Abstract
Disease relapse is the most common cause of treatment failure after allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndromes, yet treatment options for such patients remain extremely limited. Azacitidine is an important new therapy in high-risk myelodysplastic syndromes and acute myeloid leukemia but its role in patients who relapse post allograft has not been defined. We studied the tolerability and activity of azacitidine in 181 patients who relapsed after an allograft for acute myeloid leukemia (n=116) or myelodysplastic syndromes (n=65). Sixty-nine patients received additional donor lymphocyte infusions. Forty-six of 157 (25%) assessable patients responded to azacitidine therapy: 24 (15%) achieved a complete remission and 22 a partial remission. Response rates were higher in patients transplanted in complete remission (P=0.04) and those transplanted for myelodysplastic syndromes (P=0.023). In patients who achieved a complete remission, the 2-year overall survival was 48% versus 12% for the whole population. Overall survival was determined by time to relapse post transplant more than six months (P=0.001) and percentage of blasts in the bone marrow at time of relapse (P=0.01). The concurrent administration of donor lymphocyte infusion did not improve either response rates or overall survival in patients treated with azacitidine. An azacitidine relapse prognostic score was developed which predicted 2-year overall survival ranging from 3%–37% (P=0.00001). We conclude that azacitidine represents an important new therapy in selected patients with acute myeloid leukemia/myelodysplastic syndromes who relapse after allogeneic stem cell transplantation. Prospective studies to confirm optimal treatment options in this challenging patient population are required.
Introduction
Allogeneic stem cell transplantation (SCT) is an important curative option in patients with high-risk acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). The major cause of treatment failure remains disease relapse, which occurs in 40%–70% of patients.1,2 Little progress has been made to date in developing effective treatment options for patients with recurrent disease after allogeneic SCT, and the great majority remain destined to die of resistant disease.3 Although a small number of patients with disease recurrence can survive long term after a second transplant or donor lymphocyte infusion (DLI), the success of both these treatment modalities is contingent on the prior acquisition of morphological remission with salvage therapy.4,5 At present, the only established salvage option for patients who relapse post allograft is intensive chemotherapy which is variably effective, with reported complete response (CR) rates of 15%–30%, and is often poorly tolerated in this heavily pre-treated population patients.3–5 The low rates of response to myelosuppressive chemotherapy, coupled with its substantial toxicity and requirement for lengthy hospitalization, makes the identification of more effective and better tolerated re-induction therapies for patients relapsing after allogeneic SCT a significant unmet clinical need.
Azacitidine (AZA) is a DNA methyltransferase inhibitor (DNMTI) which demonstrates significant clinical activity in patients with AML and high-risk MDS.6,7 The mechanism of the anti-tumor activity of AZA has not been determined but may be due to its ability to reverse epigenetically silenced pro-apoptotic pathways. AZA also has the capacity to up-regulate the expression of epigenetically silenced tumor antigens, and can induce a CD8+ T-cell response to tumor antigens post transplant, raising the possibility that it may have the potential to augment a graft-versus-leukemia (GvL) response.8,9 A number of small series have reported that AZA can induce remissions in patients who relapse after an allogeneic transplant, raising the possibility that this agent represents a potential new treatment strategy in this challenging patient population.10–12 Furthermore, it has been suggested in single arm studies that AZA may augment the anti-tumor activity of DLI in patients who relapse after an allograft.13 However, importantly, so far there has been no systematic analysis of the clinical activity of AZA in patients who have relapsed after an allograft for AML or MDS, or of whether its activity is increased by the concurrent administration of DLI.
Methods
Patients
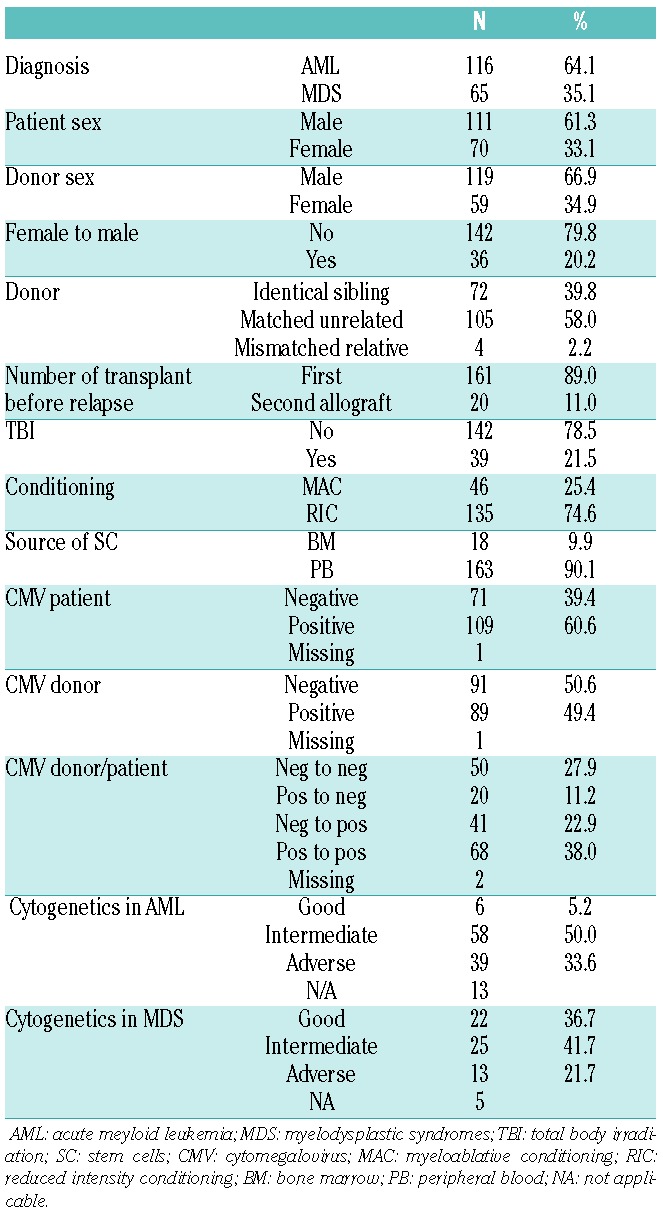
The study cohort was made up of 181 patients from the EBMT database who had received AZA for the treatment of morphological disease relapse between 2006 and 2010 after an allogeneic stem cell transplantation for AML or MDS. Only patients who received AZA within one month of disease recurrence were included. Patients who had received prophylactic or pre-emptive post-transplant AZA were specifically excluded from this analysis. Study patients’ characteristics are summarized in Table 1; 116 patients had undergone transplantation for AML and 65 for MDS. Median time from transplant to relapse was eight months (range 1–71 months); 72 patients relapsed within six months of transplant, 52 between six and 12 months post transplant, and 57 more than 12 months post transplant. In patients who had been transplanted as treatment for AML, presentation cytogenetics were classified as either good, intermediate or adverse risk according to the Medical Research Council (MRC) criteria14 and in patients transplanted for MDS utilizing the International Prognostic Scoring System.15 Seventy-two patients were transplanted using a matched sibling donor and 109 from an adult unrelated donor. Forty-six patients received a myeloablative conditioning (MAC) regimen and 135 patients received a reduced intensity (RIC) regimen according to EBMT criteria.16
Table 1.
Patients’ characteristics.
Azacitidine therapy
Azacitidine was administered at a dose of 75 mg/m2 for 5–7 consecutive days every month. Median time from relapse to commencement of AZA was 11 days (range 1–30 days). The median duration of AZA treatment was 53 days (2–1196 days) and the median total AZA dose delivered to the study population was 1050 mg/m2 (75–10,500 mg/m2). Sixty-nine patients received DLI in addition to AZA treatment, 39 of whom received DLI within two months of commencing AZA salvage and in the absence of a clinical response. Thirty-five patients proceeded to a second allogeneic transplant after AZA salvage therapy at a median of 119 days (range 12–1183 days) after the commencement of salvage AZA. Twenty-four patients were allografted before an assessment of response to AZA salvage was made (median 82 days), 6 of whom were transplanted after acquisition of a major response [complete response/partial response (CR/PR)] following AZA therapy and 5 after loss of a major response to AZA.
Non-hematologic toxicities were graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (http://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm). Hematologic toxicity was not assessable in the presence of active leukemia.
Response criteria
Response to AZA was assessed by conventional morphological criteria and evaluated in patients who had received AZA alone or AZA in combination with DLI. CR was defined as acquisition of less than 5% blasts on bone marrow assessment. PR was defined using the criteria defined by Cheson et al.17
Statistical analysis
The primary end point of the study was overall survival (OS). Secondary end points were response rate (CR or PR). Outcome parameters were measured from the date of commencement of AZA. Cumulative incidence curves were used to estimate CR and response rate, as death was a competing event.18 The probability of OS was calculated using the Kaplan-Meier method. Two Cox proportional hazards models were developed, including or not DLI within two months as a time-dependent variable. Fixed variables were diagnosis (AML or MDS), cytogenetics at time of diagnosis, time to relapse, the percentage of blasts in bone marrow at time of relapse, chronic GvHD before AZA, DLI before AZA, and year of transplantation. A stepwise procedure was then used for selection of variables with a P value of 0.05. The two remaining significant fixed factors, time interval from transplant to relapse and percentage of blasts in bone marrow at time of relapse, were used to develop a risk score in an additive way. Twenty-four patients who received AZA and proceeded to a second transplant were excluded from the prognostic factor analysis.
Results
Tolerability of AZA in patients who have relapsed post allograft
Fifty-two patients developed grade 3–4 non-hematologic toxicity during the course of AZA administration. Eighteen patients developed Grade 3–4 sepsis/infectious-related complications, 15 patients Grade 3–4 pneumonia, and 7 patients Grade 3–4 liver/gastrointestinal toxicity. It is likely that disease-related neutropenia made a substantial contribution to infection-related toxicities. Thirteen patients developed Grade 2–4 GvHD after AZA therapy, of whom 7 had received prior DLI. Five of the 112 patients who did not receive DLI developed Grade 2–4 GvHD. One patient developed GvHD after DLI followed by a second transplant.
Clinical response to AZA salvage therapy
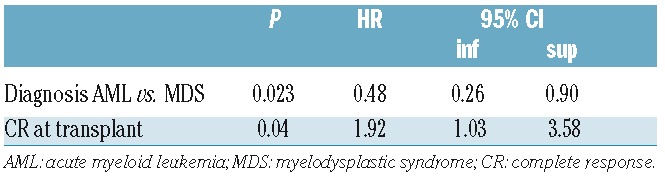
Forty-six of 157 (29.3%) assessable patients treated with AZA or AZA and DLI in combination demonstrated a major response (CR/PR) to AZA salvage therapy. Twenty-four (15.3%) patients achieved a CR and 22 (14%) a PR. Median time to achieve a CR after commencement of AZA was 108 days. In multivariate analysis, transplantation for MDS as opposed to AML was associated with a higher probability of achieving a major response [HR 0.48 (0.26–0.90); P=0.023] and transplantation in CR [HR 1.92 (1.03–3.58); P=0.04] (Table 2). The additional administration of DLI within two months of AZA administration was analyzed as a time-dependent variable and had no impact on OS [HR=1.04 (95%CI: 0.67–1.61); P=0.86].
Table 2.
Multivariable analysis of factors determining acquisition of a major clinical response in patients relapsing after an allogeneic transplant for acute myeloid leukemia or myelodysplastic syndrome who were treated with azacitidine.
Overall survival after AZA salvage therapy
The median follow up after commencement of AZA therapy was 24 months (range 2–72 months). At the time of latest follow up, 163 patients had died: 130 of disease relapse, 25 of infection-related complications, 5 of GvHD, and 3 of miscellaneous transplant-related complications. Eighteen patients are still alive.
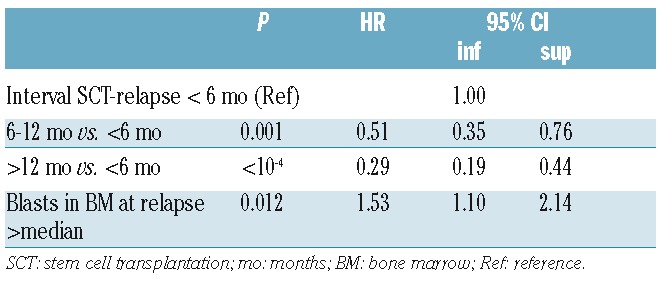
The 2-year OS for the whole group was 12.4%. The 2-year OS in patients achieving a CR after AZA salvage was 48.4%, and 28.7% in patients with a major response. In multivariable analysis, the following factors were predictive of 2-year OS at the time of relapse (Table 3): time to relapse 6–12 months versus less than six months [HR 0.51: (0.35–0.76); P=0.001] or more than 12 months [HR 0.29 (0.19–0.44); P=<10−4), respectively, and blasts in bone marrow greater than median (20%) at time of relapse [HR 1.5 (1.1–2.13); P=0.012].
Table 3.
Multivariable analysis of factors determining 2-year overall survival after azacitidine treatment.
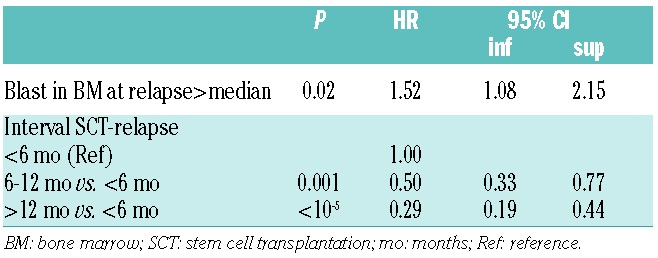
In a time-dependent multivariable analysis (Table 4), the following factors were predictive of 2-year OS: time to relapse post allograft 6–12 months versus less than six months [HR 0.5 (0.33–0.77); P=0.001] and more than 12 months, respectively [HR 0.29 (0.19–0.44); P=<10−5] and blasts in bone marrow greater than median (20%) at time of relapse [HR1.52 (1.08–2.15); P=0.02]. The administration of DLI within two months of commencement of AZA salvage had no impact on either the probability of achieving a major response or 2-year OS.
Table 4.
Time-dependant multivariable analysis of factors determining 2-year overall survival after azacitibine treatment.
The development of the AZA Relapse Prognostic Score
Using factors previously identified to determine survival after AZA therapy, it was possible to create a scoring system based on interval from transplant to relapse and blast percentage in the bone marrow at the time of relapse. In the AZA Relapse Prognostic Score (ARPS), the interval from transplant to relapse was assigned 2 points if less than six months, 1 if 6–12 months, and 0 if greater than 12 months. A blast percentage greater than the median (20%) at relapse was assigned 1 point. Utilizing the ARPS predicted the likelihood of achieving both a CR and major response as well as 2-year OS after AZA salvage therapy (Table 5 and Figure 1).
Table 5.
Two-year overall survival of 181 patients who relapsed after an allogeneic transplant for acute myeloid leukemia or myelodysplastic syndrome treated with azacitibine according to the AZA Relapse Prognostic Score.
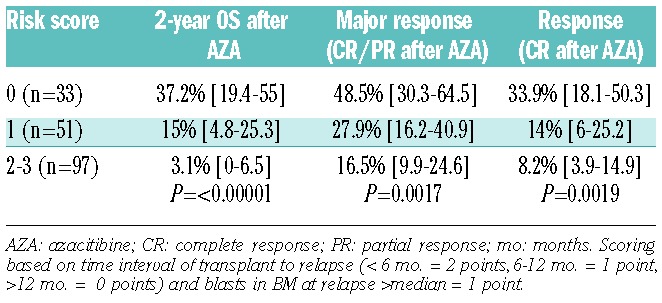
Figure 1.
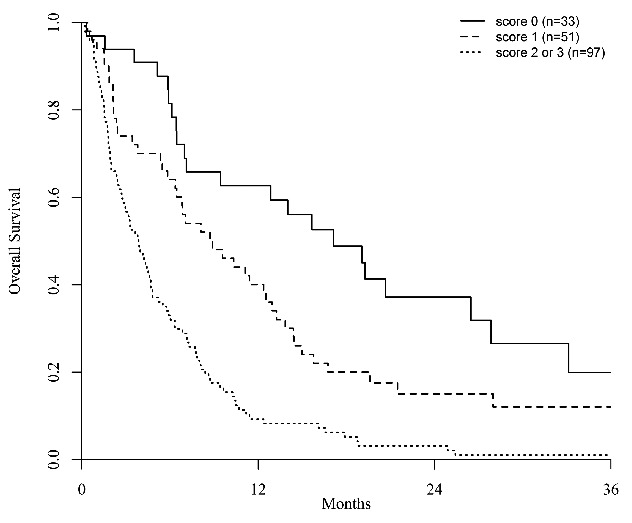
Two-year overall survival after azacitidine therapy in patients who relapsed after an allogeneic transplant for acute myeloid leukemia or myelodysplastic syndrome according to the AZA Relapse Prognostic Score.
Discussion
The ability of AZA to produce major clinical responses in a proportion of patients who relapse after an allograft for AML or MDS conclusively identifies this agent as a new treatment option in this challenging clinical setting. Furthermore, our study represents the first systematic analysis of activity of AZA in patients relapsing post transplant and it defines factors predicting the likelihood of response, which will assist its logical deployment. A second transplant or administration of DLI represent the only treatment modalities with the capacity to deliver long-term survival in patients with recurrent disease after an allogeneic transplant, but their utility is almost entirely dependent on the prior acquisition of a morphological remission.3 Currently the only established therapy in this patient population is intensive chemotherapy which has been reported to result in 2nd CR rates in the region of 15%–30% but is associated with significant toxicity and prolonged hospitalization.3–5 Our data permit, for the first time, the identification of patients with a significant chance of responding to AZA in whom DLI or a second transplant can be delivered with potential curative effect. The retrospective nature of these data, in common with previous reports of outcome after intensive chemotherapy, introduces significant potential selection bias. We have deliberately only studied patients who received AZA within a month of relapse in order to minimize this bias. It will be important, however, for future studies addressing this important clinical challenge to be performed prospectively, either in a registration study or as a randomized comparison of AZA and intensive chemotherapy.
For patients who have experienced the rigors of a previous allograft, considerations of both treatment toxicity and patient disposition are important. In general, AZA re-induction was well tolerated. Although approximately 30% of patients experienced Grade 3–4 non-hematologic toxicities, these were principally due to infection and likely to be consequent upon the cytopenias associated with disease relapse rather than being directly attributable to AZA. There was a notably low incidence of GvHD observed in this study, which is surprising given the likelihood that many patients had undergone a rapid immunosuppression taper. Although this observation requires prospective validation, it is consistent with the demonstration that AZA has the capacity to expand regulatory T cells post transplant, which may result in a reduced risk of GvHD.19,20 An additional, potentially valuable benefit of the use of AZA compared with intensive chemotherapy in this patient population is the opportunity to deliver salvage therapy as an out-patient. The other pressing therapeutic challenge in the management of patients with relapsed disease is to maximize the curative potential of a second transplant in patients who have responded to salvage therapy. Transplant toxicity remains substantial in this setting and it is possible that AZA results in less organ toxicity than conventional chemotherapy.
In the light of the significant number of patients who do not respond to AZA therapy, an important question raised by our data is whether it is possible to increase the response rate to AZA in this patient population. One proposed approach has been to combine AZA with DLI.21 This is the first study to study the impact of concurrent DLI on AZA response, and we failed to demonstrate any benefit associated with the co-administration of DLI. Co-administration of a histone deacetylase inhibitor, such as sodium valproate or vorinostat, may increase both the overall response rate and its speed in patients with AML and MDS,22–24 and it would be interesting to study such an approach in patients who relapse after an allograft. AZA has previously been shown to up-regulate the expression of epigenetically silenced tumor antigens, and one of its mechanisms of action in patients who have relapsed after an allogeneic transplant is the augmentation of a GvL effect. Consequently, combined administration of AZA with lenalidomide, an immunomodulatory drug with the capacity to activate CD8+ T cells, which also has the capacity to salvage patients with relapsed myeloid malignancies who relapse post transplant, would be of interest.25
In conclusion, our data demonstrate a potentially important role for AZA in the management of selected patients with relapsed AML or MDS after an allograft. Given its acceptable toxicity and ease of administration, these results emphasize a role for AZA as a novel treatment strategy in patients with recurrent disease. The development of cellular or pharmacological strategies with the capacity to increase response rates is a priority.
Acknowledgments
The hard work of data mangers at the BMT centers returning data for this study is gratefully acknowledged.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/7/879
Funding
A component of the data analysis presented in this manuscript was supported by an educational grant from Celgene.
References
- 1.Appelbaum FR. Optimising the conditioning regimen for acute myeloid leukaemia. Best Pract Res Clin Haematol. 2009; 22(4):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craddock C, Nagra S, Peniket A, et al. Factors predicting long-term survival after T-cell depleted reduced intensity allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica. 2010;95(6):989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood. 2012;119(6): 1599–1606. [DOI] [PubMed] [Google Scholar]
- 4.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31):4938–4945. [DOI] [PubMed] [Google Scholar]
- 5.Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31(26):3259–3271. [DOI] [PubMed] [Google Scholar]
- 6.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009; 10(3):223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–569. [DOI] [PubMed] [Google Scholar]
- 8.Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood. 2010;116(11):1908–1918. [DOI] [PubMed] [Google Scholar]
- 9.Mohty M, Chevallier P. Azacitidine after allo-SCT: the good without the bad? Blood. 2012;119(14):3199–3200. [DOI] [PubMed] [Google Scholar]
- 10.Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia. 2012;26(3):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antar A, Otrock ZK, Kharfan-Dabaja M, et al. Azacitidine in the treatment of extramedullary relapse of AML after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48(7):994–995. [DOI] [PubMed] [Google Scholar]
- 12.Tessoulin B, Delaunay J, Chevallier P, et al. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(4):567–571. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder T, Rachlis E, Bug G, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions–a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant. 2015;21(4):653–660. [DOI] [PubMed] [Google Scholar]
- 14.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 15.Onida F, Brand R, van Biezen A, et al. Impact of the International Prognostic Scoring System cytogenetic risk groups on the outcome of patients with primary myelodysplastic syndromes undergoing allogeneic stem cell transplantation from human leukocyte antigen-identical siblings: a retrospective analysis of the European Society for Blood and Marrow Transplantation-Chronic Malignancies Working Party. Haematologica. 2014; 99(10):1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003; 21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 18.Fine JP. Regression modeling of competing crude failure probabilities. Biostatistics. 2001;2(1):85–97. [DOI] [PubMed] [Google Scholar]
- 19.Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood. 2012; 119(14):3361–3369. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder T, Frobel J, Cadeddu RP, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia. 2013; 27(9):1910–1913. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder T, Czibere A, Platzbecker U, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013;27(6): 1229–1235. [DOI] [PubMed] [Google Scholar]
- 22.Craddock C, Quek L, Goardon N, et al. Azacitidine fails to eradicate leukemic stem/progenitor cell populations in patients with acute myeloid leukemia and myelodysplasia. Leukemia. 2013; 27(5): 1028–1036. [DOI] [PubMed] [Google Scholar]
- 23.Silverman LR, Verma A, Odchimar-Reissig R, et al. A PhaseI Trial of the Epigenetic Modulators Vorinostat, in Combination with Azacitidine (azaC) in Patients with the Myelodysplastic Syndrome (MDS) and Acute Myeloid Leukemia (AML): A Study of the New York Cancer Consortium. ASH Annual Meeting Abstract. 2008;3656. [Google Scholar]
- 24.Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008; 111(3):1060–1066. [DOI] [PubMed] [Google Scholar]
- 25.Sockel K, Bornhaeuser M, Mischak-Weissinger E, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97(9):e34–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


