the 2-year PFS (from randomization after ASCT) of primary refractory patients was 60% and 42% in the BV and placebo arms,27 respectively, whereas in H96, the 2-year PFS (from ASCT) of primary refractory patients in CR, PR, or SD after salvage treatment was 67% according to the intent-to-treat analysis, and 74% for patients completing tandem ACST (data not shown). In view of the results of AETHERA, early BV consolidation after ASCT may certainly be proposed to intermediate-risk patients but whether BV consolidation after the first ASCT may replace tandem ASCT in poor-risk patients remains an open question.
Besides BV, many new drugs, including histone deacetylase inhibitors (mocetinostat and panobinostat),28,29 everolimus,30 bendamustine,31 and PD-1–blocking antibodies (nivolumab32 and pembrolizumab33), have shown activity in relapsed/refractory HL and could be used either to increase the rate of CR prior to ASCT, or as consolidation therapy after ASCT.
Finally, the role of alloSCT in the management of patients who relapse after ASCT remains controversial. In our matched analysis, the 10-year OS did not differ significantly between RIC-allo and no alloSCT patients after post-ASCT relapse. However, the limited number of patients may have prevented the demonstration of a significant benefit of RIC-allo. Considering the 4-year OS (45% for RIC-allo patients and 35% for matched controls), our results are similar to those reported previously.5,34
In conclusion, with long-term follow-up, the risk-adapted strategy remains appropriate, but special attention should be paid to SPM. Integration of PET findings and new drugs such as BV or PD-1–blocking antibodies may help to refine this strategy, especially the need for a second ASCT in poor-risk patients, and possibly improve outcomes for patients with first-relapsed or refractory HL.
Acknowledgments
The authors would like to thank Janet Jacobson for editorial assistance.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/4/474
References
- 1.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852):1051–1054. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–2071. [DOI] [PubMed] [Google Scholar]
- 3.Morschhauser F, Brice P, Ferme C, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26(36):5980–5987. [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 5.Martinez C, Canals C, Sarina B, et al. Identification of prognostic factors predicting outcome in Hodgkin’s lymphoma patients relapsing after autologous stem cell transplantation. Ann Oncol. 2013;24(9):2430–2434. [DOI] [PubMed] [Google Scholar]
- 6.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–633. [DOI] [PubMed] [Google Scholar]
- 7.Sirohi B, Cunningham D, Powles R, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol. 2008;19(7):1312–1319. [DOI] [PubMed] [Google Scholar]
- 8.Smith EP, Li H, Friedberg JW, et al. SWOG S0410/BMT CTN 0703: A phase II trial of tandem autologous stem cell transplantation (AHCT) for patients with primary progressive or recurrent Hodgkin lymphoma (HL) (ClinicalTrials.gov Identifier: NCT00233987). Blood. 2014;124(21). Abstract 676. [Google Scholar]
- 9.Stiff PJ, Unger JM, Forman SJ, et al. The value of augmented preparative regimens combined with an autologous bone marrow transplant for the management of relapsed or refractory Hodgkin disease: a Southwest Oncology Group phase II trial. Biol Blood Marrow Transplant. 2003;9(8):529–539. [DOI] [PubMed] [Google Scholar]
- 10.Ng AK. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol. 2011;154(1):23–31. [DOI] [PubMed] [Google Scholar]
- 11.Rugbjerg K, Mellemkjaer L, Boice JD, Kober L, Ewertz M, Olsen JH. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943–2009. J Natl Cancer Inst. 2014;106(6):dju110. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin’s lymphoma. J Clin Oncol. 2007;25(12):1489–1497. [DOI] [PubMed] [Google Scholar]
- 13.Elkin EB, Klem ML, Gonzales AM, et al. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin’s lymphoma: a multi-institutional, matched cohort study. J Clin Oncol. 2011;29(18):2466–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton LM, Dores GM, Curtis RE, et al. Stomach cancer risk after treatment for Hodgkin lymphoma. J Clin Oncol. 2013;31(27):3369–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koontz MZ, Horning SJ, Balise R, et al. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol. 2013;31(5):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichenauer DA, Thielen I, Haverkamp H, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood. 2014;123(11):1658–1664. [DOI] [PubMed] [Google Scholar]
- 17.Goodman KA, Riedel E, Serrano V, Gulati S, Moskowitz CH, Yahalom J. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5240–5247. [DOI] [PubMed] [Google Scholar]
- 18.Forrest DL, Hogge DE, Nevill TJ, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation does not increase the risk of second neoplasms for patients with Hodgkin’s lymphoma: a comparison of conventional therapy alone versus conventional therapy followed by autologous hematopoietic stem-cell transplantation. J Clin Oncol. 2005;23(31):7994–8002. [DOI] [PubMed] [Google Scholar]
- 19.Minn AY, Riedel E, Halpern J, et al. Long-term outcomes after high dose therapy and autologous haematopoietic cell rescue for refractory/relapsed Hodgkin lymphoma. Br J Haematol. 2012;159(3):329–339. [DOI] [PubMed] [Google Scholar]
- 20.Devillier R, Coso D, Castagna L, et al. Positron emission tomography response at the time of autologous stem cell transplantation predicts outcome of patients with relapsed and/or refractory Hodgkin’s lymphoma responding to prior salvage therapy. Haematologica. 2012;97(7):1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119(7):1665–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomson KJ, Kayani I, Ardeshna K, et al. A response-adjusted PET-based transplantation strategy in primary resistant and relapsed Hodgkin Lymphoma. Leukemia. 2013;27(6):1419–1422. [DOI] [PubMed] [Google Scholar]
- 23.Josting A, Muller H, Borchmann P, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphoma. J Clin Oncol. 2010;28(34):5074–5080. [DOI] [PubMed] [Google Scholar]
- 24.Biswas T, Culakova E, Friedberg JW, et al. Involved field radiation therapy following high dose chemotherapy and autologous stem cell transplant benefits local control and survival in refractory or recurrent Hodgkin lymphoma. Radiother Oncol. 2012;103(3):367–372. [DOI] [PubMed] [Google Scholar]
- 25.Castagna L, Crocchiolo R, Giordano L, et al. High-dose melphalan with autologous stem cell support in refractory Hodgkin lymphoma patients as a bridge to second transplant. Bone Marrow Transplant. 2015;50(4): 499–504. [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. [DOI] [PubMed] [Google Scholar]
- 27.Moskowitz CH, Paszkiewicz-Kozik E, Nadamanee A, et al. Analysis of primary-refractory Hodgkin lymphoma pts in a randomized, placebo-controlled study of brentuximab vedotin consolidation after autologous stem cell transplant [ICML abstract 120]. Hematol Oncol. 2015;33(suppl 1):165. [Google Scholar]
- 28.Younes A, Oki Y, Bociek RG, et al. Mocetinostat for relapsed classical Hodgkin’s lymphoma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2011;12(13):1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes A, Sureda A, Ben-Yehuda D, et al. Panobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II study. J Clin Oncol. 2012;30(18):2197–2203. [DOI] [PubMed] [Google Scholar]
- 30.Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85(5):320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskowitz AJ, Hamlin PA, Jr, Perales MA, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013;31(4):456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskowitz CH, Ribrag V, Michot JM, et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study [ASH abstract 290]. Blood. 2014;124(21):290. [Google Scholar]
- 34.Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97(2):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]