Abstract
Standard treatment for Glanzmann thrombasthenia, a severe inherited bleeding disorder, is platelet transfusion. Recombinant factor VIIa is reported to be effective in Glanzmann thrombasthenia with platelet antibodies and/or refractoriness to platelet transfusions. We aimed to evaluate recombinant factor VIIa effectiveness and safety for the treatment and prevention of surgical bleeding in patients, with or without platelet antibodies and/or refractoriness, using data from the Glanzmann Thrombasthenia Registry, an international, multicenter, observational, post-marketing study of rFVIIa. Between 2007 and 2011, 96 patients were treated for 206 surgical procedures (minor 169, major 37). History of platelet antibodies was present in 43 patients, refractoriness in 23, antibodies+refractoriness in 17, while 47 had no confirmed antibodies/refractoriness. Treatments analyzed included antifibrinolytics, recombinant factor VIIa, recombinant factor VIIa+antifibrinolytics, platelets±antifibrinolytics and recombinant factor VIIa+platelets±antifibrinolytics. The most frequent treatment for minor procedures was recombinant factor VIIa+antifibrinolytics (n=65), and for major procedures, recombinant factor VIIa+platelets±antifibrinolytics (n=13). In patients without antibodies/refractoriness, recombinant factor VIIa, either alone or with antifibrinolytics, and platelets±antifibrinolytics were rated 100% effective for minor and major procedures. The effectiveness of treatment for minor procedures in patients with antibodies and refractoriness was 88.9% for recombinant factor VIIa, 100% for recombinant factor VIIa+antifibrinolytics, 66.7% for platelets±antifibrinolytics and 100% for recombinant factor VIIa+platelets±antifibrinolytics. One of four adverse events reported for surgery was considered recombinant factor VIIa-treatment-related (non-fatal thromboembolic event in an adult female receiving recombinant factor VIIa+platelets+antifibrinolytics). For all patients, regardless of platelet antibody or refractoriness status, recombinant factor VIIa, administered with or without platelets (±antifibrinolytics), provided effective hemostasis with a low frequency of adverse events in surgical procedures in Glanzmann thrombasthenia patients. This trial was registered at clinicaltrials.gov identifier: 01476423.
Introduction
Glanzmann thrombasthenia (GT) is an inherited, autosomal, recessive disorder of platelet function caused by quantitative or qualitative defects of the platelet membrane glycoprotein IIb/IIIa (integrin αIIbβ3) complex.1,2 GT is rare, with an incidence of approximately 1:1 million, although this is much higher in areas where marriage between close family relatives is common.1,3 Overall, GT is a severe bleeding disorder. Patients show signs of bleeding beginning in childhood. Bleeds are usually mucocutaneous and include easy bruising, purpura, epistaxis, gingival bleedings and menorrhagia.2,3 Bleeding complications frequently occur after dental extraction, surgery, childbirth and trauma.
Bleeding in GT can often be treated by local hemostatic agents and antifibrinolytics (AF).1 However, platelet transfusion is required for surgical procedures and for moderate or severe bleeds when local measures are ineffective. Repeated platelet transfusion may, however, result in the development of an allergic reaction, as well as antibodies to human leukocyte antigen (HLA) or αIIbβ3 that may render future platelet transfusion ineffective.2,4–6
Previously, we showed that recombinant human factor VIIa (rFVIIa) was a good alternative therapeutic agent for bleeding and surgical prophylaxis in GT.7,8 Data from a previous international survey of 59 patients treated for 108 bleeding episodes and 34 surgical/invasive procedures7 allowed a preliminary suggestion for a more optimal rFVIIa regimen (rFVIIa ≥80 μg/kg given at ≤2.5-h intervals for three or more doses) for the treatment of moderate/severe bleeding episodes. However, the number of evaluable surgical procedures was too small for an appropriate regimen for surgical prophylaxis to be proposed.
The Glanzmann Thrombasthenia Registry (GTR)8 was an observational, international registry established following the approval of rFVIIa in Europe in 2004. This article describes the effectiveness and safety reported for the various hemostatic agents examined in surgical procedures in GT patients, with or without antibodies and/or refractoriness, in the GTR.
Methods
The Glanzmann Thrombasthenia Registry
The GTR was a post-marketing, observational, international registry with the primary objective of collecting and evaluating data on the effectiveness and safety of rFVIIa in patients with GT for treatment and prevention (surgical prophylaxis) of bleeding.8 Data were also collected on the use of other systemic hemostatic agents. Treatment was based on local clinical practice rather than a set protocol. Data entry into the GTR was between 10th May 2007 and 16th December 2011.
Ethics
This study was conducted in accordance with the Declaration of Helsinki and Guidelines for Good Pharmacoepidemiology Practices. Each participating center complied with local regulations. Where required, ethical and/or regulatory approval was obtained before data entry into the registry. Signed informed consent to participate in the GTR was obtained from all patients (or parents/legal guardians for minors).
Inclusion and exclusion criteria
The GTR included patients (males and females of any age) with congenital GT defined as patients with lifelong bleeding tendency characterized by impaired or absent platelet aggregation to physiological stimuli, and prolonged bleeding time or prolonged platelet function analyzer closure time.
Optional diagnosis criteria were impaired clot retraction, quantitative or qualitative evaluation of glycoprotein IIb/IIIa receptors including flow cytometry and identification of gene defects. Platelet refractoriness (refractoriness) and the presence of platelet antibodies (AB) were coded initially and assessed periodically as deemed important by the investigator. Patients with acquired thrombasthenic states caused by autoimmune disorders or medications were excluded.
Definitions
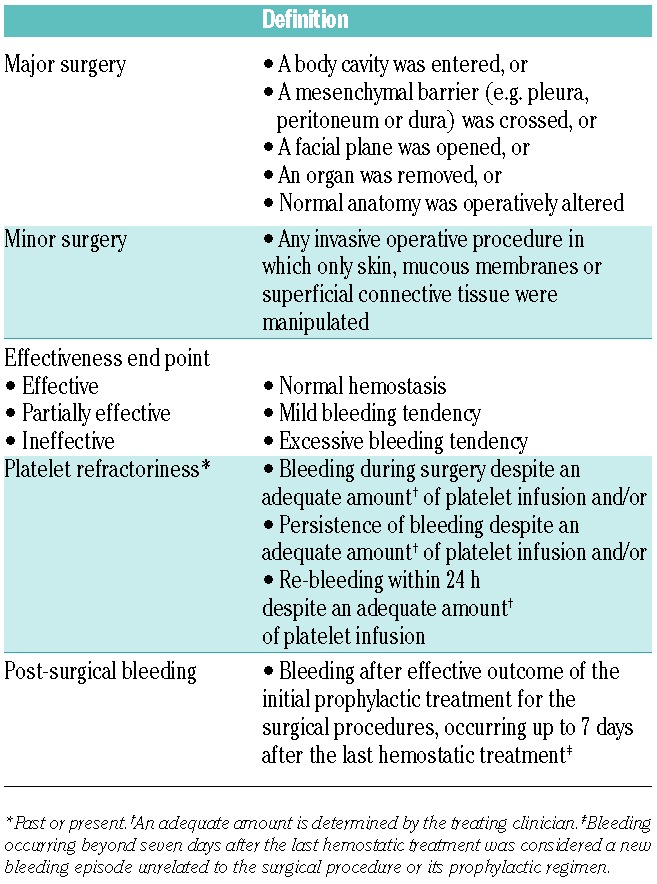
Surgical procedures were categorized post hoc as major or minor (Table 1).
Treatment was categorized into five treatment groups: 1) rFVIIa alone; 2) rFVIIa with AF (rFVIIa+AF); 3) platelets alone or with AF (P±AF); 4) combined use of rFVIIa and platelets with or without AF (rFVIIa+P±AF); and 5) AF alone (AF). AF were usually given during the pre-, intra- and post-operative periods. The history of AB and/or refractoriness was categorized as follows: no AB/refractoriness, AB+refractoriness, refractoriness only or AB only. However, as tests for AB may not have been available at all centers, AB may also have been present in some cases in the refractoriness-only group.
Definitions used for treatment effectiveness, past or present refractoriness to platelet transfusions and post-surgical bleeding are summarized in Table 1.
Safety assessments included frequency of adverse events (AEs) and serious AEs (SAEs) during and after treatment.
Table 1.
Post hoc definitions of surgical category, and definitions of treatment effectiveness, platelet refractoriness and post-surgical bleeding.
Statistical methods
The analysis of treatment effectiveness was based on all treatment-allocated patients for whom the effectiveness end point was known. All patients and treatment episodes were included in the safety analysis. All evaluations were summarized with numerical variables (mean, standard deviation, median, maximum and minimum), while categorical variables were summarized as numbers and percentages. No formal statistical comparisons were performed.
Results
Recruitment into the GTR and effectiveness and safety datasets
Data were collected from 1086 admissions (883 for bleeding and 204 for surgery; one admission was considered as both); 218 patients with GT were enrolled from 45 sites in 15 countries from Europe, Africa, Asia and North America (Figure 1). All 1086 admissions were included in the safety analysis. However, for the effectiveness evaluations, review of the data showed that four admissions were AEs and not treatments for bleeds and hence were excluded from the evaluations (Figure 1). In addition, it was found that on several occasions multiple admissions contained information on the same bleeding episode; in these instances, the admissions were collapsed and one designated as the index event. In total, 64 admissions for bleeds were considered to be linked events and were collapsed into 22 index events. Lastly, as two admissions previously considered as bleeding episodes were in fact minor surgeries, these were transferred for analysis of the surgical procedures (Figure 1). In total, the post hoc secondary effectiveness analysis was performed using data from 829 admissions for non-surgical bleeding, and 206 surgical admissions (Figure 1).
Figure 1.
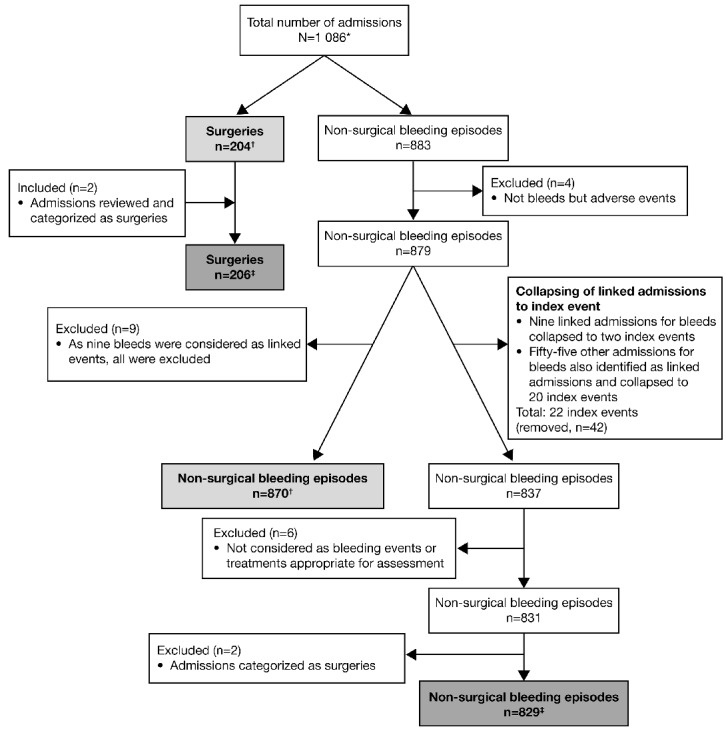
Chart depicting the flow of admissions into the GTR and the data used for primary and secondary analysis. *One admission was considered both a surgical procedure and a bleeding episode. †Data used for the primary effectiveness analysis (performed in 2012). ‡Data used for the secondary data analysis (performed in 2014). All data were included in the safety analysis.
Clinical and demographic characteristics of the GTR population undergoing surgical procedures and types of procedures
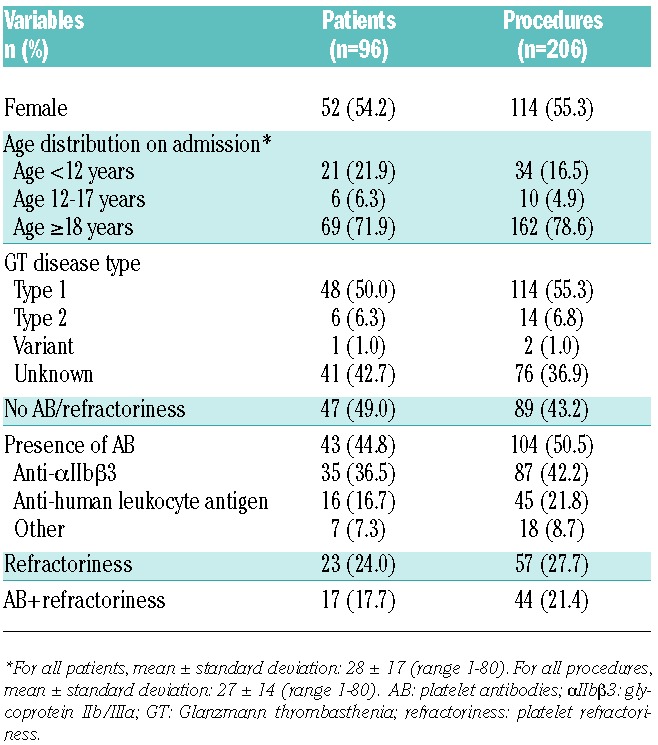
The clinical and demographic characteristics of patients entered into the GTR who underwent surgery are provided in Table 2. Of 206 invasive procedures reported in 96 patients, 169 (in 78 patients) were minor and 37 (in 29 patients) were major. The majority of surgical procedures (78.6%) (Table 2) were performed in adults aged 18 years of age or over (29 major, 133 minor). Only 21.4% of procedures (8 major and 36 minor) were performed in children under 18 years of age (Table 2).
Table 2.
Clinical and demographic characteristics of the GTR population having surgical procedures.
Minor procedures: treatment and outcome
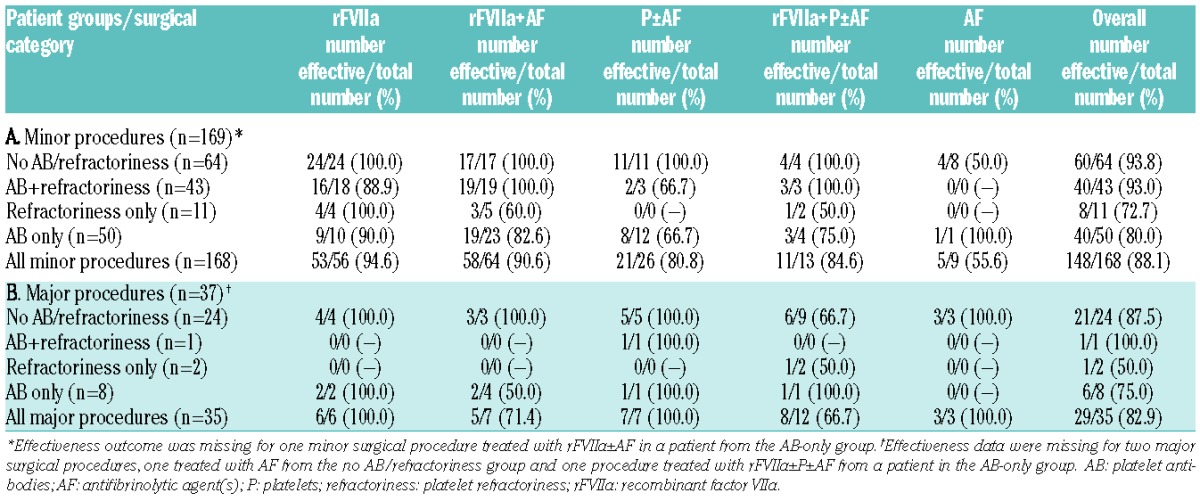
Among the 169 minor surgical procedures performed, the most common was dental (n=134, 79.3%), followed by endoscopy (n=11, 6.5%) and nasal procedures (n=8, 4.7%). Most of the minor procedures were treated with rFVIIa, either alone (56/169; 33.1%) or with AF (65/169; 38.5%) (Table 3). Data on the number of minor procedures rated as “effective” (see definition in Table 1) for the different treatment modalities (overall and stratified according to the status of platelet antibodies and platelet refractoriness) are shown in Table 3.
Table 3.
Treatments rated “effective” (see Table 1 for definition) stratified according to surgical category and to the history of platelet antibodies and/or platelet refractoriness.
Use of rFVIIa
Data on rFVIIa use were available for 133 minor procedures treated with rFVIIa, rFVIIa+AF or rFVIIa+P±AF. For all minor procedures, median rFVIIa use was 100 μg/kg (range 3.6–300 μg/kg), with very similar median values (range 90–117 μg/kg) for the different patient groups (Online Supplementary Table S1). The median dosage interval was most commonly 2 h across groups, with a median of 2.0–3.0 doses used. The median cumulative rFVIIa dose range and duration of treatment used for minor surgeries were found to be more variable across the groups (Online Supplementary Table S2).
Use of platelets
Platelets±AF were used in 26 minor procedures, with 80.8% of treatments overall being rated effective (Table 3). This treatment was rated partially effective in one of three procedures in the AB+refractoriness group and four of 12 in the AB-only group. For the 13 minor procedures treated with rFVIIa+P±AF, 84.6% of procedures were rated effective overall (Table 3).
Ineffective treatments
Only three of the 169 minor procedures were rated “ineffective.” Two were in the AB-only group (rFVIIa+AF and rFVIIa+P±AF) and one in the no AB/refractoriness group (AF only). Post-surgical bleeding (as defined in Table 1) was reported following rFVIIa treatment for two minor dental procedures at two and three days after the last dose of rFVIIa; both were successfully treated with additional rFVIIa.
Major procedures: treatment and outcome
Among the 37 major surgical procedures performed, the most common were gastrointestinal and orthopedic (n=9, 24.3% each). Major procedures were treated most frequently with rFVIIa+P±AF (n=13, 35.1%). Most of the major procedures were in the no AB/refractoriness group (n=25, 67.6%), followed by nine (24.3%) in the AB-only group, with only one (2.7%) and two (5.4%) major procedures in the AB+refractoriness and refractoriness-only groups, respectively. Effectiveness ratings for the different treatment modalities for major procedures (overall and stratified according to the status of platelet antibodies and platelet refractoriness) are shown in Table 3.
Use of rFVIIa
Data on rFVIIa use were available for analysis in 25 major procedures treated with rFVIIa, rFVIIa+AF or rFVIIa+P±AF. For all major procedures, the median rFVIIa dose was 90 μg/kg (interquartile range 90–92, range 25–240), with relatively similar median values ranging from 90–142 μg/kg reported for the different patient groups (Online Supplementary Table S1). The dosage interval was 2–6 h across the groups (median 3 h) with wider variation in the number of doses (median 2.0–14.5) (Online Supplementary Table S1). Information on the cumulative rFVIIa dose is provided in Online Supplementary Table S2.
Use of platelets
The effectiveness outcome for cases treated with platelets (P±AF, rFVIIa+P±AF) was available in 19 major surgical procedures (Table 3). Of these, all seven major procedures treated with P±AF, and eight of 12 treated with rFVIIa+P±AF, were effective, with three treated with rFVIIa+P±AF partially effective.
Ineffective treatment
Only one (in the no AB/refractoriness group, treated with rFVIIa+P±AF) of the 37 major procedures was rated “ineffective.” No post-surgical bleeding (as defined in Table 2) was reported following rFVIIa and/or platelet treatment for major procedures.
Safety outcome
Forty-six AEs in 18 patients were reported in the registry, of which 15 were SAEs. In a total of 15 AEs (in 9 patients) the treatment included rFVIIa. Seven SAEs in the GTR were entries for AEs only, all unrelated to rFVIIa treatment, and were as follows: six SAEs in one patient (angina, alternation of clinical status, hyperthermia, face and eyelid edema, and staphylococcus infection), and one SAE in a second patient (viral meningitis). Table 4 summarizes the AEs reported in treated patients.
Table 4.
Summary of adverse events reported in treated patients in the GTR*
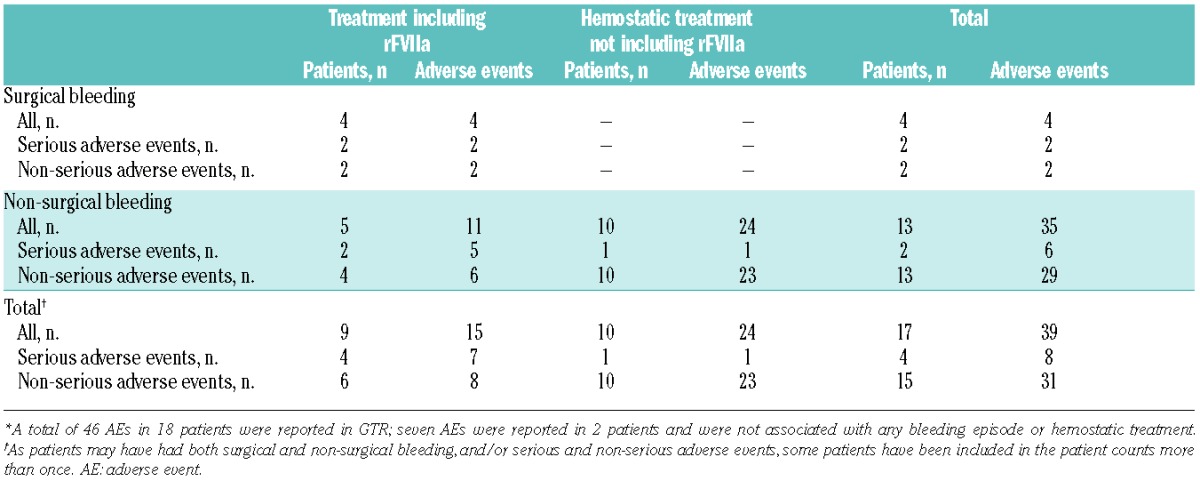
For surgery (Table 4), two SAEs and two non-serious AEs were reported in 4 rFVIIa-treated patients. One thrombotic event (deep vein thrombosis) developed in an adult female with platelet refractoriness treated with rFVIIa+P+AF after an emergency laparotomy (ovarian cyst and hematoma with bilateral ureteral compression; major surgery); the event was non-fatal and judged by the investigator to be probably or possibly related to rFVIIa. The second SAE was rectorrhagia in an adult male who underwent a colonoscopy (minor surgery) and received rFVIIa+AF, and was considered unlikely to be related to rFVIIa, and the patient recovered. The two non-serious AEs were one allergic reaction attributed to platelet transfusion in an adult male without platelet refractoriness or antibodies treated with rFVIIa+P+AF (minor surgery), and one pyrexia in a male child without platelet antibodies or refractoriness treated with rFVIIa+P+AF (major surgery); both were considered unlikely to be related to rFVIIa and the patients recovered completely.
Discussion
The GTR data reported here come from the largest observational study on GT patients and include information on the management of invasive procedures; the shortcomings of the previous survey7 are addressed in that the use of hemostatic agents other than rFVIIa is now included.
Use of rFVIIa
In general, rFVIIa, either alone or with AF, was used more frequently than platelets (P±AF), not only in patients with antibodies and/or refractoriness, but also in patients without either. The GTR results further indicate that rFVIIa has a good safety profile in GT patients.
Assessment of rFVIIa dosage and dose schedule in this large registry suggests that in minor procedures, rFVIIa dosage of 90–140 μg/kg given at approximately 2-h intervals for two or more doses (until effective hemostasis), with the first dose given immediately pre-operatively could be used. This is similar to the regimen we previously suggested for bleeding episodes (rFVIIa ≥80 μg/kg at intervals of 2.5-h or less for three or more doses),7 and is also similar to standard rFVIIa dosing in patients with congenital hemophilia A or B with inhibitors.
In major procedures, a rFVIIa dosage of 90 μg/kg was used most frequently. The median dose interval as a group was slightly longer (3 h), likely related to the tendency for dosing intervals to lengthen over time when duration of treatment is longer. For major procedures, rFVIIa at 90 μg/kg or more at intervals of 2.5-h or less should be used, at least at the beginning. The subsequent number of doses required would have to be determined by the clinical situation and dosing should be continued until hemostasis is assured.
Use of platelets
As expected, platelet transfusion was effective in covering surgical procedures in patients without platelet antibodies and refractoriness, and less effective in patients with platelet antibodies and/or platelet refractoriness. Of interest is that some of the procedures in the AB-only group had a successful outcome with P±AF, and was also successful in three out of four procedures in the AB+refractoriness group (Table 4). A possible explanation of this finding is that some patients with a history of AB and/or refractoriness were no longer refractory, or that antibodies were no longer present, at the time of surgery. It may also be due to patients with HLA antibodies only being given HLA-matched platelets; but even if HLA-unmatched platelets are given, previous data suggest that more than 50% of patients may not manifest refractoriness to platelet transfusion.9–11 These observations suggest that even in patients with a history of AB and/or refractoriness, treatment with platelets may be attempted when other agents are ineffective or not available.7 Successful platelet transfusion following removal of platelet antibodies by plasmapheresis12,13 or immunoadsorption14 has also been reported.
Combined use of platelets and rFVIIa
One of the questions raised previously is whether combined use of platelets and rFVIIa (rFVIIa+P±AF) has an advantage over rFVIIa (alone or with AF) or P±AF.7 Interestingly, rFVIIa+P±AF was less effective than rFVIIa and rFVIIa+AF. It is possible that rFVIIa+P±AF was used in patients with more difficult or challenging clinical situations; additional hemostatic agent(s) might have been added to the regimen when effectiveness was in doubt while using another hemostatic agent(s). In support of this supposition, two (one minor, one major) of the four treatment failures were in the rFVIIa+P±AF treatment group.
Use of AF without platelets or rFVIIa
The successful use of AF without platelets or rFVIIa was reported in a small number of procedures, and may be due to AF treatment being started with the intention to use other systemic hemostatic agent(s) should hemostasis not be achieved, but that these agents were subsequently considered not to be needed. The less controlled data collection process compared with actual clinical trials makes it inherently difficult to interpret or directly compare effectiveness between treatments. We do suggest, however, that AF should not be recommended as sole therapy, particularly for major procedures, without the backup availability of rFVIIa or platelet concentrates.
Safety
One potential concern regarding the use of rFVIIa in GT patients is whether rFVIIa is thrombogenic. The data reported here suggest that all of the systemic hemostatic agents used were safe for surgical procedures in GT patients. Of the 206 procedures performed in 96 patients, only one thromboembolic event was reported; this is in contrast to the higher thromboembolism rate, particularly arterial thrombosis, attributed to rFVIIa when used off-label in patients without a hemostatic defect.15–17
Limitations
The major limitation of the study reported here is that data were not obtained with defined treatment protocol(s) in a randomized manner, and treatment effectiveness and safety were not assessed at multiple consistent, predefined time points. Furthermore, due to the common use of multiple agents in GT, and delays in obtaining platelets, it is particularly difficult to ascribe effectiveness to any one or more products. In addition, as the coding of history of refractoriness or antibodies was performed at first admission and when the investigator was considered appropriate, the lack of documentation of specific antibody testing or refractoriness at the time of a particular episode limits the analysis (particularly of use of platelet-based regimens).
Formal clinical trials are unfortunately hindered by the rarity of GT. The data reported here represent the largest dataset available in the literature, including those in other databases.7,18,19 Furthermore, these registry data represent real-life clinical practices and the standards of care at the participating sites. Narrative information, as provided in the GTR, often provides additional useful insights that might differ from the coding of effectiveness at an earlier time point after surgery.
Conclusions
The number of surgical procedures performed in GT patients reported here represents the largest experience so far available in the literature. In general, both rFVIIa and platelets were observed to be effective, and rFVIIa was safe for both minor and major procedures in patients with or without antibodies and/or refractoriness.
The dosage and schedule required for surgery was very similar to the optimal regimen reported for bleeds in previous studies, being 90–140 μg/kg at intervals of 2.5 h or less for two or more doses for minor surgery, and more doses for major surgery, until hemostasis is achieved.7,20,21 The frequent use of this agent in GT patients without platelet antibodies or refractoriness suggests some investigators used this as the first-line treatment for their GT patients.
Acknowledgments
The authors would like to thank all the investigators and their participating patients for making the GTR a success. Editorial assistance was provided by Sharon Eastwood (PAREXEL) and the study was financially supported by Novo Nordisk Health Care AG. The authors would also like to thank Soraya Benchikh el Fegoun and Jens Bjerre (Novo Nordisk Health Care AG) for their comments and advice, and Anders Rosholm (Novo Nordisk Health Care AG) for statistical support, and Yves Laurian, who acted as an expert panel member for the GTR until 2008.
Footnotes
The online version of this article has a Supplementary Appendix.
GTR Participating Investigators
Algeria: Grifi Fathia, Sidi Mansour Nourredine, Mesli Naïma, Hamdi Selma, Belhani Meriem, Touhami Hadj; Austria: Max Heistinger, Paul Alexander Kyrle; Belgium: Christel Van Geet, Veerle Labarque; Bulgaria: Angelina Stoyanova, Katya Sapunarova; France: Caroline Oudot, Roseline d’Oiron, Gruel Yves, Albert Faradji, Achille Aouba, Nathalie Trillot, Alain Marquès-Verdier, Claire Pouplard; Germany: Rainer Zotz, Roswith Eisert (replaced by Ingvild Birschmann), Mario von Depka Prondzinski, Maximillian Kirchmaier, Markus Rieke, Daniele Pillitteri; Hungary: Agota Schlammadinger, Csongor Kiss, Laszlo Nemes; Italy: Gavino Piseddu, Giovanni Di Minno, Antonio Coppola, Paola Giordano, Elisabetta Sacchi, Michele Schiavulli; The Netherlands: Paula Frouke Ypma, Meijer Karina, Maria Kruip, Pieter Kamphuisen, Britta Laros Van Gorkom, Karly Hamulyak, Rienk Yde Johan Tamminga; Pakistan: Tahir Shamsi, Munira Borhany; Spain: Rosario Perez Garrido, Victor Jiménez-Yuste; Sweden: Erik Berntorp, Karin Knobe; Switzerland: Dimitrios Tsakiris, Brigitte Brand; UK: John D Grainger, Kate Khair, Jayashree Motwani, Paula Bolton-Maggs; USA: Marcella Torres.
Funding
Novo Nordisk Health Care AG was the study sponsor of the GTR and was responsible for its conduct, including engaging an external expert panel to provide advice and funding PAREXEL International to manage study operations. Novo Nordisk Health Care AG also supported several authorship meetings held to plan and discuss the manuscript and its content.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bellucci S, Caen J. Molecular basis of Glanzmann’s Thrombasthenia and current strategies in treatment. Blood Rev. 2002;16(3):193–202. [DOI] [PubMed] [Google Scholar]
- 2.George JN, Caen JP, Nurden AT. Glanzmann’s thrombasthenia: the spectrum of clinical disease. Blood. 1990;75(7): 1383–1395. [PubMed] [Google Scholar]
- 3.Toogeh G, Sharifian R, Lak M, Safaee R, Artoni A, Peyvandi F. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004;77(2):198–199. [DOI] [PubMed] [Google Scholar]
- 4.Fiore M, Firah N, Pillois X, Nurden P, Heilig R, Nurden AT. Natural history of platelet antibody formation against αIIbβ3 in a French cohort of Glanzmann thrombasthenia patients. Haemophilia. 2012;18(3):e201–e209. [DOI] [PubMed] [Google Scholar]
- 5.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008; 142(3):348–360. [DOI] [PubMed] [Google Scholar]
- 6.Santoro C, Rago A, Biondo F, et al. Prevalence of allo-immunization anti-HLA and anti-integrin1 αIIbβ3 in Glanzmann Thromboasthenia patients. Haemophilia. 2010;16(5):805–812. [DOI] [PubMed] [Google Scholar]
- 7.Poon MC, D’Oiron R, Von Depka M, et al. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann’s thrombasthenia: results of an international survey. J Thromb Haemost. 2004;2(7):1096–1103. [DOI] [PubMed] [Google Scholar]
- 8.Poon MC, Zotz R, Di Minno G, Abrams ZS, Knudsen JB, Laurian Y. Glanzmann’s thrombasthenia treatment: a prospective observational registry on the use of recombinant human activated factor VII and other hemostatic agents. Semin Hematol. 2006;43(1 Suppl 1):S33–S36. [DOI] [PubMed] [Google Scholar]
- 9.Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. The Trial to Reduce Alloimmunization to Platelets Study Group. N Engl J Med. 1997;337(26):1861–1869. [DOI] [PubMed] [Google Scholar]
- 10.Brand A, Claas FH, Voogt PJ, Wasser MN, Eernisse JG. Alloimmunization after leukocyte-depleted multiple random donor platelet transfusions. Vox Sang. 1988;54(3): 160–166. [DOI] [PubMed] [Google Scholar]
- 11.Legler TJ, Fischer I, Dittmann J, et al. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol. 1997;74(4):185–189. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Yoshida H, Hatoyama H, et al. Antibody removal therapy used successfully at delivery of a pregnant patient with Glanzmann’s thrombasthenia and multiple anti-platelet antibodies. Vox Sang. 1991; 61(1):40–46. [DOI] [PubMed] [Google Scholar]
- 13.Vivier M, Treisser A, Naett M, et al. Glanzmann’s thrombasthenia and pregnancy. Contribution of plasma exchange before scheduled cesarean section. J Gynecol Obstet Biol Reprod (Paris). 1989;18(4):507–513. [PubMed] [Google Scholar]
- 14.Martin I, Kriaa F, Proulle V, et al. Protein A Sepharose immunoadsorption can restore the efficacy of platelet concentrates in patients with Glanzmann’s thrombasthenia and anti-glycoprotein IIb-IIIa antibodies. Br J Haematol. 2002;119(4):991–997. [DOI] [PubMed] [Google Scholar]
- 15.Diringer MN, Skolnick BE, Mayer SA, et al. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010;41(1):48–53. [DOI] [PubMed] [Google Scholar]
- 16.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352(8):777–785. [DOI] [PubMed] [Google Scholar]
- 17.O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295(3):293–298. [DOI] [PubMed] [Google Scholar]
- 18.Chitlur M, Ewing N, Karut EH, Cooper DL. Recombinant factor VIIa (rFVIIa) use in Glanzmann’s thrombasthenia (GT) and other platelet disorders (OPDS): Haemophilia and Thrombosis Research Society (HTRS) registry data [abstract]. J Thromb Haemost. 2011;9(Suppl 2):340. [Google Scholar]
- 19.Lak M, Scharling B, Blemings A, et al. Evaluation of rFVIIa (NovoSeven) in Glanzmann patients with thromboelastogram. Haemophilia. 2008;14(1):103–110. [DOI] [PubMed] [Google Scholar]
- 20.Di Minno G, Coppola A, Di Minno MN, Poon MC. Glanzmann’s thrombasthenia (defective platelet integrin αIIb-β3): proposals for management between evidence and open issues. Thromb Haemost. 2009;102(6):1157–1164. [DOI] [PubMed] [Google Scholar]
- 21.Poon MC, Demers C, Jobin F, Wu JW. Recombinant factor VIIa is effective for bleeding and surgery in patients with Glanzmann thrombasthenia. Blood. 1999; 94(11):3951–3953. [PubMed] [Google Scholar]


