The mutation status of the B-cell receptor (BCR) is a strong prognostic factor in chronic lymphocytic leukemia (CLL), dividing patients into BCR mutated and unmutated CLL (CLL-Mut, CLL-UM), the latter predicting for worse prognosis.1–3 Furthermore, the occurrence of highly similar BCRs was observed in CLL, grouped as stereotyped BCRs, which was interpreted as an indication for a role of specific antigen as well as cognate T-cell help for disease development and progression.4
As a thorough analysis of the functional significance of CLL/T-cell interactions may provide an insight into disease pathogenesis, we aimed to characterize the clonal CD4+ T-cell repertoire in conjunction with BCR characteristics in chemo-naive CLL patients. By matching T-cell diversity with the BCR usage of the respective CLL clone, we found that clonal T cells seemed functionally silenced and that they accumulated in non-stereotyped CLL-UM. Our data support an involvement of T-cell help and cognate antigen in CLL growth, which is likely determined by BCR stereotype and mutation status.
To determine the clonality of CD4+ T cells, we analyzed CDR3 length polymorphism of TCR Vβ genes in RNA of purified CD4+ T cells from 55 unselected, chemo-naive CLL patients (Online Supplementary Table S1). While a number of patients showed completely polyclonal patterns in all their TCR Vβ genes, others showed either single or multiple mono- or oligoclonal TCR families. The occurrence of monoclonal CD4+ T-cell populations was not restricted to certain TCR Vβ gene families (Online Supplementary Figure S1). In summary, 28 of 55 (51%) analyzed patients showed a monoclonal pattern of at least one TCR Vβ CDR3 region, 15 (27%) showed at least one oligoclonal pattern, and 12 (22%) exhibited exclusively polyclonal TCR Vβ CDR3 regions (Figure 1A).
Figure 1.
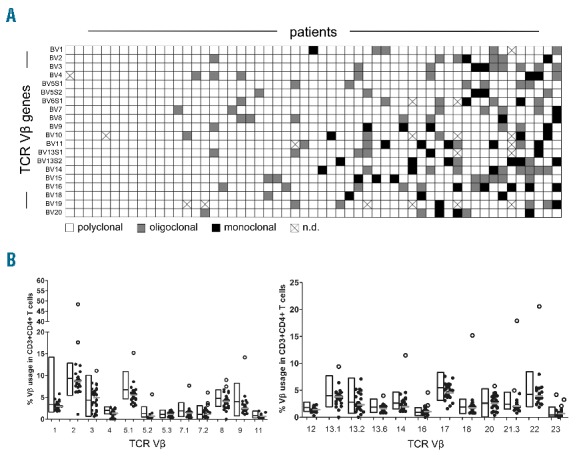
T-cell clonality of CD4+ T cells from 55 chemonaive CLL patients. (A) CDR3 length polymorphism of CD4+ T cells from 55 untreated CLL patients showing monoclonal, oligoclonal or polyclonal TCR Vβ gene families. (B) The frequency of TCR Vβ specific CD4+ T cells was determined by flow cytometry with regard to CDR3 length polymorphism. The relative abundance of monoclonal (white dots) or non-monoclonal (black dots) CD4+ T-cell populations is given in comparison to the range of values from healthy donors shown within the bars. The median values are indicated within the plots as horizontal bars.
To be able to obtain information about clone size, we determined the relative frequency of individual TCR Vβ specific CD4+ T cells by flow cytometry, as described in the Online Supplementary Appendix. Twenty-six of 55 patient samples provided sufficient material for this assay. A value of any TCR Vβ specific CD4+ T-cell population higher than the maximum value from 85 healthy specimens (except for Vb4, Vb7.2 and Vb13.2, which were tested on 46 healthy specimens) was considered an over-representation. Overall, 14 of 26 (54%) analyzed CLL patients showed an over-representation of a TCR Vβ CD4+ T-cell population and 6 patients (23%) showed an over-representation of more than one TCR Vβ CD4+ T-cell clone (Figure 1B). By comparing the relative frequency of TCR Vβ specific CD4+ T cells with CDR3 length polymorphism, we found that over-represented CD4+ T cells were monoclonal in all cases analyzed (Figure 1B). Clone sizes ranged from 2.0% to 48.4% of total CD4+ T-cell numbers (median 9.0%).
We next determined the IgHV mutation status, IgHV usage and IgHV stereotype in our cohort (Figure 2A and Online Supplementary Table S2). While overall no strong association with any specific IgHV gene was found (Online Supplementary Figure S1), we observed that CLL with unmutated stereotyped IgHV genes were associated with a significantly lower rate of clonal CD4+ cells than unmutated non-stereotyped subsets (P<0.0001) (Figure 2B). In the IgHV mutated subset, there was no association of CD4+ T-cell clonality with BCR stereotype. Importantly, the frequency of monoclonal or over-represented T cells was independent from CMV serostatus (Online Supplementary Figure S2).
Figure 2.
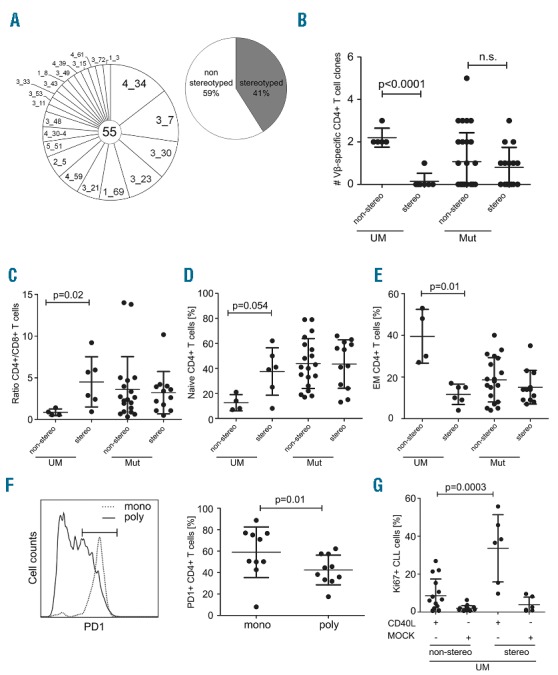
CD4+ T-cell clonality associates with BCR stereotypy in IgHV unmutated CLL. (A) Pie chart showing IgHV gene usage and the occurrence of stereotyped BCR of CLL clones. The segment size gives the percentage of IgHV elements (left chart) or stereotyped BCRs (right chart) as indicated within the respective segments. The center of the pie shows the number of samples analyzed. (B) The occurrence of over-represented CD4+ T cells was analyzed with regard to stereotyped BCR and IgHV mutation status. On the y-axis, the number of over-represented Vβ-specific CD4+ T-cell clones per patient sample is indicated. On the x-axis, samples are classified according to stereotype and IgHV mutation status. The ratio of CD4+/CD8+ T cells (C) as well as the percentage of naïve (D) and effector memory T cells (E) was analyzed in the different CLL subsets. (F) A representative FACS plot is shown for the PD-1 expression on clonally overrepresented (mono; dotted) versus non-over-represented (poly; solid) CD4+ T cells from a CLL patient. Graph showing the percentage of PD-1 expression on over-represented clonal CD4+ T cells versus polyclonal CD4+ T cells (mono: monoclonal; poly: polyclonal). (G) Stereotyped or non-stereotyped CLL-UM cells were stimulated in vitro on a layer of CD40L expressing or mock transfected fibroblasts. The graph shows the percentage of CLL cells positive for the proliferation associated antigen Ki67 three days after stimulation.
We speculated whether the appearance of clonal CD4+ T cells might reflect changes in the T-cell functionality and T-cell subset distribution. Strikingly, we found that while T cells in the peripheral blood of CLL patients were usually skewed towards a high CD4+/CD8+ ratio, CD4+ T cells from IgV-UM non-stereotyped CLL patients approximately equaled the number of CD8+ T cells (Figure 2C). Moreover, CD4+ T cells from IgV-UM non-stereotyped CLL showed lower percentages of naïve and higher percentages of antigen experienced effector memory T cells (Figure 2D and E). As recent reports suggested T-cell exhaustion, a functional silencing of T cells, to be involved in the development of CLL,5–7 we also assessed the expression of the exhaustion marker programmed death-1 (PD-1) on over-represented T cells. Thereby, we found that clonally over-represented CD4+ T cells were more abundantly PD-1 positive than their polyclonal counterparts (53.9% PD1+CD4+ vs. 40.7% PD1+CD4+; P=0.04) (Figure 2F and Online Supplementary Table S3), suggesting that clonal T cells are likely to be an exhausted T-cell fraction. Based on these data, we suspected that CLL cells from patients with clonally exhausted T cells would in turn be less responsive to T-cell-derived stimulation. To test this assumption, we stimulated CLL cells in vitro using CD40L expressing fibroblasts that mimic T-cell help, and measured subsequent upregulation of the proliferation-associated antigen Ki67. We found that stereotyped CLL-UM cells were significantly more responsive to CD40 stimulation than non-stereotyped counterparts, resulting in the appearance of a higher fraction of Ki67+CLL cells (8.6% Ki67+ CLL vs. 33.6% Ki67+ CLL; P=0.0003) (Figure 2G and Online Supplementary Table S3). In addition, we found that the stereotyped CLL-UM fraction was associated with the expression of the risk factors CD38 and ZAP70 (Figure 3A and B). Finally, we analyzed time to first treatment (TTFT) of patients according to BCR stereotype and the occurrence of monoclonal CD4+ T cells including their BCR mutation status. Patients with unmutated BCR showed an expected shortened TTFT compared to patients with mutated BCR (median TTFT: UM 76 months vs. Mut median not reached; log rank P=0.0001) (data not shown). BCR stereotype did not per se predict any difference in TTFT (median TTFT 168 months vs. 164 months; log rank P=0.848) (Figure 3C). When we included the mutation status, CLL-UM patients with a non-stereotyped BCR showed a significantly shorter TTFT compared to those with a stereotyped BCR (median TTFT 64 months vs. 110 months; log rank P=0.029) (Figure 3D). Within the CLL-Mut patient cohort, BCR stereotype did not affect TTFT (median TTFT not reached; log rank P=0.949) (Figure 3D). In patients bearing at least one monoclonal CD4+ T-cell clone we observed a tendency towards shortened TTFT compared to patients with no monoclonal CD4+ T cells [median TTFT: not reached (polyclonal T cells) vs. 164 months (monoclonal T cells); log rank P=0.190] (Figure 3E). By combining the CD4+ T-cell clonality with the mutation status of the patients we found a tendency of shortened TTFT in CLL-UM patients with monoclonal CD4+ T cells versus polyclonal CD4+ T cells (median TTFT 64 months vs. 76 months; log rank P=0.112) (Figure 3F). Again, within the CLL-Mut cohort we observed no difference in TTFT with regard to the occurrence of monoclonal CD4+ T cells (median TTFT not reached; log rank P=0.533) (Figure 3F).
Figure 3.
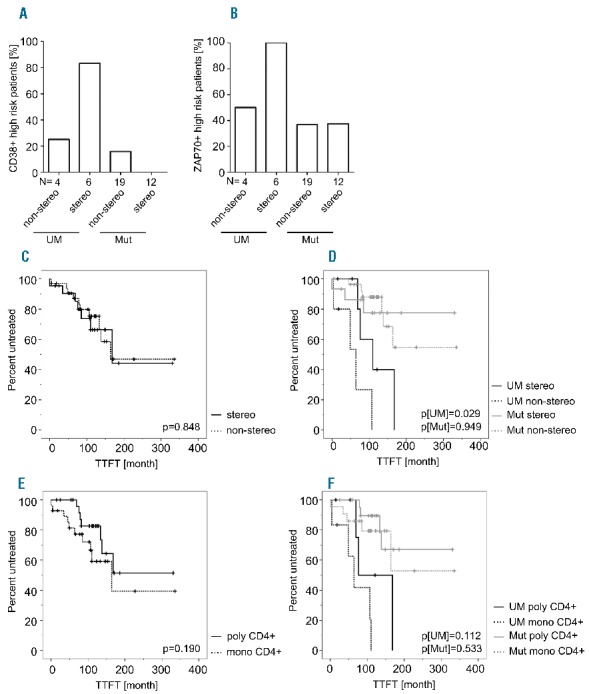
Clinical parameters of patients according to BCR stereotype and IgHV mutation status. Expression of the risk factors CD38 (A) and ZAP70 (B) was determined for the respective CLL subsets. The numbers of patients analyzed are given as numbers below the bars. Time to first treatment (TTFT) was investigated using Kaplan-Meier analysis in chemo-naive, stereotyped (stereo) versus non-stereotyped (non-stereo) patients (C and D) as well as in patients harboring at least one monoclonal CD4+ T-cell clone (mono) versus patients without monoclonal T cells (poly) (E and F). In (D) and (F), patients were further stratified according to IgHV mutation state (CLL-UM: n=12; CLL-Mut: n=33). Patient details are provided in Online Supplementary Tables S1 and S2.
T-cell exhaustion has been proposed as an important mechanism involved in CLL pathogenesis,5,6,8 leading to a general hyporesponsiveness of T cells in CLL. As such, the clones observed in our study may be functionally silent and not necessarily provide relevant T-cell help for CLL. Fittingly, we also find that the responsiveness of CLL cells to CD40L stimulation is inversely correlated with the presence of CD4+ T-cell clones in the unmutated subgroup, with a higher level of proliferation induced by CD40L stimulation in stereotyped CLL-UM. Whether this can be attributed to the presence of clonal and possibly exhausted T cells in the non-stereotyped subgroup will be tested in future studies. Accordingly, while in accordance with our data recent studies revealed that BCR stereotype can substantially affect treatment-free survival in CLL,9 our result on slightly shortened TTFT in CLL-UM patients with monoclonal CD4+ T cells could well reflect the existence of CLL subtypes with differential CLL/T cell crosstalk, but this certainly needs to be tested in a larger patient cohort.
In summary, we present the presence of sizeable CD4+ T-cell clones in CLL and find a significant association of clones with an exhausted T-cell phenotype. Furthermore, we show that the presence of clonal T cells is not independent from BCR characteristics. Our work suggests a relevant crosstalk between T cells and CLL cells that seems to involve characteristics of antigen recognition. While the limited number of patients analyzed with our laborious approach does not allow more general conclusions to be drawn, we propose a concept that may be more readily addressable in the future using novel sequencing technologies for the analysis of clonal diversity in B and T cells.
Footnotes
Funding: the authors would like to thank the Austrian science fund FWF (T516-B13 to NZ; P24619 to RGe; P24100 to RGr), the Austrian National Bank fund ÖNB (10990 to AE), the Paracelsus Medical University (PMU-FFF 12/16/084-EGG to AE and RGe), the SCRI-LIMCR GmbH, and the province and city of Salzburg for funding and grants.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 2.Weinberg JB, Volkheimer AD, Chen Y, et al. Clinical and molecular predictors of disease severity and survival in chronic lymphocytic leukemia. Am J Hematol. 2007;82(12):1063–1070. [DOI] [PubMed] [Google Scholar]
- 3.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94(6):1848–1854. [PubMed] [Google Scholar]
- 4.Darzentas N, Stamatopoulos K. The Significance of Stereotyped B-Cell Receptors in Chronic Lymphocytic Leukemia. Hematol Oncol Clin North Am. 2013;27(2):237–250. [DOI] [PubMed] [Google Scholar]
- 5.Gassner FJ, Zaborsky N, Neureiter D, et al. Chemotherapy-induced augmentation of T cells expressing inhibitory receptors is reversed by treatment with lenalidomide in chronic lymphocytic leukemia. Haematologica. 2014;99(5):67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunes C, Wong R, Mason M, et al. Expansion of a CD8(+)PD-1(+) Replicative Senescence Phenotype in Early Stage CLL Patients Is Associated with Inverted CD4:CD8 Ratios and Disease Progression. Clin Cancer Res. 2012;18(3):678–687. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay AG, Johnson AJ, Lee AM, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118(7):2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9):1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baliakas P, Hadzidimitriou A, Sutton L, et al. Clinical effect of stereotyped B-cell receptor immunoglobulins in chronic lymphocytic leukaemia: a retrospective multicentre study. Lancet Haematol. 2014; 1(2):e74–e84. [DOI] [PubMed] [Google Scholar]


