Abstract
Interferon-α is a potent antiviral agent and a vigorous adjuvant in the induction of T-cell responses but its use is limited by hematologic toxicity. Interferon-α alters hematopoietic stem cell dormancy and impairs myelocytic and erythrocytic/megakaryocytic differentiation from hematopoietic progenitors. However, the effect of chronic interferon-α exposure on hematopoietic precursors has still not been well characterized. Here, we transduced the liver of mice with an adenoassociated vector encoding interferon-α to achieve sustained high serum levels of the cytokine. The bone marrow of these animals showed diminished long-term and short-term hematopoietic stem cells, reduction of multipotent progenitor cells, and marked decrease of B cells, but significant increase in the proportion of CD8+ and CD4+CD8+ T cells. Upon adoptive transfer to RAG−/− mice, bone marrow cells from interferon-α-treated animals generated CD4+ and CD8+ T cells while CD19+, CD11b+ and NK1.1+ lineages failed to develop. These effects are associated with the transcriptional downregulation of transcription factors involved in B-cell differentiation and modulation of key factors for T-cell development. Thus, sustained interferon-α exposure causes hematopoietic stem cells exhaustion and drives common lymphoid progenitors towards T-cell generation.
Introduction
Interferon-α (IFNα) is an antiviral, immunomodulatory and antiproliferative cytokine which is produced in response to a variety of infectious agents including viruses and bacteria.1 It constitutes a key component of natural immunity linking innate and adaptive immune responses. IFNα activates macrophages, induces dendritic cell maturation, enhances CD4+ T helper-1 and NK cell–mediated immunity, supports B-cell differentiation to antibody-secreting plasma cells and promotes the generation of effector T cells.2 In line with these activities, IFNα has been utilized in the treatment of chronic viral infections and diverse neoplastic conditions including hematologic malignancies and solid tumors.3–5 Furthermore, IFNα has been shown to function as a potent adjuvant in a variety of animal models acting as a third signal in the induction of CD8+ T-cell immune response6 and is currently being used in a number of vaccination trials.7 On the other side, IFNα treatment may cause immune-mediated tissue damage and induces the development of autoimmune diseases.8,9 Moreover, IFNα alters hematopoiesis and during high-dose IFNα therapy, 26–60% of patients develop neutropenia, anemia and thrombocytopenia requiring discontinuation of the therapy.10
Several mechanisms responsible for hematologic toxicity have been identified. It has been shown that IFNα impairs the replication and differentiation of megakaryocytic and erythrocytic progenitor cells resulting in thrombocytopenia and anemia.11–14 It also blocks granulopoietic differentiation leading to accumulation of granulocyte-macrophage colony forming cells (GM-CFC).15 In addition, IFNα causes lymphopenia, an effect that has been ascribed to redistribution of lymphocytes from the peripheral circulation to lymphoid organs.16
Furthermore, IFNα acts on hematopoietic stem cells (HSCs) altering their dormancy. HSCs constitute a minute cell population of pluripotent cells capable of generating all blood cell lineages for a lifetime. Under steady-state conditions, HSCs are mainly in dormancy to avoid exhaustion. Upon hematopoietic stress, HSCs rapidly and transiently expand and differentiate to replenish blood cells. It has been shown that lymphocytic choriomeningitis virus (LCMV)-induced transient bone marrow (BM) aplasia was due to IFN type I produced shortly after viral infection.17 The same authors demonstrated that LCMV infection caused depletion of pluripotent and lineage committed hematopoietic progenitors in WT but no in IFNα/β receptor deficient animals. Thus, type I IFN can act directly on quiescent long-term hematopoietic stem cells (LT-HSC) forcing them to enter the cell cycle. In fact, it is suggested that interferon may play a role in the mechanism of the acute erythroblastopenic crisis occasionally observed in patients with chronic anemia following viral infections.18 More recently it has been shown that IFNα induces proliferation of HSCs and that maintained exposure to this cytokine by repeated poly(I:C) administration leads to HSC exhaustion.19,20 However, these results have recently been questioned by studies showing that upon poly(I:C) administration, the HSC pool proliferates transiently to enter subsequently into quiescence, thus being protected from the killing effects of IFNs.21 Therefore, the consequences on HSC function of chronic exposure to IFNα still need to be characterized.
In the present work, we have investigated the consequences of long-term IFNα treatment on blood cell homeostasis using an adenoassociated viral vector (AAV) expressing murine IFNα under the control of a constitutive promoter. We showed that sustained IFNα exposure depletes the LT-HSC and short-term HSCs (ST-HSC) reservoir and, at the same time, drives BM lymphopoiesis towards generation of T-cell precursors at the expense of other lymphocyte subsets. This effect is associated with the transcriptional modulation of a number of factors involved in blood cell lineage specification.
Methods
Mice and treatment
Experiments were performed with 6–8-week old male C57BL/6 mice purchased from Harlan Laboratories (Barcelona, Spain). RAG-1-deficient (RAG1−/−) mice were bred and maintained under pathogen-free conditions in the animal facility of the University of Navarra. The experimental design was approved by the Ethical Committee for Animal Testing of the University of Navarra. Mice were injected intravenously with AAV vectors. For all procedures, animals were anesthetized by intraperitoneal injection of a mixture of xylacine (Rompun 2%, Bayer) and ketamin (Imalgene 500, Merial) 1:9 v/v.
Viral construction, production and purification
The expression cassette contained in the AAV-IFNα plasmid consist of the murine interferon-alpha-2 gene (GenBank accession number NM_010503) under the transcriptional control of the constitutive and ubiquitous promoter of human elongation factor-1 (GenBank accession number J04617.1). As control, IFNα gene was replaced by luciferase to obtain the AAV-luc. rAAV8 vectors were produced, as previously described.22 Viral titers in terms of viral genome per milliliter (vg/mL) were determined by Q-PCR.
Flow cytometry and cell sorting
All flow cytometry and cell sorting (FACS) studies were performed on single-cell suspensions and cells were stained using standard protocols. Details of the flow cytometry methods used are provided in the Online Supplementary Appendix. All antibodies used for blood cells analysis and HSCs staining are presented in Online Supplementary Tables S1 and S2. Flow cytometry was performed on an 8 color FACS Canto II (BD Bioscience) and sorting on FACSAria II cell sorter (BD Bioscience). Data were analyzed with FlowJo (TreeStar) and DIVA software.
Colony-forming unit assay
Colony-forming unit (CFU) assays were performed according to the manufacturer’s instructions (Stem Cell Technologies) using Mouse Methylcellulose Complete Media (HSC007). BM cells were plated at 7×104 cells in 6-well plates and incubated at 37°C in a humidified atmosphere of 5% CO2. Cells were cultured for 15 days and monitored by phase-contrast microscopy.
Quantitative PCR analysis
One microgram of total RNA prepared from snap-frozen mouse BM cells were reverse transcribed according to the manufacturer’s instructions of M-MLV RT (Invitrogen). Real-time PCR based quantification was performed using SYBR Green master mix (Applied Biosystems, Foster City, CA, USA). Primers are presented in Online Supplementary Table S3.
Bone marrow transplantation
Bone marrow cells from CD45.1+ C57BL/6 (5×106 cells) treated with AAV-IFNα or AAV-luc for 21 days were isolated and transplanted by retroorbital injection into RAG1−/− or CD45.2+ C57BL/6 recipient mice.
Statistical analysis
RT-PCR data analysis details are provided in the Online Supplementary Appendix. For the rest of experiments the data are presented as mean values±standard deviation and were analyzed for significance by Student t-test (P<0.05 was considered significant) with the GraphPad Prism 5.0 software.
Results
Chronic IFNα exposure induces a dramatic change in composition of the leukocyte population in peripheral blood and in bone marrow
We administered intravenously increasing doses of the AAV encoding IFNα (AAV-IFNα), low: 1.5×1012 viral genomes/kg (vg/kg), intermediate: 5×1012 vg/kg and high: 1.5×1013 vg/kg. As control, mice were injected with 1.5×1013 vg/kg of an AAV expressing luciferase (AAV-luc). IFNα levels in serum were quantified by ELISA at day 7, 14, 21 and 28 after vector injection. No IFNα was detected in AAV-luc treated mice while sustained levels of the cytokine were detected in the animals receiving the intermediate and the high dose. In animals treated with the low dose, IFNα expression disappeared with time (Figure 1A). Thus, for most posterior experiments we used the intermediate AAV-IFNα dose.
Figure 1.

Sustained IFN-α expression provokes hematologic toxicity accompanied by important changes in the blood population distribution. C57BL/6 mice (4–8 mice/group) intravenously injected with different doses of AAV-IFNα, low (1.5×1012 vg/Kg), intermediate (5×1012 vg/Kg), high (1.5×1013 vg/Kg) or AAV-luc at high dose. (A) IFN-α serum levels before and 7, 14, 21 and 28 days post injection. (B–D) Analysis of white blood cell (WBC) (B), red blood cell (RBC) (C) and platelet (PLT) (D) counts at the indicated time point after virus injection (5–6 mice/group). (E) Relative numbers of CD11b+, CD19+, NK1.1+, CD8+, CD4+, in peripheral blood (5–6 mice/group) at day 21 after injection of AAV-IFNα intermediate dose. Results are expressed as mean+SD. Statistical significance was determined by Student t-test (*P≤0.05; **P≤0.01; ***P≤0.001). Results are representative of three independent experiments.
In a second study, mice were treated as described above and blood cell count analysis was performed on different days. We observed a fast and dramatic decrease of leukocytes in all the animals receiving IFNα probably due to the redistribution from the peripheral circulation to marginal and tissue pools.16 Thereafter, leukocyte count very slowly diminished over time (Figure 1B). Red blood cell and platelet numbers showed a progressive decrease with time, especially at intermediate and high doses (Figure 1C and D). Flow cytometry analysis of the peripheral leukocyte populations at day 28 following administration of the intermediate dose of AAV-IFNα showed no significant differences in the relative numbers of CD8+, CD4+, or NK1.1+ but the proportion of B cells was severely reduced and the percentage of CD11b+ cells (monocytes) was increased (Figure 1E). The study by flow cytometry of BM cells isolated from mice treated with this same dose of AAV-IFNα or AAV-luc also demonstrated a highly significant reduction of B cell in IFNα-treated mice compared to controls (Figure 2A) which was accompanied by decreased number of erythroblasts and a tendency to a reduction in megakaryocytes (Figure 2B). Interestingly, while monocyte and NK numbers remained unaltered (Figure 2B) and CD4+ T cells showed a non-significant tendency to increase, CD8+ T cells and CD4+ CD8+ double positive cells, a T-lymphocyte precursor population, were markedly elevated in IFNα-treated animals (Figure 2B and C). Cell cycle analysis revealed that IFNα-treatment induces the proliferation of CD8+ T cells in the BM but not of CD4+ T cells (Figure 2D). Next, we analyzed the T cells in the thymus in both groups of animals, and found that the percentage of CD4+ and CD8+ single positive cells significantly increased while the number of DP cells decreased (Online Supplementary Figure S1A and B). Furthermore, the percentage of DN3 cells is significantly higher in IFNα-treated mice (Supplementary Figure S1C and D). These data suggest that IFNα induces the differentiation of T cells in the thymus and at the same time induced the production of T-cell precursors in the BM (Figure 2C).
Figure 2.

Chronic IFNα expression affects the composition of bone marrow differentiated cell population. Three weeks after vector injection (5–6 mice/group) the composition of BM cells from AAV-IFNα-treated mice was compared with the AAV-luc-treated mice. (A) (left) The mean numbers of CD19+ cells (B cells) per femur are represented. (right) Representative flow cytometry histogram analysis of the CD19+ cells in BM of AAV-treated mice at the end of the experiment. (B) Mean numbers of CD11b+ (monocytes), NK1.1+ (NK-cells), Ter-119+ (erythrocytes), CD41+ (platelets) per femur. (C) (left) Mean numbers of CD4+, CD8+ (T cells) and double-positive cells per femur. (right) Representative flow cytometry analysis of the CD4+ and/or CD8+ cells in BM obtained from AAV-treated mice at the end of the experiment. (D) (left) Mean percentages of CD8+ T cells per femur in G0-G1, S or G2-M phase. (right) Representative flow cytometry analysis of CD8+ cells cycle in BM AAV-treated mice at the end of the experiment. The inserts report the percentages of cells for each quadrant. Results are expressed as the mean+SD. Statistical significance was determined by Student t-test (*P≤0.05; **P≤0.01; ***P≤0.001).
Sustained IFNα expression diminished hematopoietic stem cell compartment
Homeostasis of blood cells is dependent on dormant BM HSCs possessing long-term self-renewal capacity. Thus, we analyzed the effect of sustained IFNα production on BM HSCs. One month after vector injection, mice were sacrificed and the total number of BM cells was determined. Mice treated with AAV-IFNα experienced a dose-dependent BM hypoplasia (Figure 3A). To determine the impact of IFNα treatment on hematopoietic precursors, we quantified the cells expressing the stem cell marker c-kit and Sca-1 while lacking the canonical marker of lineage differentiation Lin- (so-called LSK cells). We found, however, that Sca-1 is aberrantly induced by IFNα (Online Supplementary Figure S2) preventing the use of this marker for HSC characterization. Using Lin- c-kit+ (LK) as identification markers for HSC, our analysis revealed a dose-dependent decrease of LK population in mice treated with AAV-IFNα (Figure 3B). The analysis of the LK cells with anti-CD48 and anti-CD150, which discriminates between LT-HSCs (LK, CD48−, CD150+), ST-HSCs (LK, CD48−, CD150−), and multipotent progenitors (MPPs: LK, CD48+, CD150−), revealed a profound and dose-dependent decrease of all three populations in animals treated with IFNα (Figure 3C–F). In particular, LT-HSCs nearly disappeared after high-dose treatment (Figure 3D).
Figure 3.

Long-term IFN-α expression induces bone marrow hypoplasia characterized by LT-HSCs and ST-HSCs disappearance and MPPs reduction in a dose dependent manner. Three weeks after vector injection (5–6 mice/group) the composition of BM cells from AAV-IFNα-treated mice was compared with the AAV-luc-treated mice. (A) Mean BM cellularity per femur of AAV-treated mice three weeks after vector injection (5–6 mice/group). (B) Bar graphs represent the numbers of Lin− c-Kit+ (LK) cells per femur (5–6 mice/group) (C) Representative flow cytometric analysis of the BM progenitor cells of AAV-treated mice at the end of the experiment. (D–F) Bar graphs represent the numbers of long-term (D) and short-term (E) hematopoietic stem cell (HSCs) and multipotent progenitor (F) cell population per femur (5–6 mice/group). Results are expressed as the mean+SD. Statistical significance was determined by Student t-test (*P≤0.05; **P≤0.01; ***P≤0.001). Results are representative of three independent experiments.
We further investigated the influence of IFNα on the more differentiated progenitor cells. We used flow cytometry to analyze the percentage of common lymphoid progenitors (CLPs: Lin− cKitlow CD127+ Thy1.2−), using the gating strategy described in Online Supplementary Figure S3,23 observing a significant decrease in CLP (Figure 4A). In addition, this analysis revealed a significant reduction in the expression levels of CD127, the receptor for IL-7 (IL7R), in the IFNα-treated group (Figure 4C), a finding that was confirmed by RT-PCR (Figure 4D). The exclusion of Sca-1 marker from the analysis impaired the correct phenotypic analysis of common myeloid progenitors (CMP) and granulocyte/monocyte progenitors (GMP), thus, a functional analysis to determine the number of granulocytes/monocytes CFU (CFU-GM), monocytes CFU (CFU-M) and granulocyte CFU (CFU-G) was performed, showing a clear reduction in IFNα-treated mice (Figure 4B).
Figure 4.

IFNα influences the population of progenitors cells. Three weeks after vector injection (5–6 mice/group) the composition of progenitor population in BM cells from AAV-IFNα-treated mice was compared with the AAV-luc-treated mice. (A) Mean percentage of common lymphoid progenitors (CLPs) in Lin−cKitlow population of AAV-treated mice three weeks after vector injection (4–5 mice/group). (B) Mean number of the CFU-GM + CFU-M + CFU-G in methylcellulose in one representative experiment performed in triplicate. (C) Representative flow cytometry analysis of the CLPs in BM AAV-treated mice showing the phenotypic downregulation of the CD127 marker. Results are representative of two independent experiments. (D) Bar graphs showing the expression of IL-7Rα messenger RNA in BM cells of AAV-treated mice three weeks after vector injection (5–6 mice/group). Results are expressed as the mean+SD. Statistical significance was determined by Student t-test (*P≤0.05; **P≤0.01; ***P≤0.001).
Long term IFNα treatment guides multipotent hematopoietic progenitor cells toward a T-cell fate
To analyze the functionality of MPPs obtained from IFNα treated mice we carried out the experiment represented in Figure 5A. C57BL/6 CD45.1+ mice received 5×1012 vg/kg of AAV-IFNα or AAV-luc and three weeks later animals were sacrificed. First, a colony formation assay was performed using BM cells. This study showed a significant reduction in the number of CFUs and in the size of colonies obtained from IFNα treated mice compared to control animals (Figure 5B). We then investigated the repopulation capacity of MPPs; for that purpose the same number of BM cells from each group of animals was transferred to CD45.2 RAG-1 deficient mice (RAG−/−). At different time points after cell transfer the number of CD45.1+ and CD3+, CD4+, CD8+, CD19+, CD11b, and NK+ cells were quantified by flow cytometry in peripheral blood of recipient animals. We observed that the percentage of CD45.1+ cells in RAG−/− mice that received the cells from AAV-luc mice was higher than in the mice given BM from AAV-IFNα mice (Figure 5C). Furthermore, while BM cells obtained from AAV-luc mice generated CD4+, CD8+, CD19+ CD11b, and NK cells (Figure 5D–I and Online Supplementary Figure S4) only CD4+ and CD8+ single positive T cells were detected in RAG−/− given BM cells from IFNα-treated mice (Figure 5D–I and Online Supplementary Figure S4). Next we analyzed the T-cell population in the thymus of recipient animals. First, we observed that the engraftment of T cells in the thymus of the recipient mice is significantly lower when the mice received BM cells from IFNα-treated animals than from luc-treated mice (Online Supplementary Figure S5A and B). Furthermore, in the thymus of mice that received BM from IFNα-treated mice, we found significantly higher numbers of single positive T cells and lower numbers of DP and DN T cells in comparison to the thymus of mice receiving BM from luc-treated mice (Online Supplementary Figure S5C and D), indicating once again a more differentiated T-cell phenotype.
Figure 5.
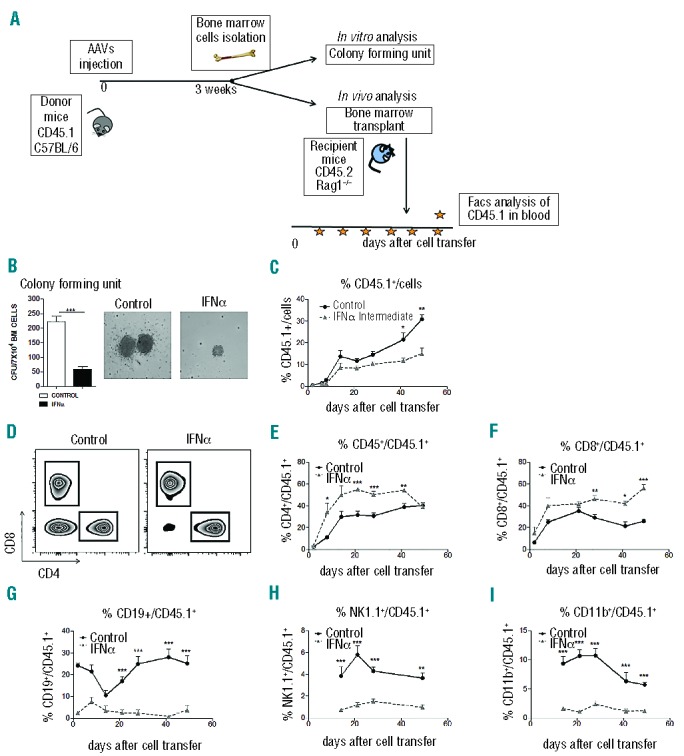
Sustained IFNα expression affects the functionality of MPPs cells. (A) Experimental design for MPPs functional testing. Three weeks after vector injection BM cells from C57/BL6 CD45.1 AAV-treated mice were isolated and plated in methylcellulose medium and colony forming unit (CFU) number was calculated. BM cells from AAV-treated CD45.1 C57/BL6 mice were transplanted by retroorbital injection into RAG-1-deficient (RAG−/−) CD45.2 recipient mice to evaluate the repopulation capacity after the treatment. (B) Mean number (left) and representative photograph of the colonies (right) grown in methylcellulose in one representative experiment performed in triplicate. (C) Analysis of CD45.1+ donor cells from AAV-treated mice in blood of recipient CD45.2 mice (4–5 mice/group). (D) Representative flow cytometry analysis of CD45.1+ CD4+ and CD8+ donor cells from AAV-treated mice in blood of CD45.2 recipient mice (4–5 mice/group). (E–I) Analysis of CD45.1+CD4+ (E), CD45.1+CD8+ (F), CD45.1+CD19+ (G), CD45.1+NK1.1+ (H), CD45.1+CD11b+ (I) in blood of recipient CD45.2 mice (4–5 mice/group). Results are expressed as the mean+SD. Statistical significance was determined by Student t-test (*P≤0.05; **P≤0.01; ***P≤0.001).
The adoptive transfer experiment was repeated in lethally irradiated RAG−/− mice. In these studies we found that BM recipients from CD45.1 IFNα-treated mice (but not from control vector treated mice) died within two weeks. Interestingly, when RAG−/− mice received AAV-IFNα treated BM cells together with BM cells from untreated CD45.2 mice, CD45.1 cells were hardly detected in peripheral blood but these few cells were mainly T cells (data not shown).
To determine if the CD45.1+ T cells developed in the RAG−/− mice that received the BM cells from IFNα- treated mice are derived from the DP T cell population found in the BM (Figure 2C), we performed a second adoptive transfer experiment. CD45.1+ cells purified from RAG−/− mice previously transplanted (Recipient-1) were transferred to a second group of RAG−/− mice (Recipient-2) (for a schematic representation of the experiment see Online Supplementary Figure S6A). First, we analyzed the presence of CD4 CD8 DP cells in the BM of Recipient-1 mice. Flow cytometry analysis showed no DP population in these mice (Online Supplementary Figure S6B). After adoptive transfer, the Recipient-2 mice developed both CD45.1 positive CD4 and CD8 single positive T cells and no other cell lineages (Online Supplementary Figure S6C). In conclusion, our data showed that the T cells detected in recipient mice are not exclusively developed from DP T cells.
Effect of IFNα on the expression of transcription factors involved in lineage specification of hematopoietic precursors
To understand the way IFNα modulates the commitment of HSCs, we analyzed by quantitative RT-PCR the expression levels of key transcription factors (TF) involved in MPPs lineage specification in: total BM cells, in purified LK cells and in differentiated cells (Lin+). The genes involved in B-cell lineage commitment, such as E2A, EBF-1, PAX-5 and FOXO-124,25 were markedly down-regulated in all the cases (Figure 6A–C). Thus, B-cell differentiation seems to be inhibited at the precursor levels. On the other hand, the expression of those genes involved in T-lymphocyte differentiation such as GATA-3 and DDL-4 appears to be up-regulated in the differentiated BM population (Lin+) whilst DELTEX-1, which inhibits T-cell differentiation, was significantly down-regulated (Figure 6C).26 The expression of these genes is not significantly altered in the progenitor population (LK). Furthermore, the expression of genes that stimulate megakaryocyte/erythrocyte progenitor (MEP) differentiation into erythrocyte and platelets like RUNX-1 and MEIS-1 were repressed in progenitor cells (Figure 6B) while SOCS-1 that negatively regulates this process was significantly induced in differentiated cell (Figure 6C). We found a reduction of NOTCH-1 expression in progenitor cells that might be related to the exhausted phenotypes of HSCs, but more studies need to be performed to clarify this aspect. No alteration in NOTCH-1 expression was detected in lineage committed cells. Our data suggest that IFNα directly down-regulates the expression of B-cell TFs in lymphocyte precursor cells and this effect alters the differentiation of these cells driving them to the production of T lymphocytes.
Figure 6.

Chronic IFNα expression transcriptionally reprograms hematopoiesis towards a T-cell fate. (A–C) Bar graphs showing the expression of genes involved in the modulation of lineage-choice from BM (A), LK cells (B) Lin+ cells (C) of AAV-treated mice three weeks after vector injection. Data are shown as log2 of the ratio between IFNα treated and Luc treated mice (5 mice/group, experiment performed in duplicate).
IFNα directly modulates the expression of transcription factors implicated in hematopoietic stem cell lineage commitment
To determine whether IFNα can directly control the expression of transcription factors that mediate lineage specification of HSCs, purified LK cells were treated with 0.5 U/μL of recombinant IFNα during 8 h and 24 h, and transcriptional levels of these transcription factors were determined by RT-PCR. Eight hours after IFNα treatment we found a marked decrease in the expression of key factors involved in B-cell development like EBF-1, PAX-5, and FOXO-1 while no differences were observed in T- or Mk/E-cell fate factors except for downregulation in SOCS-1 expression (Figure 7A). After 24 h of IFNα treatment, we observed a persistent decrease of B-cell fate TFs, and a tendency to increase in the expression levels of GATA-3 and DDL-4, and a decrease in DELTEX-1 expression similarly to the data obtained after IFNα in vivo treatment. No change was observed in the expression pattern of genes that stimulate MEP differentiation (Figure 7B). These data indicate that IFNα has a direct effect over the expression of B cell TFs and indirectly affects the expression of T-cell fate molecules.
Figure 7.

IFNα expression directly regulates the expression of B-cell fate transcription factors. (A and B) Bar graphs showing the expression of genes involved in the modulation of lineage choice in LK cells obtained from C57/BL6 mice and treated ex vivo for 8 h (A) or three days (B) with 0.5 U/μL of recombinant murine-IFNα. Data are shown as log2 of the ratio between IFNα treated and control cells. Value are means, n=4. Results are expressed as the mean+SD. Statistical significance was determined by Student t-test (*P≤0.05; **P≤0.01; ***P≤0.001).
Discussion
IFNα exerts potent antiviral, antitumor and adjuvant effects but these activities are counterbalanced by the induction of peripheral pancytopenia that frequently limits its clinical use.10 It has also been shown that viral infections repress hematopoiesis in an IFNα-dependent manner.17 Moreover, recently it has been reported that repeated administrations of interferon inducers, like poly(I:C), alter HSCs quiescence provoking HSCs exhaustion.19,20 Thus, IFNα exerts a complex impact on hematopoiesis, it compromises the stemness of HSCs but also redirects the function of the hematopoietic precursors. In this sense IFNα is known to induce lymphopenia but, at the same time, it is a vigorous stimulator of T-cell immunity.6,16 Although a large amount of information is available about the beneficial and deleterious effects of IFNα, the modulation of hematopoiesis by IFNα still remains poorly understood.
Here we analyzed the hematopoietic changes occurring in mice subjected to chronic IFNα exposure. This was achieved by transducing the liver with AAV-IFNα. These animals developed profound peripheral leukopenia, anemia and thrombocytopenia. As far as leukocytes are concerned, not all cell populations were affected in the same manner. While the relative frequency of T cells or NK cells was similar in IFNα-treated and control animals, in the former there was a dramatic reduction of B cells, while CD11b+ cells were markedly increased. The deleterious effect of IFNα on B cells has been previously reported in patients with chronic hepatitis C virus (HCV) infection receiving PEG-IFN.27 Similarly, it has been shown that an inappropriate activation of type I IFN plays a role in the pathogenesis of autoimmune diseases and that this alteration is associated with a profound change in B-cell development.28 However, the mechanisms underlying the modulation of B-cell population by IFNα have not yet been explored. Moreover, the descent of B cells was also observed in BM of animals treated with AAV-IFNα. Interestingly, the impairment of B-cell lymphopoiesis was associated with an increase of CD8+ T cells and of double positive CD4+ CD8+ T cells. Cell cycle analysis revealed that IFNα increase the proliferation of CD8+ T cells in the BM. Interestingly, in the thymus, IFNα pushed T-cell differentiation. In line with this observation, we noticed that while the BM from AAV-luc-treated CD45.1+ mice transferred to RAG−/− mice resulted in development of different hematopoietic lineages, the transplanted BM of AAV-IFNα-treated CD45.1+ mice gave rise only to T cells but not to B, NK or CD11b+ cells. These data clearly indicate that IFNα influences MPPs differentiation and promotes lineage skewing towards T cells. The analysis of the MPP subpopulations revealed a significant decrease in the relative amount of the CLP subpopulation which showed marked downregulation of IL-7 receptor. The fact that these CLPs are not able to generate B cells but only T cells is concordant with the reported fact that CLPs generated in the absence of IL-7-signal have normal T-differentiation potential, but severely impaired B potential.29,30
The plasticity of hematopoietic progenitor cells is controlled by the expression of different transcription factors and interacting molecules. To understand the mechanisms underlying the ability of IFNα to skew MPPs differentiation towards generation of T cells, we analyzed the expression of the TFs and additional factors involved B-and T-cell lymphopoiesis in BM progenitors and differentiated cells. We found that those factors promoting the generation of pro B cells from CLPs including E2A, EBF-1, PAX-5 and FOXO-1,25 were markedly repressed by IFNα in progenitor and lineage committed cells. On the other hand, while no variation in the expression of factors involved in T-cell differentiation were detected in progenitor cells (except for the significant reduction of NOTCH-1 expression, which is probably associated to the exhausted status of progenitor cells), significant differences were observed in differentiated BM cells. IFNα treatment results in an enhanced expression of GATA-3 and DLL-4, which promotes T-cell lineage specification and reduces the expression of DELTEX-1 which inhibits T-cell differentiation.31–33 It has been described that activation of NOTCH-1 by DLL-4 initiates the process of T-cell commitment while blocking B lymphopoiesis.34 In contrast, interaction of NOTCH-1 with DELTEX-1 inhibits T-cell differentiation.26 These events, which normally occur in the thymus, can be recapitulated in BM cells.34 Indeed, we found that the percentage of T cells in the BM of IFNα-treated mice was significantly higher than in controls. The former group of animals exhibited 2-fold higher percentage of CD4 and CD8 single positive cells and 10-fold higher proportion of CD4+ CD8+ double positive cells. These findings are in agreement with the observation that in Notch-induced T-cell acute lymphoblastic leukemia (T-ALL) the tumor cells were largely CD4+CD8+ T cells.35,36 Regarding the mechanism of action of IFNα, in vitro studies performed to analyze how IFNα modulates the expression of the factors involved in B- and T-cell fate development showed that IFNα directly down-regulates the expression of B-cell fate factors, while the effect over T-cell fate transcription factor is secondary to the initial IFNα effect.
Myeloid cells in the BM originate from CMP which can give rise to monocytes, mast cells, and granulocytes via GMP and to platelets and erythrocytes via MEP. We found that IFNα exposure affects the expression of factors involved in MEP differentiation. This finding is consistent with the reduction in erythrocytes and platelets occurring during IFNα therapy and shows that, besides the reported effect of IFNα on replication and differentiation of megakaryocytic and erythrocytic progenitor cells11–14 the reduction of these two populations might be also due to the alteration of MEP differentiation.
Several hematopoietic cytokines regulate hematopoiesis by controlling the proliferation, differentiation, and maturation of primitive hematopoietic cells.37 The effect of IFNα on HSCs is under debate. Essers et al. reported that poly(I:C) promotes HSC proliferation in an IFNα receptor-dependent manner leading to HSC exhaustion19 but recent data from Pietras et al.21 indicate that poly(I:C) induces HSC transient proliferation followed by rapid return to quiescent state. Here we found that LT-HSC and ST-HSC are dramatically reduced in IFNα-treated animals causing a progressive and lethal pancytopenia indicative of the exhaustion of the HSC compartment.
In conclusion, our results demonstrate that long-term exposure to IFNα produces exhaustion of HSCs and triggers a unique genetic program in hematopoietic progenitor cells favoring the generation of T cells while blocking the development of B cells and the differentiation of MEP. Furthermore, regulation of the transcriptional activity of transcription factors like SOCS-1 could play an important role in the development of anemia and thrombocytopenia observed in a high percentage of IFNα-treated patients. Our data underscore the role of IFNα in the promotion of adaptive T-cell immune responses and provide specific targets to counteract the hematologic toxicities of this cytokine while maintaining its immunostimulatory properties.
Acknowledgments
We want to thank Dr Estanislao Nistal-Villan, Dr Rafael Aldabe, Dr Felipe Prosper, Dr Jesús San Miguel for comments on the manuscript. We would like to thank Dr Sandra Hervas-Stubbs and Idoia Rodríguez Serrano for their assistance on Flow cytometry analysis as well as Elizabeth Guruceaga Martínez for statistical analysis. The authors also thank Elena Ciordia and CIMA’s animal facility staff for animal care and vivarium management.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by grants; UTE project CIMA, Fundación Barrié de la Maza y Condesa de Fenosa, CIBERehd Instituto de Salud Carlos III, SAF SAF2009-08524 and SAF2012-39578 (to G. Gonzalez-Aseguinolaza) from the Spanish Department of Science. MDS is in receipt of a fellowship from Fondo de Investigaciones Sanitarias, IGF and LV were in receipt of FPI grants. The authors have no conflicting financial interests.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Biron CA. Interferons alpha and beta as immune regulators–a new look. Immunity. 2001;14(6):661–664. [DOI] [PubMed] [Google Scholar]
- 2.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013; 57(1):399–408. [DOI] [PubMed] [Google Scholar]
- 4.Rohon P. Biological therapy and the immune system in patients with chronic myeloid leukemia. Int J Hematol. 2012;96(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5.Tarhini AA, Gogas H, Kirkwood JM. IFN-alpha in the treatment of melanoma. J Immunol. 2012;189(8):3789–3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber JP, Farrar JD. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132(4):466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizza P, Capone I, Moretti F, Proietti E, Belardelli F. IFN-alpha as a vaccine adjuvant: recent insights into the mechanisms and perspectives for its clinical use. Expert Rev Vaccines. 2011;10(4):487–498. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Davidson A. IFNalpha Inducible Models of Murine SLE. Front Immunol. 2013;4:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29(12):3948–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirkwood JM, Bender C, Agarwala S, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. 2002;20(17):3703–3718. [DOI] [PubMed] [Google Scholar]
- 11.Dukes PP, Izadi P, Ortega JA, Shore NA, Gomperts E. Inhibitory effects of interferon on mouse megakaryocytic progenitor cells in culture. Exp Hematol. 1980;8(8):1048–1056. [PubMed] [Google Scholar]
- 12.Ganser A, Carlo-Stella C, Greher J, Volkers B, Hoelzer D. Effect of recombinant interferons alpha and gamma on human bone marrow-derived megakaryocytic progenitor cells. Blood. 1987;70(4):1173–1179. [PubMed] [Google Scholar]
- 13.Mamus SW, Oken MM, Zanjani ED. Suppression of normal human erythropoiesis by human recombinant DNA-produced alpha-2-interferon in vitro. Exp Hematol. 1986;14(11):1015–1022. [PubMed] [Google Scholar]
- 14.Ortega JA, Ma A, Shore NA, Dukes PP, Merigan TC. Suppressive effect of interferon on erythroid cell proliferation. Exp Hematol. 1979;7(3):145–150. [PubMed] [Google Scholar]
- 15.Verma DS, Spitzer G, Zander AR, et al. Human leukocyte interferon preparation-mediated block of granulopoietic differentiation in vitro. Exp Hematol. 1981;9(1):63–76. [PubMed] [Google Scholar]
- 16.Shiow LR, Rosen DB, Brdickova N, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. [DOI] [PubMed] [Google Scholar]
- 17.Binder D, Fehr J, Hengartner H, Zinkernagel RM. Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med. 1997;185(3):517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitt LJ, Reyes GR, Moonka DK, Bensch K, Miller RA, Engleman EG. Human T cell leukemia virus-I-associated T-suppressor cell inhibition of erythropoiesis in a patient with pure red cell aplasia and chronic T gamma-lymphoproliferative disease. J Clin Invest. 1988;81(2):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essers MA, Offner S, Blanco-Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458(7240):904–908. [DOI] [PubMed] [Google Scholar]
- 20.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10(3):201–209. [DOI] [PubMed] [Google Scholar]
- 21.Pietras EM, Lakshminarasimhan R, Techner JM, et al. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. J Exp Med. 2014;211(2):245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanrell L, Di Scala M, Blanco L, et al. Development of a liver-specific Tet-on inducible system for AAV vectors and its application in the treatment of liver cancer. Mol Ther. 2011;19(7):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schurch C, Riether C, Amrein MA, Ochsenbein AF. Cytotoxic T cells induce proliferation of chronic myeloid leukemia stem cells by secreting interferon-gamma. J Exp Med. 2013;210(3):605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seet CS, Brumbaugh RL, Kee BL. Early B cell factor promotes B lymphopoiesis with reduced interleukin 7 responsiveness in the absence of E2A. J Exp Med. 2004;199(12):1689–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nechanitzky R, Akbas D, Scherer S, et al. Transcription factor EBF1 is essential for the maintenance of B cell identity and prevention of alternative fates in committed cells. Nat Immunol. 2013;14(8):867–875. [DOI] [PubMed] [Google Scholar]
- 26.Izon DJ, Aster JC, He Y, et al. Deltex1 redirects lymphoid progenitors to the B cell lineage by antagonizing Notch1. Immunity. 2002;16(2):231–243. [DOI] [PubMed] [Google Scholar]
- 27.Soldevila B, Alonso N, Martinez-Arconada MJ, et al. A prospective study of T- and B-lymphocyte subpopulations, CD81 expression levels on B cells and regulatory CD4(+) CD25(+) CD127(low/−) FoxP3(+) T cells in patients with chronic HCV infection during pegylated interferon-alpha2a plus ribavirin treatment. J Viral Hepat. 2011;18(6):384–392. [DOI] [PubMed] [Google Scholar]
- 28.Palanichamy A, Bauer JW, Yalavarthi S, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol. 2014;192(3):906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JP, Izon D, DeMuth W, Gerstein R, Bhandoola A, Allman D. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002; 196(5):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201(6):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosoya T, Kuroha T, Moriguchi T, et al. GATA-3 is required for early T lineage progenitor development. J Exp Med. 2009; 206(13):2987–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L. T lineage progenitors: the earliest steps en route to T lymphocytes. Curr Opin Immunol. 2006;18(2):121–126. [DOI] [PubMed] [Google Scholar]
- 34.Mohtashami M, Shah DK, Kianizad K, Awong G, Zuniga-Pflucker JC. Induction of T-cell development by Delta-like 4-expressing fibroblasts. Int Immunol. 2013; 25(10):601–611. [DOI] [PubMed] [Google Scholar]
- 35.Chiang MY, Shestova O, Xu L, Aster JC, Pear WS. Divergent effects of supraphysiologic Notch signals on leukemia stem cells and hematopoietic stem cells. Blood. 2013;121(6):905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giambra V, Jenkins CR, Wang H, et al. NOTCH1 promotes T cell leukemia-initiating activity by RUNX-mediated regulation of PKC-theta and reactive oxygen species. Nat Med. 2012;18(11):1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]


