Although 90–95% of adults recover completely from Hepatitis B (HBV) infection, a minority are unable to clear the virus.1 Epidemiological studies have demonstrated an increased risk of B-NHL among those with persistent HBV and B-NHL.2–5 However, the roles of exposure per se, occult infection, antibody response and viral clearance remain unclear.
Occult HBV infection (OBI) signifies persistence of the viral genome in blood and liver tissue in patients without hepatitis B surface antigen (HBsAg), with or without antibodies to hepatitis B core (anti-HBc) or hepatitis B surface (anti-HBs). Most subjects with OBI are in fact positive for anti-HBc and negative for HBsAg, while some are seronegative for all HBV markers.6 The association between OBI and B-NHL is clinically important due to the risk of reactivation of HBV following immunotherapy or chemotherapy. Few studies, however, have addressed OBI as a risk factor for NHL.7–9
Immune response to HBV (defined as anti-HBs) is elicited either by natural response to viral exposure (anti-HBc+ with anti-HBs+) or by vaccination (anti-HBc− with anti-HBs+). Limited data have suggested a negative association between the presence of anti-HBs and B-NHL.7,8
Heredity plays a role in NHL etiology, as evidenced by the doubling of risk in first-degree relatives of NHL patients.10 It is unknown whether host genetic factors related to viral clearance are also related to NHL susceptibility.
The role of HBV infection in B-NHL has not previously been evaluated in Israel or the West Bank, where HBsAg seroprevalence has been estimated at 0.22% and 1.8%, respectively.11,12 In a case-control study (Online Supplementary Methods), we explored associations between exposure, persistence, and immune response to HBV with overall B-NHL as well as with two major subtypes: diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL). Additionally, we assessed whether a family history of hematopoietic malignancies were associated with HBV persistence.
We recruited 823 (516-Israelis/307-Palestinians) incident cases of B-NHL (median time from diagnosis- three months). DLBCL was the most common histology, comprising 427 (52%) cases, while FL was diagnosed in 186 (23%). We recruited 808 healthy controls (414-Israelis/394-Palestinians) from individuals accompanying patients to hospital or health centers. Cases and controls (Online Supplementary Table S1) differed in distributions of sex, age, marital status and family history of hematopoietic malignancies (P<0.01 for all); we adjusted for these variables in our models accordingly.
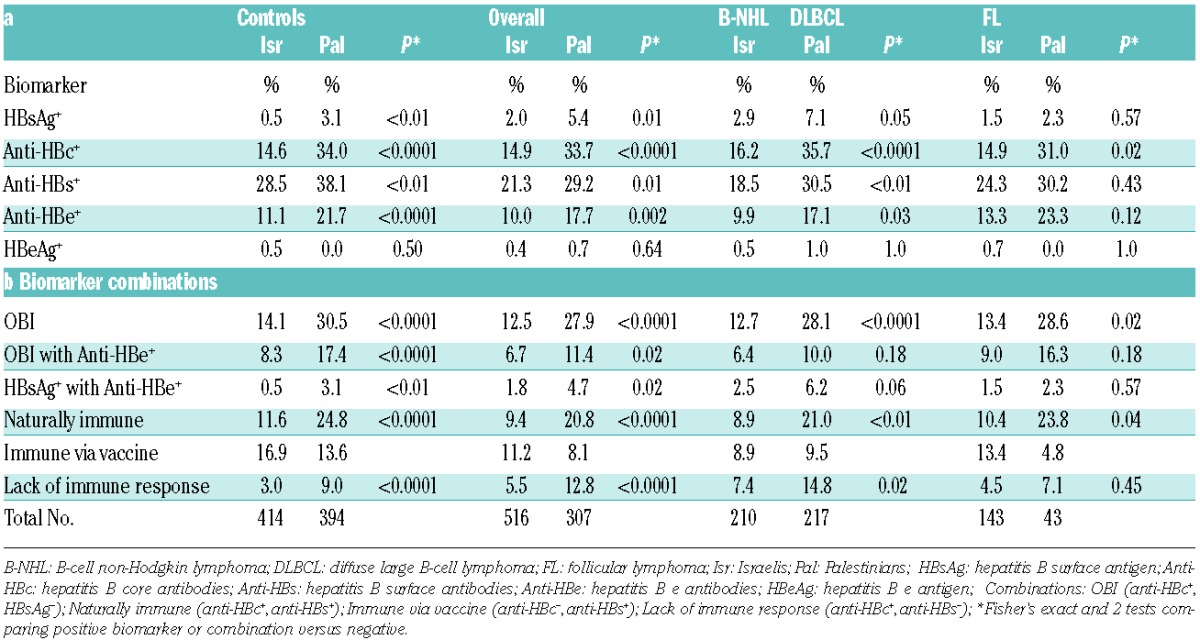
HBV serology was performed in 96.8% of participants (see results of individual markers, Table 1a). Most seromarkers (with the exception of eAg), including anti-HBc, HBsAg, anti-HBs and e-antibodies (anti-HBe), were more prevalent among Palestinians than Israelis. In contrast, antibody response to vaccine was more prevalent among Israelis. In addition, the combination of anti-HBc+ without anti-HBs, representing lack of immune response to HBV, was more common in Palestinians (9.0% vs. 3.0% in Israelis, P<0.0001) (Table 1b). Case-control association patterns were similar in the two groups.
Table 1.
Seropositivity for HBV and HCV biomarkers in Israelis versus Palestinians for Overall B-NHL, DLBCL, FL patients and controls.
Comparing cases and controls we found that persistence of HBV, as evidenced by HBsAg+, was associated with DLBCL (odds ratio (OR)=2.39, 95% confidence-interval (CI):1.13–5.06). Moreover, the prevalence of HBsAg+ among those exposed to HBV (anti-HBc+) was higher in cases than in controls (15% vs. 7.8%, P=0.03), indicating lower viral clearance rates among cases. However, no case-control differences were found regarding exposure to HBV (anti-HBc+) (OR=0.94, CI:0.72–1.23) or OBI (OR=0.84, CI:0.64–1.12) (Figures 1–2), anti-HBe or HBeAg prevalence (data not shown).
Figure 1.
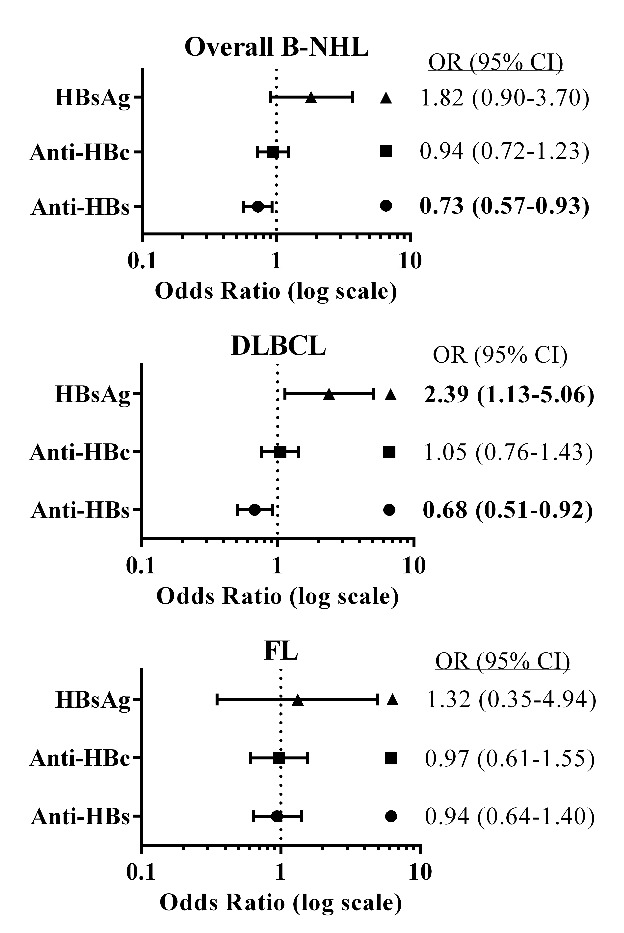
Forest plots showing the odds ratio (OR) and 95% confidence interval (CI) for combinations of hepatitis B (HBV) biomarkers and their associations with overall B-cell non-Hodgkin lymphoma (B-NHL), diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) for the pooled populations (Israelis and Palestinians). ORs were stratified by population (Israelis, Palestinians), sex and age categories (four-year grouping); adjusted for marital status, education (yrs), family history of hematopoietic malignancies in first-degree relatives. Hepatitis biomarkers: hepatitis B surface antigen (HBsAg), hepatitis B core antibodies (anti-HBc) and hepatitis B surface antibodies (anti-HBs). Bold font indicates P<0.05.
Figure 2.
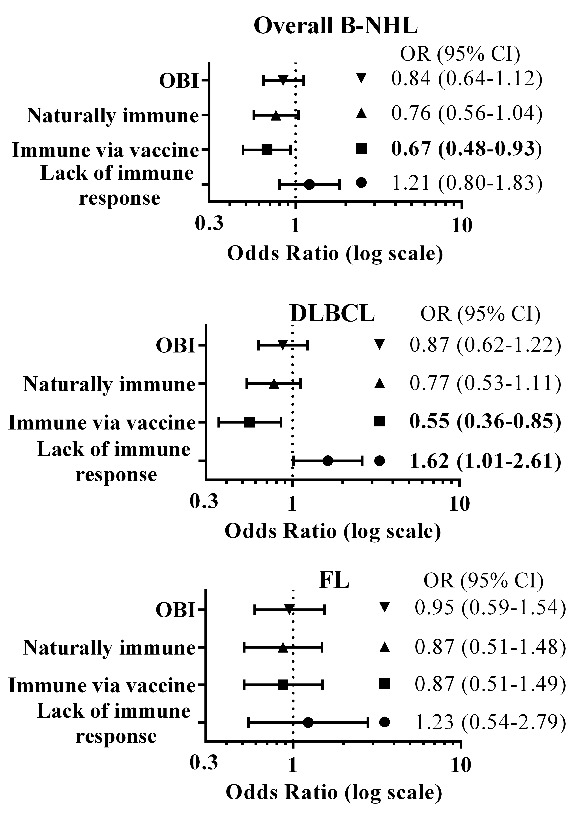
Forest plots showing the odds ratio (OR) and 95% confidence interval (CI) for combinations of hepatitis B (HBV) biomarkers and their associations with overall B-cell non- Hodgkin lymphoma (B-NHL), diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) for the pooled populations (Israelis and Palestinians). ORs were stratified by population (Israelis, Palestinians), sex and age categories (four-year grouping); adjusted for marital status, education (yrs), family history of hematopoietic malignancies in first-degree relatives. HBV biomarkers: hepatitis B surface antigen (HBsAg), hepatitis B core antibodies (anti-HBc) and hepatitis B surface antibodies (anti-HBs). Combinations: Occult hepatitis B infection (OBI: anti-HBc+, HBsAg−; Naturally immune (anti- HBc+, anti-HBs+); Immune via vaccine (anti-HBc−, anti-HBs+); Lack of immune response (anti-HBc+, anti-HBs−); Bold font indicates P<0.05.
The presence of anti-HBs antibodies was inversely associated with B-NHL (OR=0.73, CI:0.57–0.93) and DLBCL (OR=0.68, CI:0.51–0.92). This pattern held for antibody response to vaccine (OR=0.67, CI:0.48–0.93 for overall B-NHL; OR=0.55, CI:0.36–0.85 for DLBCL), but was not statistically significant for natural antibody response (OR=0.76, CI:0.56–1.04). Conversely, a lack of antibody response was positively associated with DLBCL (OR=1.62, CI:1.01–2.61).
As expected, having a first-degree relative with hematopoietic cancer was associated with overall B-NHL (OR=1.69, CI:1.16–2.48) and DLBCL (OR=1.83, CI:1.17–2.87). Interestingly, this variable was also strongly associated with persistent HBV infection among controls (OR=6.80, CI:1.14–23.8).
In summary, we confirm that persistent HBsAg carriers had a significantly increased risk of DLBCL and a non-significant increased risk for overall B-NHL. The European Epilymph study reported a non-significant increased risk of B-NHL in HBsAg carriers (OR=1.58, CI:0.69–3.64) and DLBCL (OR=1.50, CI:0.47–4.82),2 while a Turkish study reported OR=1.26, CI:0.65–2.46 and OR= 2.68, CI:1.19–6.01 for B-cell lymphoid tumors and DLBCL, respectively, and a strong relation for FL (OR=5.48, CI:1.02–29.5).3 Korean investigators reported a significant positive association between HBsAg and NHL (hazard ratio=1.74, CI:1.45–2.09) and a twofold risk of DLBCL in a cohort study4 and adjusted OR (2.09, CI:1.11–3.92) in a case-control study,5 respectively. In the latter study, investigators did not detect HBV-DNA in lymphoma tissues, but found HBV S, X, and C genes in DNA extracted from peripheral blood mononuclear cells, suggesting an indirect effect on lymphomagenesis.5
In the current study, the presence of anti-HBc was not associated with B-NHL or its subtypes, implying that exposure to HBV per se is not a risk factor for B-NHL. This finding is supported by a single Japanese cohort study of 20,360 subjects.13
Chen et al., defining OBI as HBsAg− and HBV-DNA+, reported a non-significant positive association with B-NHL.9 DNA extracted from tumor samples also failed to detect the HBV genome.
The inverse association between the presence of anti-HBs with B-NHL and DLBCL is consistent with Marcucci et al.’s report of an OR=0.61 (CI:0.44–0.85), both in indolent (OR=0.57, CI:0.38–0.88) and aggressive (OR=0.63, CI:0.44–0.93) B-NHL.7 Similarly, Wang et al. reported an OR of 0.60 (CI:0.40–0.70).8 In contrast, Kim et al. found no significant difference in anti-HBs+ prevalence comparing NHL patients with subjects with non-hematological malignancies and non-malignant conditions.14
Distinguishing between naturally acquired and vaccine-associated immunity is not clear cut as HBV DNA was not available to confirm actual exposure status. Nevertheless, we found a significant inverse association of B-NHL and DLBCL with anti-HBs overall. Similarly, Wang et al. reported a lower proportion of immune individuals among B-NHL cases.8 In contrast, the Epilymph study found no association between vaccine immunity and B-NHL.2 However, the OR for DLBCL (OR=0.56, CI:0.29–1.06) was similar to the current study’s point estimate.
Lack of immune response showed a significant positive association with DLBCL in our study. Likewise, Wang et al. concluded that patients with B-NHL may show lower clearance of the virus.8 Marcucci et al. also demonstrated that lack of an antibody response was positively associated with B-NHL (OR=2.05, CI:1.24–3.37).7 These findings suggest that immune competence may be protective against B-NHL. Alternative explanations include diminished immune response to HBV in B-NHL patients due to the lymphoma itself or reduction in antibody titres due to treatment, especially rituximab,15 or waning immunity with age. Most of the sera among cases (75%) were collected post-treatment. However, in a subgroup with available paired samples, pre- and post-treatment sera showed agreement [(Kappa=1.0 for HBsAg and anti-HBs biomarkers, and Kappa=0.81 (CI:0.55–1.00) for anti-HBc)] due to two patients with positive pre-treatment anti-HBc+ becoming seronegative post-treatment. Wang et al.’s study, in which 85% of cases were recruited before treatment, also demonstrated a significant negative association between anti-HBs+ and B-NHL.8
Intriguingly, we found a higher proportion of individuals with positive family history among HBsAg carriers than non-carriers in the control group. This association has not previously been reported and indicates the possibility of a joint inherited susceptibility to both diminished viral clearance and hematologic malignancy. Alternatively, it could reflect increased NHL risk in families where vertical transmission has occurred.
Limitations of this study include the inclusion of spouses as controls for 12% of enrolled cases. Use of spouse controls could, theoretically, alter case-control comparisons and bias the study toward the null. However, no couples were concordant for HBsAg+; in one couple, both members were anti-HBc+. Sensitivity analysis excluding spouse controls did not alter any of the reported associations (not shown). Other drawbacks are the lack of information on specific HBV genotypes, which may influence type and degree of immune responses.
The study’s strengths include its unique population and the examination of several hepatitis biomarkers, enabling assessment of combinations and their association with B-NHL. Although a number of studies have examined associations between HBV seromarkers and B-NHL, few have clearly distinguished the roles of exposure, persistence and viral clearance in disease etiology.
Higher seroprevalence observed among Palestinians implies that this population is at increased risk for HBV complications, including hepatocellular carcinoma and, potentially, B-NHL. It is particularly important to screen this population and all groups with high levels of exposure prior to immunosuppressive treatments in order to prevent potentially fatal flare-ups of pre-existing hepatitis infection.
In conclusion, our findings support an association between persistent HBV infection and B-NHL. We raise the possibility that hereditary factors may be related both to susceptibility to lymphoma and viral persistence, and that exposure per se does not explain the observed association between HBV and B-NHL. Prospective studies may clarify both the role of HBV vaccination in the prevention of B-NHL in endemic populations, and long-term B-NHL risk in individuals with poor antibody response to vaccine. The results of this study prompt a rethinking of the mechanism of virus-associated B-NHL beyond effects of chronic antigenic stimulation or viral integration, to include host response to infection as a marker of disease susceptibility.
Footnotes
Funding: this study was made possible by the generous support of the American people through the United States Agency for International Development (USAID), MERC grant #TA-MOU-11-M31-025. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government; Israel Science Foundation (ISF) grant #877/10; and the Hadassah University Hospital Compensatory Fund. We also gratefully acknowledge funding support for the 2003 Epilymph study by IARC.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.World Health Organization. Hepatitis B. World Health Organization; [cited 2015 Dec 23]. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/index3.html
- 2.Becker N, Schnitzler P, Boffetta P, et al. Hepatitis B virus infection and risk of lymphoma: results of a serological analysis within the European case-control study Epilymph. J Cancer Res Clin Oncol. 2012;138(12):1993–2001. [DOI] [PubMed] [Google Scholar]
- 3.Okan V, Yilmaz M, Bayram A, et al. Prevalence of hepatitis B and C viruses in patients with lymphoproliferative disorders. Int J Hematol. 2008;88(4):403–408. [DOI] [PubMed] [Google Scholar]
- 4.Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11(9):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SC, Jeong S-H, Kim J, et al. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J Med Virol. 2008;80(6):960–966. [DOI] [PubMed] [Google Scholar]
- 6.Bréchot C, Thiers V, Kremsdorf D, et al. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology. 2001;34(1):194–203. [DOI] [PubMed] [Google Scholar]
- 7.Marcucci F, Mele A, Spada E, et al. High prevalence of hepatitis B virus infection in B-cell non-Hodgkin’s lymphoma. Haematologica. 2006;91(4):554–557. [PubMed] [Google Scholar]
- 8.Wang F, Xu R-H, Han B, et al. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109(7):1360–1364. [DOI] [PubMed] [Google Scholar]
- 9.Chen M-H, Hsiao L-T, Chiou T-J, et al. High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin’s lymphoma. Ann Hematol. 2008;87(6):475–480. [DOI] [PubMed] [Google Scholar]
- 10.Wang ASS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma: a pooled analysis of 10,214 cases and 11,906 controls from the InterLymph Consortium. Blood. 2007;109(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palestinian Ministry of Health, Health Annual Report 2014. [cited 2016 Mar 4]. Available from: http://www.moh.ps/?lang=1&page=4&id=939
- 12.Novack L, Sarov B, Goldman-Levi R, et al. Impact of pooling on accuracy of hepatitis B virus surface antigen screening of blood donations. Trans R Soc Trop Med Hyg. 2008;102(8):787–792. [DOI] [PubMed] [Google Scholar]
- 13.Abe SK, Inoue M, Sawada N, et al. Hepatitis B and C virus infection and risk of lymphoid malignancies: A population-based cohort study (JPHC Study). Cancer Epidemiol. 2015;39(4):562–566. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Bang Y-J, Park BJ, et al. Hepatitis B virus infection and B-cell non-Hodgkin’s lymphoma in a hepatitis B endemic area: a case-control study. Jpn J Cancer Res. 2002;93(5):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsutsumi Y, Yamamoto Y, Shimono J, et al. Hepatitis B virus reactivation with rituximab-containing regimen. World J Hepatol. 2013; 5(11):612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]


