Abstract
Ruxolitinib and lenalidomide may target distinct clinical and pathological manifestations of myelofibrosis and prevent therapy-related worsening of blood cell counts. To determine the efficacy and safety of the combination in patients with myelofibrosis, patients were given 15 mg ruxolitinib orally twice daily in continuous 28-day cycles, plus 5 mg lenalidomide orally once daily on days 1–21. Thirty-one patients were treated, with a median followup of 28 months (range, 12 – 35+). Due to failure to meet the predetermined efficacy rules for treatment success the study was terminated early. Simultaneous administration of ruxolitinib and lenalidomide was difficult: 20 of the 23 dose interruptions occurred within the first 3 months of therapy. Lenalidomide was interrupted in all 20 cases. Fourteen patients (45%) were completely off lenalidomide within 3 months of initiation. Responses were noted in 17 patients (55%). The median time to response was 1.8 months (range, 0.4 – 31). All responses were International Working Group for Myelofibrosis Research and Treatment–defined clinical improvement in palpable spleen size. One spleen responder also met the criteria for clinical improvement in hemoglobin. The response rate was higher (73%) among patients who did not require early dose interruption than among those who required early interruption (45%). Improvements in bone marrow fibrosis and serial reductions in lactate dehydrogenase >50% were noted in 17% and 50% of evaluable responders, respectively. Alternate approaches such as sequential dosing need to be evaluated when considering novel combination strategies for myelofibrosis. This trial was registered with clinicaltrials.gov identifier: NCT01375140
Introduction
Myeloproliferative neoplasms, including primary myelofibrosis (MF) and MF evolving from a pre-existing myeloproliferative neoplasm (post-polycythemia vera-MF and post-essential thrombocythemia-MF), are myeloid progenitor cell–derived conditions characterized by bone marrow fibrosis, osteosclerosis and pathological angiogenesis.1,2 Over the last 3 decades many therapies have been evaluated in MF, including JAK inhibitors (e.g., ruxolitinib, fedratinib, pacritinib), immunomodulatory drugs (e.g., thalidomide, lenalidomide), DNA methyltransferase inhibitors (e.g., 5-azacytidine, decitabine), chemotherapeutic agents (e.g, hydroxyurea, cladribine), and biologic-response modifiers (e.g., androgens, erythropoietin).3 Unfortunately, no one therapy has demonstrated an ability to produce complete remissions or even rapid and consistent reversal of fibrosis in many patients with MF.
The discovery of the JAK2V617F mutation in MF, resulting in constitutive activation of the JAK-STAT pathway, led to the development of the potent and selective JAK1/2 inhibitor ruxolitinib. Ruxolitinib greatly reduces the signs and symptoms associated with MF, reducing spleen size, promoting weight gain, improving performance status and controlling constitutional symptoms, leading to prolonged survival; in selected patients it can, after prolonged therapy, decrease the degree of marrow fibrosis.4–7 Based on the positive outcomes in two phase III studies (COMFORT-I, COMFORT-II)8,9 ruxolitinib was approved for use in the USA in patients with intermediate- or high-risk MF. While ruxolitinib significantly abrogates splenomegaly and constitutional symptoms, it has little to no effect on improving erythropoiesis in patients with MF. Furthermore, some patients achieve a less than optimal response to ruxolitinib while others lose a response after some time. Alternative therapeutic approaches, including the development of rational combinations with ruxolitinib to concurrently target other potential drivers of MF may overcome these therapeutic hurdles.
Thalidomide, lenalidomide and pomalidomide are immunomodulatory agents that have been demonstrated to improve anemia, thrombocytopenia and splenomegaly in selected patients with MF.10–18 Lenalidomide is a derivative of thalidomide and is more effective and less toxic than thalidomide.19 This improved safety profile has made lenalidomide an attractive choice for combination regimens in multiple myeloma,20,21 myelodysplastic syndrome22 and high-risk myelodysplastic syndrome/acute myeloid leukemia.23,24 In MF, lenalidomide alone or in combination with prednisone elicits response rates of approximately 30%,13,14,18 and was particularly effective at improving erythropoiesis. Furthermore, in a study of 40 patients treated with lenalidomide plus prednisone, 91% and 100% of evaluable patients who had a clinical response had reductions in bone marrow fibrosis and JAK2 allele burden, respectively.14 These types of improvements warrant further evaluation of lenalidomide in combination with other active drugs in MF.
The combination of lenalidomide and ruxolitinib may target distinct clinical and pathological manifestations of MF as well as prevent therapy-related worsening of blood cell counts, allowing proper administration of ruxolitinib in high-risk MF patients with significant cytopenias at presentation. We, therefore, sought to evaluate the efficacy and safety of this combination in patients with MF in a phase 2 study. To our knowledge this is the first published report of a clinical trial testing a JAK2 inhibitor in combination with another agent.
Methods
Eligibility
Patients ≥18 years of age with a diagnosis of MF requiring therapy;25 including previously treated, relapsed, refractory, or if newly diagnosed, with intermediate or high risk according to the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) dynamic-international prognostic scoring system (D-IPSS) criteria (risk factors: age >65 years, presence of constitutional symptoms, hemoglobin <10 g/dL, white blood count >25×109/L, and circulating blast cells ≥1%).26 Other eligibility criteria included Eastern Cooperative Oncology Group performance status ≤2; serum creatinine ≤2.0 mg/dL; serum direct bilirubin <2.0 times the upper limit of the normal range; blood transaminase level ≤3 times the upper limit of the normal range; absolute neutrophil count ≥1.0×109/L and platelet count ≥50×109/L. Patients with thromboembolic disease (i.e., deep vein thrombosis or pulmonary embolism) within 6 months of study entry or known hypercoagulability syndrome were not eligible. Patients must not have been treated with growth factors, cytotoxic chemotherapeutic agents, corticosteroids, or experimental therapy for at least 14 days or five half-lives, whichever was longer, prior to study enrollment. Women of childbearing age must have had a negative pregnancy test and all patients were required to use effective methods of contraception during study participation. All patients signed an informed consent form and the protocol was approved by the Institutional Review Board at MD Anderson Cancer Center.
Treatment schedule
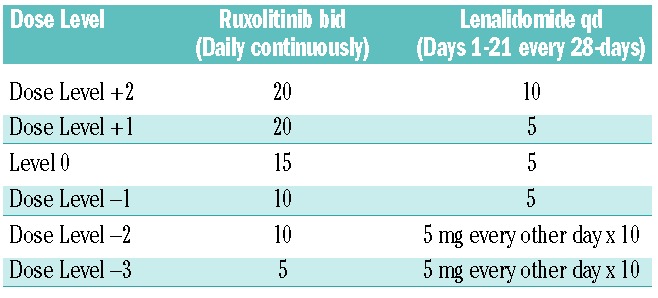
Ruxolitinib and lenalidomide were given in 28-day cycles, with 15 mg ruxolitinib given orally twice daily continuously and 5 mg lenalidomide given orally daily on days 1–21 of each cycle. In our previous phase 2 study of lenalidomide given at a dose of 10 mg/day on days 1–21 of a 28-day cycle in combination with a tapering dose of prednisone for the first three cycles, there was an overall response rate of 30% for anemia and 42% for splenomegaly. However, grade 3 to 4 neutropenia, anemia, and thrombocytopenia occurred in 23 (58%), 17 (42%) and 5 patients (13%), respectively. Twenty-four patients (60%) had their lenalidomide dose reduced to dose level −1 (5 mg/day on days 1–21) predominantly because of myelosuppression. These data suggested that the 10 mg/day dose of lenalidomide would result in prohibitive myelosuppression when combined with ruxolitinib. A dose of 5 mg/day dose of lenalidomide was, therefore, chosen for this combination. The doses of ruxolitinib and lenalidomide could be reduced based on adverse events, or escalated in patients with proliferative disease (Table 1). Ruxolitinib and lenalidomide were continued for at least 6 months unless significant toxicity was observed, to account for the delayed time to response observed with biologic agents. If drug-related grade 3 or 4 non-hematologic toxicity was attributable to one or both of the drugs, that particular drug(s) was interrupted for the remainder of the cycle. The offending agent could be resumed at the next lower dose level in subsequent cycles after resolution of toxicity to grade ≤1. Patients who developed a platelet count ≤35×109/L or an absolute neutrophil count ≤0.5×109/L during a cycle of therapy had one dose level reduction for subsequent cycles. Patients could be given the next higher dose level if: no dose interruptions or dose reductions were needed in the preceding two cycles; the current cycle and prior cycle were not delayed due to toxicity; the platelet count was ≥100×109/L and absolute neutrophil count was ≥1.0×109/L; they did not develop new onset transfusion dependency; and if they had sub-optimal benefit defined as <50% reduction in spleen length from baseline and/or no improvements in cytopenia. Low-dose aspirin prophylaxis was recommended for patients with a platelet count >75×109/L to reduce the risk of thrombosis. Details of the patients’ evaluation on study are included in the Online Supplementary Data.
Table 1.
Dose modification table.
Study design
This study was designed as a two-stage (MiniMax), prospective, single center, phase II trial. The primary efficacy endpoint of the trial was IWG-MRT-defined objective response rate, including complete remission, partial remission or clinical improvement, within 6 months of study initiation.27 The combination would be considered unpromising if ≤35% of patients achieved an objective response, which in general corresponds to the objective response rate with single-agent ruxolitinib.8,9 The smallest proportion of patients achieving a response that would support subsequent studies was 50%. Given the response rates stated above, if the probability of inappropriately accepting a poor therapy is 10% (α=0.1), a total sample size of 49 patients would result in 80% power (β=0.2).
The study included one interim analysis after 31 evaluable patients had been observed for at least 6 months to permit early stoppage if there was strong evidence that the study regimen was inactive. If ten or fewer patients responded to the combination therapy after being treated for 6 months or discontinued due to excessive toxicity or lack of efficacy, then the study would be terminated. Those patients who stopped receiving one of the two study drugs due to safety reasons were counted as failures in the efficacy analysis. If 11 or more treatment successes were observed in these 31 patients, an additional 18 patients would be accrued. After 49 patients had been accrued, if 21 or fewer patients responded to the combination therapy or discontinued due to excessive toxicity, the therapy would be declared ineffective. The patients were simultaneously monitored for toxicity.
Results
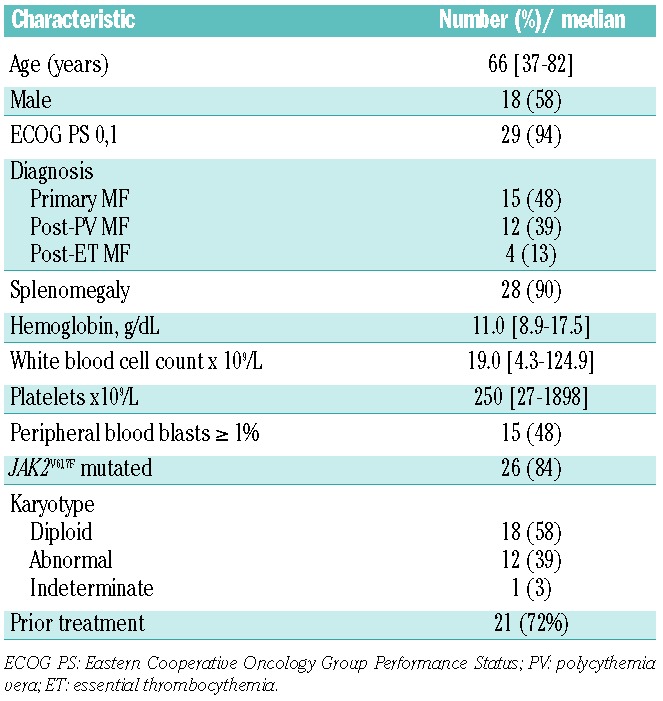
Thirty-one patients with primary, post-polycythemia vera-MF or post-essential thrombocythemia-MF with anemia were enrolled. The patients’ characteristics are summarized in Table 2. Twenty-one patients (68%) had received a median of two prior therapies (range, 1–3) for MF. Eleven patients (35%) had received hydroxyurea alone, five (16%) had received anagrelide and hydroxyurea, seven (23%) had received interferon (pegylated-interferon in 3 cases and interferon-α in 4), three (10%) had received DNA methyltransferase inhibitors, two (6%) had received thalidomide, one had received danazol, and five had received a variety of investigational therapies (including 2 investigational agents in 1 patient): AZD1840 (JAK1/2 inhibitor), LY2784544 (selective JAK2 inhibitor), IPI-926 (oral hedgehog inhibitor), AB0024 (humanized monoclonal antibody against lysyl oxidase-like 2), one patient received both BMS911543 (selective JAK2 inhibitor) and CYT387 (JAK1/2 inhibitor). Of 30 patients with available pretreatment cytogenetic analyses, 14 (40%) had an abnormal karytotype, including del(20) in seven patients, del(13) in three, del(6) in two, del(5)/del(7) in one, and inv(9) in one. Six patients were defined as high-risk, 11 as intermediate-2 risk and 14 as intermediate-1 risk according to the D-IPSS criteria.28
Table 2.
Patients’ characteristics (n=31).
Concomitant administration of ruxolitinib and lenalidomide
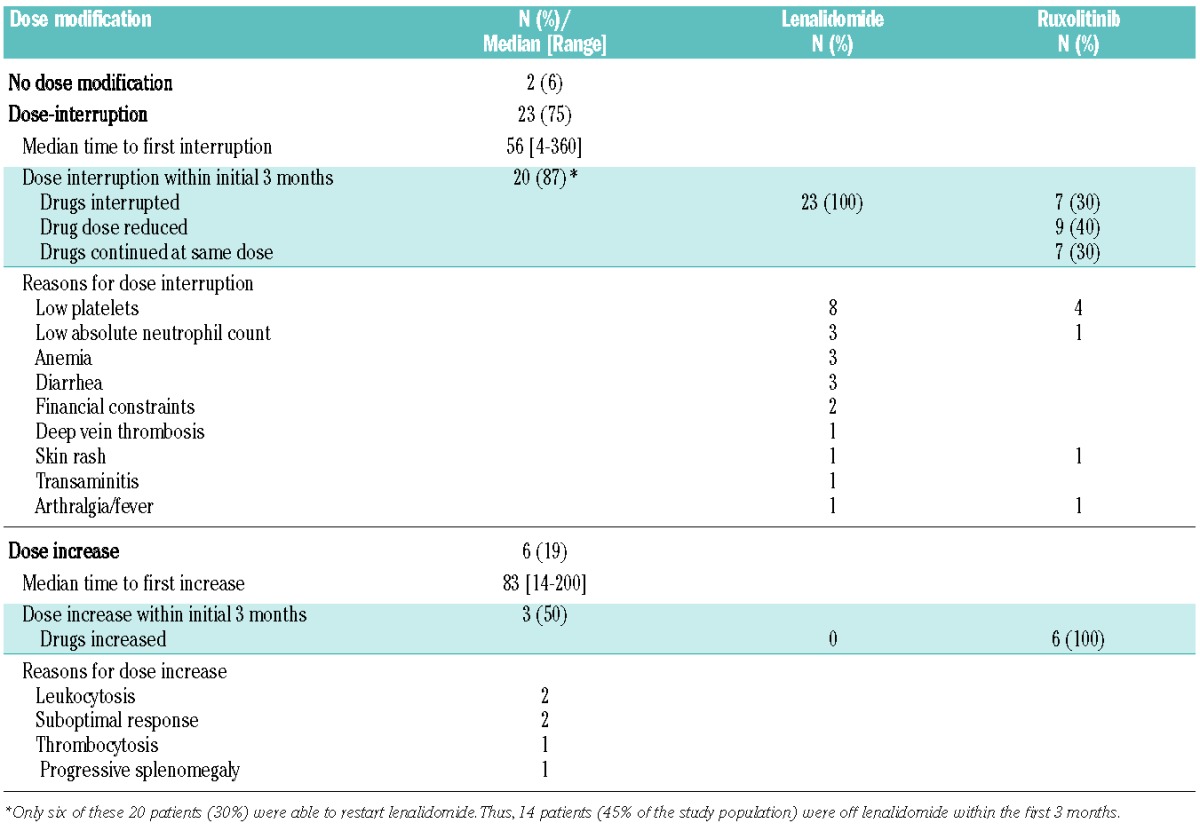
Simultaneous administration of ruxolitinib and lenalidomide was difficult: 23 patients required a dose interruption with or without dose decrease due to toxicity. On the other hand, six patients required a dose increase of one or both drugs due to lack of satisfactory efficacy. Dose-interruptions occurred early, with 20 of the 23 dose interruptions occurring within 3 months of initiation of the combination. Only six of these 20 patients were able to restart lenalidomide. Thus, 14 patients (45% of all the study patients) were completely off lenalidomide within 3 months of initiation of therapy. The details of dose adjustments/interruptions and the reasons for dose adjustment/interruption are given in Table 3.
Table 3.
Dose modifications on protocol.
Response to ruxolitinib and lenalidomide therapy
Twenty-five patients (81%) remain alive after a median follow-up of 28 months (range, 12–35+ months); 16 patients remain on study with a median time on study of 26 months (range, 13–35+). The median time on study was 20 months (range, 1–35+ months) and the median number of cycles administered was 22 (range, 1–37+ cycles). Of the 31 patients enrolled, 17 patients (55%) achieved an IWG-MRT-defined response. No complete or partial responses were documented. All 17 responses included clinical improvement in palpable spleen size, including 100% spleen reduction (i.e. non-palpable spleen) in seven patients and ≥50% spleen reduction in ten patients. Responses occurred in ten of 21 (48%) previously treated patients and seven of ten (70%) untreated patients (P=0.28). Responses occurred in 17/28 (61%) JAK inhibitor-naïve patients and 0/3 patients previously treated with a JAK inhibitor. The two patients previously treated with thalidomide did not respond. The median time to clinical improvement in spleen size was 1.8 months (range, 0.4–31 months). Delayed responses were seen more frequently than would be expected with single agent ruxolitinib, with six of the responses occurring ≥6 months after initiation of therapy. Responses have been durable, with a median response duration of 19 months (range, 3–32+ months). Thus far, only two of the responders have come off study due to disease progression heralded by elevated bone marrow blasts (1 after 22 months and the other after 24 months of therapy). Among the patients who achieved clinical improvement in spleen size, one also achieved an IWG-MRT-defined clinical improvement in hemoglobin (sustained improvement in hemoglobin ≥2 g/dL that was maintained for >8 weeks). The time to clinical improvement in hemoglobin was 28 months and the response was maintained for 6 months. None of the patients had a baseline platelet count <100 × 109/L. Thus, none of the patients was evaluable for IWG-MRT-defined clinical improvement in platelet counts. The median time on study for all patients who responded was 28 months (range, 18–35+ months), with a median of 31 cycles (range, 21–37+ cycles). At this time, 15 of the patients who responded remain on therapy: 11 are taking both drugs and four are taking ruxolitinib only. The four patients who remain on study on ruxolitinib alone were taking the combination at the time of response; lenalidomide was subsequently discontinued in all four patients due to myelosuppression. As these four patients were on the combination at the time of response it is possible that they achieved therapeutic benefit from the addition of the lenalidomide. Furthermore, these patients continue to be monitored on study. Hence, we included these patients in the response and endpoint analysis.
Although an IWG-MRT-defined response was achieved in 17 patients, only seven patients met the predetermined definition of efficacy: response to the combination within 6 months of initiation without discontinuation of one of the study drugs. Six patients who achieved the response after 6 months and ten patients who had discontinued lenalidomide at the time of response due to drug-related toxicities were counted as failures in accordance with the predefined efficacy analysis. Since fewer than ten patients met the predetermined efficacy rules for treatment success the study was terminated after the accrual of 31 patients.
Impact of combination therapy on response
The ability to receive the combination of ruxolitinib and lenalidomide influenced the response rate. As noted in Table 3, only 11 patients did not require a dose interruption within 3 months of initiation of therapy. Eight of the 11 patients (73%) who did not require early dose interruption achieved a response as compared to 11 of 20 (45%) who required a dose interruption within the initial 3 months (P=0.26). The median time to response (2.5 months versus 1.4 months, P=0.5) and median response duration (22.1 months versus 16.7 months) were not significantly different between the patients who did not require early dose interruption and those who did. Similarly, the overall survival was not different between the two groups (28 versus 29 months).
Molecular, cytogenetic, bone marrow fibrosis, and cellularity changes
Of the 14 JAK2V617F-positive patients who responded, six had serial measurements of JAK2V617F allele burden available for review. Five patients (83%) had a reduction in the JAK2V617F allele burden from baseline and stable levels were noted in one patient. The reduction in the JAK2V617F allele burden was less than 50% in all cases. The median time to first documented reduction was 3 months. All five patients were on both drugs at the time of documented reduction.
A response was noted in 12 of 17 patients with diploid cytogenetics, and five of 14 patients with cytogenetic abnormalities. Of the five responders with aberrant cytogenetics at enrollment none achieved a cytogenetic remission. The median baseline European Myelofibrosis Network (EUMNET)29 fibrosis score among the 17 patients who responded was MF-2 (MF-3 in 4, MF-2 in 11, MF-1 in 1). Serial evaluation of bone marrow fibrosis was available in 12 of the 17 patients who responded. Only two of the 12 (17%) had a documented reduction in EUMNET fibrosis score, one from MF-2 to MF-1 after 27 months and the other from MF-2 to MF-0 after 20 months on therapy. Both of these patients had an initial interruption of lenalidomide but were able to restart at the same dose of lenalidomide and were taking both drugs at the time of documented improvement in bone marrow fibrosis. Furthermore, six of the 12 (50%) patients who responded and had assessable bone marrow specimens had reductions in bone marrow cellularity. The median bone marrow cellularity before treatment was 98% and the best median cellularity after treatment was 50%, including a >50% reduction in three of the patients. Serial reductions in lactate dehydrogenase levels were noted in 15 of the 17 (88%) patients who responded, including a >50% reduction in nine (53%). The median time to >50% reduction in lactate dehydrogenase was 8 months. A >50% reduction was noted in eight of 11 (73%) responders who continued on both drugs compared to only one of four patients who continued on ruxolitinib alone.
Toxicity
The most common non-hematologic toxicities involved the gastrointestinal system, including diarrhea in eight patients (grade 3 in 1), nausea and vomiting in three (all grade 1 or 2), abdominal pain in three (all grade 1 or 2) and constipation in three (all grade 1 or 2). Grade 3 or 4 myelosuppression was noted in 16 patients. Five patients experienced grade 3 or 4 non-hematologic toxicity (irrespective of attribution), including diarrhea, edema, transaminitis, bilirubinemia, and acute kidney injury. Two patients discontinued treatment due to drug-related toxicities including grade 2 persistent nausea and grade 3 diarrhea. One episode of lower extremity thrombosis was noted. Three deaths were documented (two on study and one after discontinuation of treatment) and were attributed to pneumonia in one patient, kidney failure in one, and possible stroke in one.
Of the 15 patients who have come off study, three did so because of concurrent disease (lymphoma, emphysema, and pericarditis with renal failure), two, who initially responded, because of disease progression, two because of myelosuppression (grade 4 anemia and grade 3 thrombocytopenia), three because of refractory disease, two because of toxicities (grade 2 persistent nausea and grade 3 diarrhea), one because of persistent and severe lower extremity cellulitis, one because of non-compliance, and one for financial reasons. Among the 16 patients who remain on study, ten are still taking both ruxolitinib and lenalidomide and six are taking ruxolitinib only. None of the patients is taking only lenalidomide. The ruxolitinib dose ranges from 10 mg bid to 25 mg bid continuously, and the lenalidomide dose ranges from 5 mg daily to 15 mg daily for 21 days of the 28-day cycle.
Discussion
In our study, the combination of lenalidomide and ruxolitinib elicited an IWG-MRT-defined spleen response (≥50% reduction in spleen size by palpation) in 57% of patients, at any time point during the study conduct. Long-term follow up of patients treated with ruxolitinib in the phase 3 COMFORT-1 study revealed similar results: 59% of patients (91/155) originally randomized to ruxolitinib achieved a ≥35% reduction in spleen volume (corresponding to ≥50% reduction in spleen size by palpation) at any time during the study follow-up.30 Responses were durable, with a median response duration of 19 months, and to date only two patients have lost their response. IWG-MRT-defined clinical improvement in hemoglobin concentration was noted in only one patient. Unfortunately, symptom assessment was not part of the therapy evaluation.
Concomitant initiation and continuation of both drugs was difficult due to toxicity with most discontinuations occurring early when the hematologic toxicities were at their peak: two-thirds of patients required interruption of either lenalidomide or both drugs within 3 months of initiation. The main reasons for early interruption were myelosuppression (70%) or gastrointestinal toxicities attributable to lenalidomide (15%). In cases in which the toxicity was more attributable to one agent that agent was discontinued e.g. diarrhea, deep vein thrombosis and skin rash were more likely attributable to lenalidomide. Ruxolitinib likely plays a more central role in the treatment of myelofibrosis. Thus, when toxicities occurred that could not be clearly attributed to either of the drugs (e.g. myelosuppression) ruxolitinib was preferentially continued and lenalidomide was the first drug to be interrupted, as was done in all 23 cases that needed interruption. The specific reasons for lenalidomide and ruxolitinib interruptions are listed in Table 3. Seventy percent of the patients who required an early interruption were unable to restart lenalidomide, resulting in 45% of the patients being treated on single-agent ruxolitinib within the first 3 months. Among the patients who did not require early interruption, eight of 11 (73%) achieved a response, suggesting that the combination - when deliverable – might be associated with an improved response rate. This appeared to be associated with reductions in bone marrow cellularity and lactate dehydrogenase but, disappointingly, not with improvements in anemia and bone marrow fibrosis or a decrease in JAK2V617F allele burden.
This is the first clinical trial to determine the feasibility and efficacy of combining a JAK inhibitor with another disease-modifying agent in MF. An improved understanding of the complex pathobiological mechanisms underlying MF and a better understanding of the downstream mediators of the JAK-STAT pathway suggest that rationally selected combinations may achieve a degree of disease modification that has so far eluded single agent JAK inhibitor therapy.31 In addition to our study presented here, a number of combination studies with ruxolitinib are currently ongoing. The goal of these combinations is to enhance responses by targeting non-JAK-STAT drivers of MF, to address therapeutic limitations of JAK2 inhibitors, or to alleviate the anti-JAK2-mediated myelosuppression. Enhanced response may be obtained by combining ruxolitinib with drugs that target multiple levels of the JAK2 signaling cascade as being tested in ongoing trials in combination with the Pi3K-inhibitor BKM-120 (NCT01730248) or drugs that inhibit parallel pro-survival pathways such as the histone deacetylse-inhibitor pracinostat (NCT02267278) or panabinostat (NCT01693601, NCT01433445), Hedgehog-inhibitor LDE225 (NCT01787552) and DNA methyltransferase inhibitors azacytidine (NCT01787487) and decitabine (NCT02076191). Similarly, agents such as PRM-1 and GS-6624, which have been shown to reverse bone marrow fibrosis in preclinical models, are being evaluated in combination with ruxolitinib (NCT01981850, NCT01369498). In addition to our study with lenalidomide, other agents such as pomalidomide and danazol may improve hematopoiesis thereby mitigating JAK2 inhibitor-mediated myelosuppression and are being evaluated in combination with ruxolitinib (NCT01644110, NCT01732445).
Our current experience with the lenalidomide-ruxolitinib combination teaches us that although there is likely a clinical benefit from combining other agents with JAK2 inhibitors, the cumulative or overlapping toxicities (e.g. myelosuppression), optimal dosage and treatment schedule need to be carefully evaluated. A sequential rather than concomitant approach could be considered when contemplating combination regimens with ruxolitinib, which may further increase the response rate and tolerability of such combinations. For example, a run-in phase with ruxolitinib for 3 months followed by a cautious introduction and gradual escalation of lenalidomide could improve the tolerability and efficacy of this combination. We are currently exploring such an approach in ongoing novel combination strategies at our institution (NCT01787487, NCT02267278).
Acknowledgments
We would like to thank Incyte corporation for supporting the clinical trial.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
Supported in part by the MD Anderson Cancer Center Support Grant (CCSG) CA016672 and by the Incyte corporation.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Spivak JL. The chronic myeloproliferative disorders: clonality and clinical heterogeneity. Semin Hematol. 2004;41(2 Suppl 3):1–5. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol. 2005;23(33):8520–8530. [DOI] [PubMed] [Google Scholar]
- 3.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent and how? Blood. 2014;124(24)3529–3537. [DOI] [PubMed] [Google Scholar]
- 4.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. [DOI] [PubMed] [Google Scholar]
- 7.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. [DOI] [PubMed] [Google Scholar]
- 10.Mesa RA, Steensma DP, Pardanani A, et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood. 2003;101(7):2534–2541. [DOI] [PubMed] [Google Scholar]
- 11.Marchetti M, Barosi G, Balestri F, et al. Low-dose thalidomide ameliorates cytopenias and splenomegaly in myelofibrosis with myeloid metaplasia: a phase II trial. J Clin Oncol. 2004;22(3):424–431. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DA, Giles FJ, Albitar M, et al. Thalidomide therapy for myelofibrosis with myeloid metaplasia. Cancer. 2006;106(9): 1974–1984. [DOI] [PubMed] [Google Scholar]
- 13.Tefferi A, Cortes J, Verstovsek S, et al. Lenalidomide therapy in myelofibrosis with myeloid metaplasia. Blood. 2006;108(4): 1158–1164. [DOI] [PubMed] [Google Scholar]
- 14.Quintas-Cardama A, Kantarjian HM, Manshouri T, et al. Lenalidomide plus prednisone results in durable clinical, histopathologic, and molecular responses in patients with myelofibrosis. J Clin Oncol. 2009;27(28):4760–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tefferi APF, Barbui T, Barosi G, et al. Phase 3 study of pomalidomide in myeloproliferative neoplasm (MPN)-associated myelofibrosis with RBC-transfusion-dependence. Blood. 2013; 122(21):394a.23687088 [Google Scholar]
- 16.Daver N, Shastri A, Kadia T, et al. Modest activity of pomalidomide in patients with myelofibrosis and significant anemia. Leuk Res. 2013;37(11):1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daver N, Shastri A, Kadia T, et al. Phase II study of pomalidomide in combination with prednisone in patients with myelofibrosis and significant anemia. Leuk Res. 2014;38(9):1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mesa RA, Yao X, Cripe LD, et al. Lenalidomide and prednisone for myelofibrosis: Eastern Cooperative Oncology Group (ECOG) phase 2 trial E4903. Blood. 2010;116(22):4436–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dredge K, Dalgleish AG, Marriott JB. Thalidomide analogs as emerging anti-cancer drugs. Anticancer Drugs. 2003;14(5):331–335. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood. 2012; 120(25):4945–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollyea DA, Kohrt HE, Gallegos L, et al. Safety, efficacy and biological predictors of response to sequential azacitidine and lenalidomide for elderly patients with acute myeloid leukemia. Leukemia. 2012;26(5): 893–901. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Manero G, Daver NG, Borthakur G, et al. Phase I study of the combination of 5-azacitidine sequentially with high-dose lenalidomide in higher-risk myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML). Blood. 2011;118(21):1122. [Google Scholar]
- 25.Vardiman JW BR, Harris NL, ed. WHO histological classification of chronic myeloproliferative diseases. Lyon, France, International Agency for Research on Cancer Press, 2001. [Google Scholar]
- 26.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. [DOI] [PubMed] [Google Scholar]
- 27.Gale RP, Barosi G, Barbui T, et al. What are RBC-transfusion-dependence and -independence? Leuk Res. 2011;35(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. [DOI] [PubMed] [Google Scholar]
- 29.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]
- 30.Verstovsek S, Mesa RA, Gotlib J, et al. Three-year efficacy, overall survival, and safety of ruxolitinib therapy in patients with myelofibrosis from the COMFORT-I study. Haematologica. 2015;100(4):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascarenhas J. Rationale for combination therapy in myelofibrosis. Best Pract Res Clin Haematol. 2014;27(2):197–208. [DOI] [PubMed] [Google Scholar]
- 32.McClure R, Mai M, Lasho T. Validation of two clinically useful assays for evaluation of JAK2 V617F mutation in chronic myeloproliferative disorders. Leukemia. 2006;20(1): 168–171. [DOI] [PubMed] [Google Scholar]
- 33.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108(5): 1497–1503. [DOI] [PubMed] [Google Scholar]


