Abstract
Pomalidomide + low-dose dexamethasone is effective and well tolerated for refractory or relapsed and refractory multiple myeloma after bortezomib and lenalidomide failure. The phase III trial MM-003 compared pomalidomide + low-dose dexamethasone with high-dose dexamethasone. This subanalysis grouped patients by baseline creatinine clearance ≥ 30 − < 60 mL/min (n=93, pomalidomide + low-dose dexamethasone; n=56, high-dose dexamethasone) or ≥ 60 mL/min (n=205, pomalidomide + low-dose dexamethasone; n=93, high-dose dexamethasone). Median progression-free survival was similar for both subgroups and favored pomalidomide + low-dose dexamethasone versus high-dose dexamethasone: 4.0 versus 1.9 months in the group with baseline creatinine clearance ≥ 30 − < 60 mL/min (P<0.001) and 4.0 versus 2.0 months in the group with baseline creatinine clearance ≥ 60 mL/min (P<0.001). Median overall survival for pomalidomide + low-dose dexamethasone versus high-dose dexamethasone was 10.4 versus 4.9 months (P=0.030) and 15.5 versus 9.2 months (P=0.133), respectively. Improved renal function, defined as an increase in creatinine clearance from < 60 to ≥ 60 mL/min, was similar in pomalidomide + low-dose dexamethasone and high-dose dexamethasone patients (42% and 47%, respectively). Improvement in progression-free and overall survival in these patients was comparable with that in patients without renal impairment. There was no increase in discontinuations of therapy, dose modifications, and adverse events in patients with moderate renal impairment. Pomalidomide at a starting dose of 4 mg + low-dose dexamethasone is well tolerated in patients with refractory or relapsed and refractory multiple myeloma, and of comparable efficacy if moderate renal impairment is present. This trial was registered with clinicaltrials.gov identifier 01311687 and EudraCT identifier 2010-019820-30.
Introduction
Renal impairment is a common presenting feature for patients with multiple myeloma (MM). Approximately 20% to 40% of patients with newly diagnosed MM, who are primarily aged over 65 years, have renal impairment at diagnosis, and this rate increases during the course of disease.1–4 Renal impairment as a complication of MM often leads to cast nephropathy, potentially resulting in renal tube atrophy and tubulointerstitial fibrosis.5,6 In addition, impaired renal function may be a result of aging and can also be caused by comorbidities unrelated to MM, such as diabetes, hypertension, vascular disease, or prior non-myeloma therapies.3 Renal impairment in patients with MM is associated with shortened survival, but recovery of renal function during treatment may improve survival in these patients and even achieve similar outcomes in patients with a history of normal renal function.3,4,7
The past several decades have seen advances in the treatment of MM, and outcomes have improved for patients with varying degrees of renal impairment;3,8 despite this, most patients will ultimately relapse.9,10 Control of MM via effective therapy has been shown to improve renal function in a large proportion of patients.11,12 However, patients who are refractory to bortezomib and have relapsed following treatment with an immunomodulatory agent, or patients who are refractory to or ineligible to receive an immunomodulatory agent, have a poor prognosis (median survival 9 months).13 Physicians may perceive that treatment options in these patients may be further limited by the presence of renal impairment, as some novel agents rely on metabolism/excretion by the kidneys.7
Pomalidomide (POM) acts via direct antimyeloma, stromal-support inhibitory, and immunomodulatory effects.14,15 POM in combination with low-dose dexamethasone (LoDEX) was evaluated for the treatment of relapsed and refractory MM in patients after lenalidomide and bortezomib failure in the MM-002 and MM-003 studies. The phase I component of MM-002 established the maximum tolerated dose for POM at 4 mg daily with or without LoDEX (40 mg weekly).16 This dose was moved forward into an open-label, randomized, phase II component, which found POM + LoDEX to be more efficacious than POM alone [overall response rate (ORR) 33% vs. 18%; median progression-free survival (PFS) 4.2 vs. 2.7 months; median overall survival (OS) 16.5 vs. 13.6 months]. The pivotal, multicenter, open-label, randomized, phase III study MM-003 verified the safety and efficacy of POM + LoDEX (n=302) compared with high-dose dexamethasone (HiDEX; n=153) in patients with refractory or relapsed and refractory MM previously treated with (and 74% refractory to) lenalidomide and bortezomib.17 Overall trial results included significant improvements in ORR (31% vs. 10%; P<0.0001), PFS (4.0 vs. 1.9 months; P<0.0001), and OS (12.7 vs. 8.1 months; P=0.0285).17 Preliminary pharmacokinetic data suggest that renal impairment probably does not impact on POM exposure.18
The aim of the current analysis was to examine efficacy and safety of POM + LoDEX versus that of HiDEX in patient subgroups enrolled in MM-003 by renal function at baseline [creatinine clearance (CrCl) ≥ 30 − < 60 mL/min vs. ≥ 60 mL/min]. In addition, we examined median PFS and OS in patients who had improvement of renal function from CrCl ≥ 30 − < 60 mL/min at baseline to ≥ 60 mL/min at any point during study treatment.
Methods
MM-003 was an open-label, randomized, phase III trial conducted in 93 centers in Europe, Russia, Australia, Canada, and the United States (clinicaltrials.gov identifier: 01311687; EudraCT 2010-019820-30). Full details have been described previously.17 All authors and the study sponsor were involved in data collection and analysis, review and interpretation of results, and the writing of the report.
Patients
Eligible patients were aged 18 years or over with refractory or relapsed and refractory MM, had measurable serum or urine M protein, and were refractory to their last prior treatment [documented progressive disease (PD) during or within 60 days of last therapy]. Prior bortezomib and lenalidomide treatment (≥ 2 consecutive cycles, alone or in combination) must have failed [i.e. refractory (never experienced a response), PD within six months after at least a partial response (PR), or intolerant (to bortezomib only)]. Adequate prior alkylator therapy was required.
Exclusion criteria included: absolute neutrophil count < 1×109/L; platelets < 75×109/L (< 30×109/L if ≥ 50% of bone marrow nucleated cells were plasma cells); CrCl < 45 mL/min; peripheral neuropathy grade ≥ 2; prior exposure to POM; hypersensitivity to thalidomide, lenalidomide, or DEX; or resistance to DEX.
Study design and treatment
Patients were randomized 2:1 to POM + LoDEX (28-day cycles; oral POM: 4 mg/day, days 1–21; LoDEX: 40 mg/day, days 1, 8, 15, and 22) or HiDEX (40 mg/day, days 1–4, 9–12, and 17–20). For patients aged over 75 years, DEX dose was reduced to 20 mg/day in both treatment arms. Patients continued treatment until PD or unacceptable toxicity. Thromboprophylaxis was required for all patients receiving POM and any patient at high risk of developing thrombosis.
Patients provided written informed consent. The study received institutional review board or independent ethics committee approval at all participating centers according to the Declaration of Helsinki and the International Conference on Harmonisation Guidelines on Good Clinical Practice.
Subgroup analyses
In this retrospective analysis, renal impairment cohorts included patients with moderate renal impairment (baseline CrCl ≥ 30 − < 60 mL/min) or without renal impairment (baseline CrCl ≥ 60 mL/min), based on the Cockroft-Gault formula.19,20 CrCl data were collected prospectively and were estimated at the start of each treatment cycle and upon discontinuation.
Assessments
The primary end point was investigator-assessed PFS. Survival distribution functions for each treatment group were estimated with the Kaplan-Meier product-limit method and compared with the log-rank test. Key secondary end points included OS, ORR [≥ PR by International Myeloma Working Group (IMWG) criteria], safety [adverse events (AEs) graded according to National Cancer Institute Common Terminology Criteria for Adverse Events v.4.0], and improvement of renal function from CrCl ≥ 30 − < 60 mL/min at baseline to ≥ 60 mL/min at any point during study treatment (only assessed in patients with baseline and post-baseline CrCl data). As an additional retrospective analysis, renal response was assessed according to IMWG criteria.5 Efficacy was assessed in the intent-to-treat population (all patients randomized to treatment) using IMWG criteria, and tolerability was assessed in the safety population (all patients who received ≥ 1 dose of study drug). PFS and OS for patients with renal improvement were calculated according to European Medicines Evaluation Agency criteria. Statistical analyses were performed using SAS software v.9.2.
Results
Patients’ characteristics
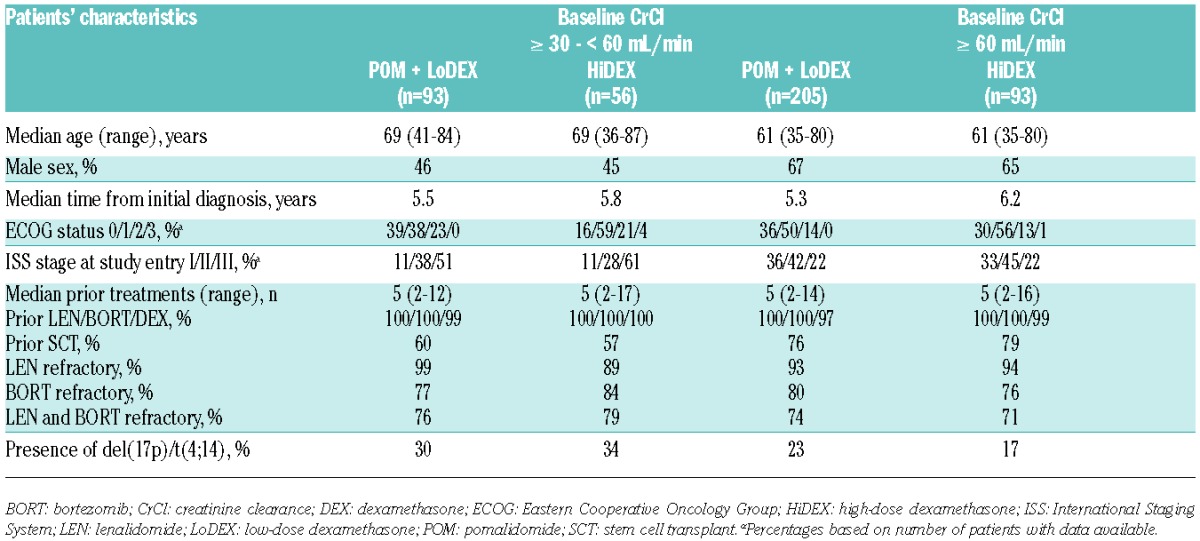
This analysis was performed using a data cut off of September 1st, 2013, corresponding to a median follow up of 15.4 months. A total of 302 patients received POM + LoDEX, and 153 patients received HiDEX (Online Supplementary Figure S1). Patients were heavily pre-treated, with a median of 5 prior lines of treatment in all groups. For patients with baseline CrCl ≥ 30 − < 60 mL/min, data were available for 93 POM + LoDEX and 56 HiDEX patients. For those with baseline CrCl ≥ 60 mL/min, data were available for 205 POM + LoDEX and 93 HiDEX patients. Eight patients were not included in the analysis: 3 patients had missing baseline CrCl values (2 in the POM-LoDEX arm and 1 in the HiDEX arm), and 5 patients had baseline CrCl levels below 30 mL/min (2 in the POM-LoDEX arm and 3 in the HiDEX arm) (Online Supplementary Figure S1). Patients with baseline CrCl ≥ 30 − < 60 mL/min were more likely to be older and less likely to have undergone prior SCT compared with patients with baseline CrCl ≥ 60 mL/min (Table 1). The moderate renal impairment cohort also exhibited higher rates of International Staging System stage II-III disease, Eastern Cooperative Oncology Group status of 3, and high-risk cytogenetics.
Table 1.
Baseline patients’ characteristics and prior therapies.
Efficacy
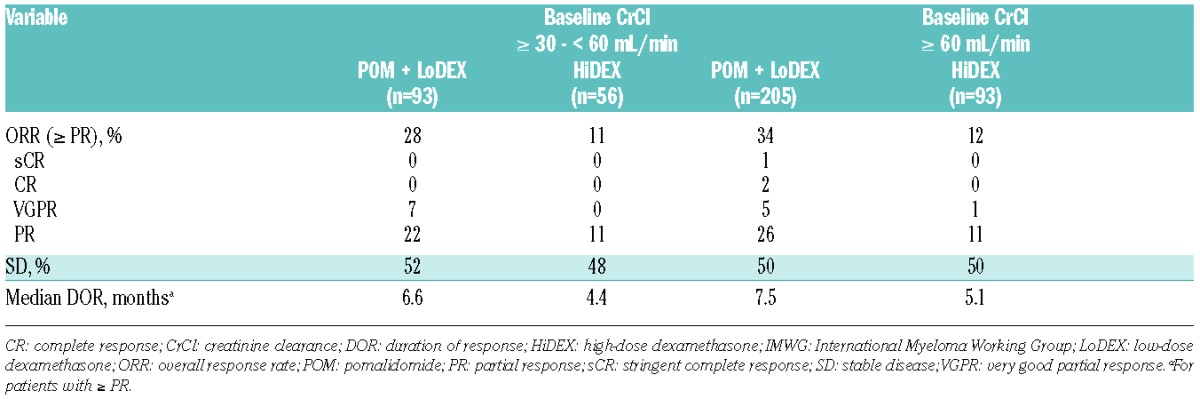
Median PFS with POM + LoDEX versus HiDEX was consistent in patients with baseline CrCl ≥ 30 − < 60 mL/min (4.0 vs. 1.9 months; P<0.001) (Figure 1A) and ≥ 60 mL/min (4.0 vs. 2.0 months; P<0.001) (Figure 1B). POM + LoDEX also improved median OS compared with HiDEX in patients with baseline CrCl ≥ 30 − < 60 mL/min (10.4 vs. 4.9 months; P=0.030) (Figure 2A) and ≥ 60 mL/min (15.5 vs. 9.2 months; P=0.133) (Figure 2B). The OS advantage of POM + LoDEX over HiDEX was achieved despite a substantial proportion of HiDEX patients receiving subsequent POM (50% of HiDEX patients with baseline CrCl ≥ 30 − < 60 mL/min and 60% of HiDEX patients with baseline CrCl ≥ 60 mL/min crossed over to POM + LoDEX). POM + LoDEX significantly improved ORR versus HiDEX regardless of baseline renal function (Table 2). Duration of response (for patients achieving ≥ PR) was consistently longer for POM + LoDEX versus HiDEX in both groups.
Figure 1.
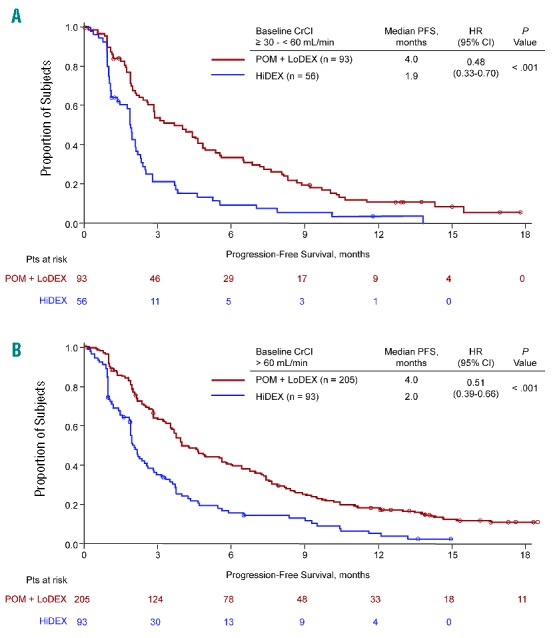
Progression-free survival for patients with baseline creatinine clearance ≥ 30 − < 60 mL/min (A) or ≥60 mL/min (B).
Figure 2.
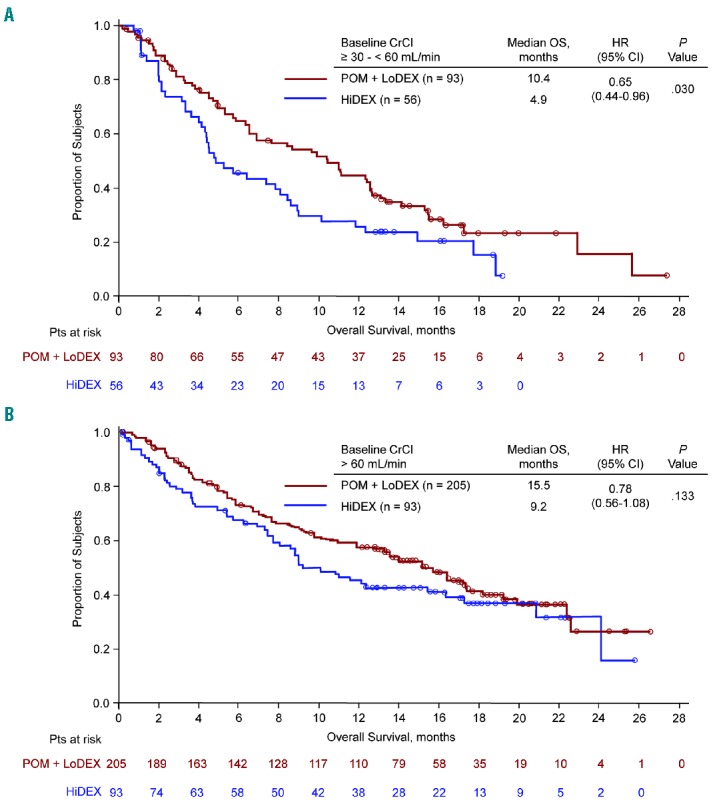
Overall survival for patients with baseline creatinine clearance ≥ 30 − < 60 mL/min (A) or ≥ 60 mL/min (B).
Table 2.
Response to treatment (IMWG criteria).
Improvement in renal function
A total of 273 patients in the POM + LoDEX arm and 128 patients in the HiDEX arm had CrCl data for baseline and ≥ 1 post-baseline assessment and were thus evaluable for change in renal function (for patients with > 1 post-baseline assessment, the best value during the first 6 cycles was used). Renal improvement to CrCl ≥ 60 mL/min was noted in 42% of patients (33 of 79) treated with POM + LoDEX who had renal impairment (CrCl < 60 mL/min) at baseline (Table 3). Renal improvement was seen in 47% (20 of 43) of HiDEX-treated patients. The median time to improvement was similar for each treatment arm (POM + LoDEX: 1.0 month; HiDEX: 0.9 months). In these POM + LoDEX and HiDEX-treated patients with renal improvement, median PFS was 6.5 (95%CI: 4.6, 8.4) and 3.2 (95%CI: 2.1, 5.5) months, respectively; median OS was 12.6 (95%CI: 7.6, 25.7) and 10.1 (95%CI: 5.7, 17.7) months, respectively.
Table 3.
Improvement in renal function.
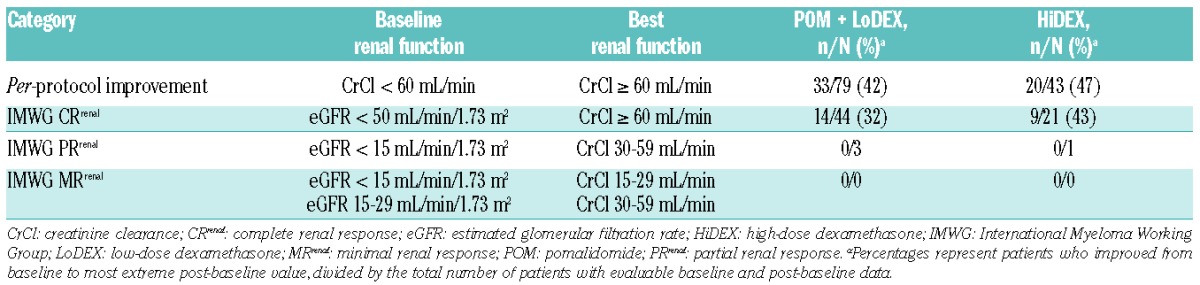
According to IMWG criteria for renal response,5 32% (14 of 44) of POM + LoDEX-treated patients with estimated glomerular filtration rate (eGFR) < 50 mL/min/1.73 m2 achieved complete response of CrCl ≥ 60 mL/min (Table 3). In a similar analysis of the HiDEX-treated patients, 43% (9 of 21) achieved a complete response. Few or no patients in either treatment arm were eligible for partial or minimal response by having baseline eGFR < 30 mL/min/1.73 m2.
Safety
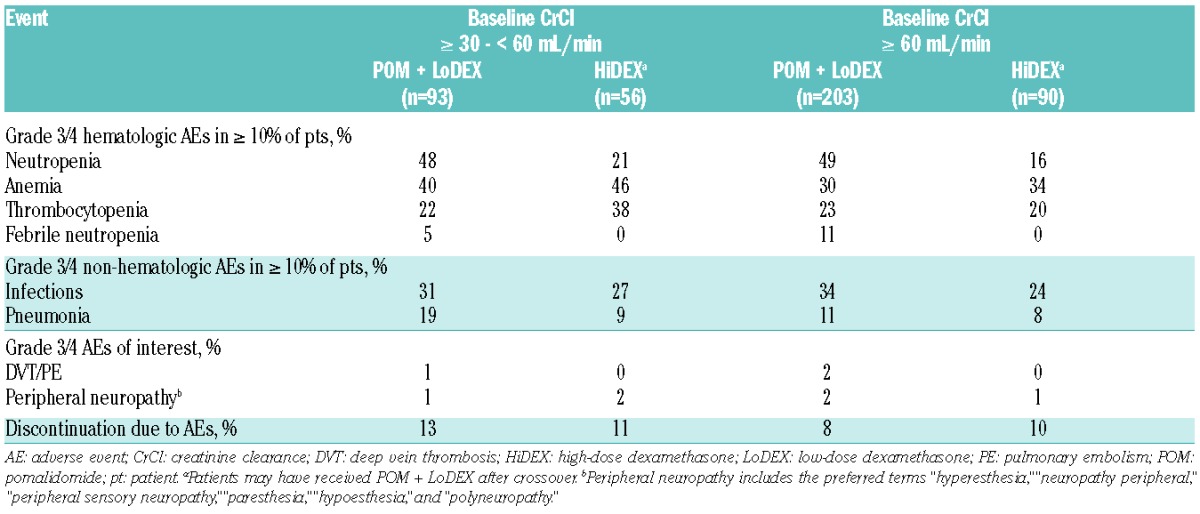
The AE profiles for POM + LoDEX and HiDEX were similar across both renal function subgroups (Table 4). The most common grade 3/4 AEs for the POM + LoDEX treatment arm were neutropenia (48% for baseline CrCl ≥ 30 − < 60 mL/min and 49% for baseline CrCl ≥ 60 mL/min), anemia (40% and 30%, respectively), and infections (31% and 34%, respectively). With mandatory thromboprophylaxis, incidence of grade 3/4 deep vein thrombosis/pulmonary embolism was low (≤ 2% in both groups).
Table 4.
Safety profile.
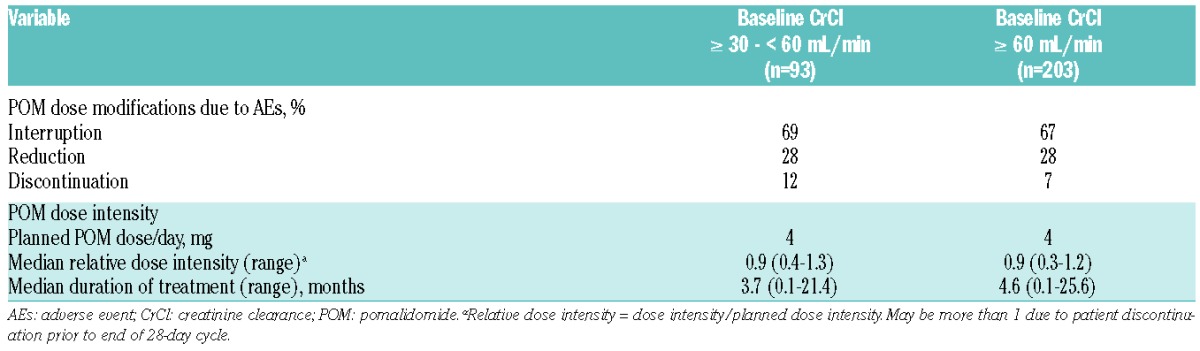
POM discontinuations and dose modifications due to AEs were similar regardless of moderate renal impairment (Table 5). Median duration of POM treatment was similar in patients with baseline CrCl ≥ 30 − < 60 mL/min (3.7 months) and ≥ 60 mL/min (4.6 months). Renal function did not affect frequency of dose reductions and interruptions. Median relative dose intensity was consistent at 90% for both renal function subgroups.
Table 5.
Pomalidomide dose modification due to adverse events and dose intensity.
Discussion
POM + LoDEX was efficacious and well tolerated in patients with refractory or relapsed and refractory MM and moderate renal impairment. POM + LoDEX significantly extended PFS versus HiDEX for all patients regardless of renal impairment (baseline CrCl ≥ 30 − < 60 mL/min, 4.0 vs. 1.9 months; baseline CrCl > 60 mL/min, 4.0 vs. 2.0 months; P<0.001 for both groups), similar to the benefits observed in the general study population (4.0 vs. 1.9 months; P<0.001).17 OS results showed a similar 5- to 6-month benefit for POM + LoDEX in both renal function subgroups (baseline CrCl ≥ 30 − < 60 mL/min, 10.4 vs. 4.9 months; baseline CrCl > 60 mL/min, 15.5 vs. 9.2 months) compared with those of the overall patient population (13.1 vs. 8.2 months),17 although these results were only statistically significant for patients with reduced renal function (baseline CrCl ≥ 30 − < 60 mL/min, P=0.030; baseline CrCl > 60 mL/min, P=0.133). This finding is likely to be confounded by the high number (56% overall) of HiDEX patients crossing over to receive POM after progression, as per protocol.
A substantial number of renally impaired patients treated with POM + LoDEX had improved renal function (42% per protocol criteria and 32% per IMWG criteria), which was similar to the improvement rate noted in the HiDEX arm. HiDEX treatment can rapidly suppress M-protein and light-chain excretion leading to recovery of renal function and can be used for acute myeloma treatment.12,21,22 In the present study, however, response rates in renally impaired patients greatly favored the POM+LoDEX arm compared with the HiDEX arm: 28% versus 11%, respectively. In patients treated with POM + LoDEX whose kidney function improved from moderate impairment to normal, PFS reached 6.5 months, which exceeded the results of patients with normal baseline kidney function (4.0 months). Despite a similar proportion of patients with renal improvement noted in the HiDEX arm, their PFS was only 3.2 months. The OS improvement observed in these patients was in the same range as that in patients with normal renal function.
Tolerability profiles were consistent regardless of baseline renal function. Rates of discontinuation due to AEs were similar in both subgroups, indicating that patients with moderate renal impairment did not experience increased toxicity. Slightly increased incidences of pneumonia were observed in patients with baseline CrCl ≥ 30 − < 60 mL/min. Duration of treatment and dose intensity were not affected by baseline renal function. These findings demonstrate that up-front dose modification is not required in patients with moderate renal impairment, and that 4 mg is a safe and appropriate starting dose of POM in combination with LoDEX for these patients.
The efficacy results of POM + LoDEX in renal function subgroups of MM-003 confirm those found previously. In the phase II component of MM-002, patients without renal impairment (baseline CrCl > 60 mL/min) had an ORR of 34%, median PFS of 5.4 months, and median OS of 16.9 months, and patients with moderate renal impairment (baseline CrCl ≥ 45 to ≤ 60 mL/min) had an ORR of 43%, median PFS of 4.7 months, and median OS of 19.5 months. It should be noted, however, that there were only 14 patients in this subgroup in that study.23 Safety profiles and relative dose intensities for these subgroups were consistent between the phase II study and the one presented here.23 This cumulative body of evidence regarding POM + LoDEX further supports use of a 4-mg starting dose for patients with mild or moderate renal impairment without up-front dose reduction.
The finding that POM does not require dose adjustment in patients with moderate renal impairment versus patients with normal renal function is related to its metabolism and excretion. In contrast to lenalidomide, which is excreted primarily via the kidneys,24 POM is extensively metabolized (with only 2.2% excreted as the parent drug in urine) and, therefore, does not require dose reductions in patients with impaired renal function;18,25 the same observation applies to thalidomide.26,27 The safety profile of POM + LoDEX as assessed in the study presented here demonstrated no difference in frequency of dose reductions in patients with moderate renal impairment and only slightly higher rates of anemia and pneumonia.
However, this analysis was limited by the fact that it concerns only patients with normal or moderately impaired renal function, as the study excluded patients with CrCl < 45 mL/min at the time of screening [although 28 (9%) POM + LoDEX and 15 (10%) HiDEX patients had baseline CrCl below this cut off due to the time that had elapsed between screening and the first treatment cycle]. To address this, 2 trials are in progress to assess the use of POM + LoDEX in patients with severe renal impairment, including those requiring dialysis: MM-008 in the United States (clinicaltrials.gov identifier 01575925) and MM-013 in the European Union (clinicaltrials.gov identifier 02045017).
Newer treatment options, including lenalidomide, thalidomide, and bortezomib, have improved outcomes and survival for many patients with MM in recent years.28,29 In addition, although newer agents can improve outcomes, including renal function, for many patients with MM with renal impairment,5,7 it remains a significant risk factor for early death in MM.3,30 Thus, the efficacy of POM + LoDEX in renally impaired relapsed/refractory MM populations is of particular importance. This analysis has demonstrated that a starting dose of POM 4 mg may be used safely regardless of moderate renal impairment, with no unexpected toxicities observed and no additional dose modifications or discontinuations required when compared with the overall trial population. PFS and OS benefits achieved by patients with moderate renal impairment were also consistent with the overall MM-003 trial population.
Acknowledgments
The authors would like to thank the patients who took part in the trial, clinical staff at the participating study sites, and representatives of the sponsors who were involved in data gathering and analyses. We also thank Peter Simon, PhD, and Nicola Hanson, PhD, of MediTech Media for writing assistance, which was funded by Celgene Corporation.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/7/872
References
- 1.Alexanian R, Barlogie B, Dixon D. Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med. 1990;150(8):1693–1695. [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. [DOI] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25(1):195–200. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65(3): 175–181. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010; 28(33):4976–4984. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki K. Diagnosis and treatment of multiple myeloma and AL amyloidosis with focus on improvement of renal lesion. Clin Exp Nephrol. 2012;16(5):659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res. 2012; 18(8):2145–2163. [DOI] [PubMed] [Google Scholar]
- 8.Uttervall K, Duru AD, Lund J, et al. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One. 2014; 9(7):e101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. [DOI] [PubMed] [Google Scholar]
- 10.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bladé J, Rosiñol L. Renal, hematologic and infectious complications in multiple myeloma. Best Pract Res Clin Haematol. 2005; 18(4):635–652. [DOI] [PubMed] [Google Scholar]
- 12.Kastritis E, Anagnostopoulos A, Roussou M, et al. Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica. 2007; 92(4):546–549. [DOI] [PubMed] [Google Scholar]
- 13.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter International Myeloma Working Group study. Leukemia. 2012;26(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDs) in multiple myeloma. Leukemia. 2010;24(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mark TM, Coleman M, Niesvizky R. Preclinical and clinical results with pomalidomide in the treatment of relapsed/refractory multiple myeloma. Leuk Res. 2014;38(5):517–524. [DOI] [PubMed] [Google Scholar]
- 16.Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121(11):1961–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann M, Kasserra C, Reyes J, et al. Absorption, metabolism and excretion of [14C]pomalidomide in humans following oral administration. Cancer Chemother Pharmacol. 2013;71(2):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 20.Luke DR, Halstenson CE, Opsahl JA, Matzke GR. Validity of creatinine clearance estimates in the assessment of renal function. Clin Pharmacol Ther. 1990;48(5):503–508. [DOI] [PubMed] [Google Scholar]
- 21.Bayraktar UD, Warsch S, Pereira D. High-dose glucocorticoids improve renal failure reversibility in patients with newly diagnosed multiple myeloma. Am J Hematol. 2011;86(2):224–227. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa H, Tanaka H, Iwato K, et al. Effect of glucocorticoids on the biologic activities of myeloma cells: inhibition of interleukin-1 beta osteoclast activating factor-induced bone resorption. Blood. 1990;75(3):715–720. [PubMed] [Google Scholar]
- 23.Siegel DS, Richardson PG, Baz R, Chen M, Zaki M, Anderson KC. Pomalidomide (POM) with low-dose dexamethasone (LoDEX) in patients with relapsed and refractory multiple myeloma (RRMM): impact of renal function on patient outcomes. Blood. 2012;120(21):4072.22927249 [Google Scholar]
- 24.Chen N, Lau H, Kong L, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47(12):1466–1475. [DOI] [PubMed] [Google Scholar]
- 25.Pomalyst® (pomalidomide) [package insert]. Summit, NJ: Celgene Corporation; 2015. [Google Scholar]
- 26.Thalomid® (thalidomide) [package insert]. Summit, NJ: Celgene Corporation; 2013. [Google Scholar]
- 27.Eriksson T, Hoglund P, Turesson I, et al. Pharmacokinetics of thalidomide in patients with impaired renal function and while on and off dialysis. J Pharm Pharmacol. 2003;55(12):1701–1706. [DOI] [PubMed] [Google Scholar]
- 28.Liwing J, Uttervall K, Lund J, et al. Improved survival in myeloma patients: starting to close in on the gap between elderly patients and a matched normal population. Br J Haematol. 2014;164(5):684–693. [DOI] [PubMed] [Google Scholar]
- 29.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16(11):1600–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002: Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36): 9219–9226. [DOI] [PubMed] [Google Scholar]


