The effects of a normal bone marrow (BM) microenvironment compared to a malignant microenvironment on multiple myeloma (MM) are significantly different, where BM stromal cells (BMSCs) play a key role. We established, characterized and compared a myeloma-derived stromal cell line (Myeloma Stromal Puente-1, MSP-1) with two normal stromal cell lines (HS-5 and HS-27A). MSP-1 was found to affect MM proliferation, adhesion, migration and drug resistance in a more profound manner than HS-5 and HS-27A. These results demonstrated the importance of malignant versus normal BM microenvironment on several key MM processes, providing a new myeloma-derived stromal cell line to study the effect of tumor microenvironment on MM.
The BM microenvironment plays a pivotal role in disease progression, metastasis and drug resistance in MM.1,2 The interactions of MM cells with the cellular- and non-cellular components of the BM were shown to play a critical role in MM disease progression and drug resistance.3–5 In particular, the interactions between MM cells and BMSCs were shown to play a crucial role in MM proliferation, in which myeloma patient-derived BMSCs supported the growth of myeloma cells.6 BMSCs have a paracrine function on MM growth and dissemination by their production of interleukin-6 (IL-6) and stromal-cell derived factor (SDF-1).7,8 Roccaro et al. discovered that MM cells uptake exosomes released by BMSCs, the likes of which increased MM proliferation.4 Moreover, BMSCs played a major role in MM cell adhesion and mobilization in the BM.9,10 Mobilization of myeloma cells involves SDF-1/CXCR4 signaling and downregulation of VLA-4.11 SDF-1 is highly expressed in active MM, as well as in the BM niches of tumor metastases.12 BM microenvironmental changes such as hypoxia regulated the secretion of SDF-1 from BMSCs, influenced tumor progression, reduced adhesion of MM cells and induced egress of MM cells to new niches.9,10 Importantly, BMSCs play a critical role in cell adhesion-mediated drug resistance (CAM-DR); MM cells in the BM remain in a protective environment which confers resistance to therapeutic agents.3,5,15 Nefedova et al. showed direct cell contact between MM cells and BMSCs, and the contribution of soluble factors produced by this cell-cell interaction contribute to drug resistance.5 Co-cultures of MM cells with BMSCs showed CAM-DR of about 50% to melphalan, doxorubicin, dexamethasone, and bortezomib.14 Azab et al. revealed that disruption of MM-BMSCs interactions by a CXCR4 inhibitor (AMD3100) induced sensitization to drugs.9
It has been shown that the effects of a normal BM microenvironment compared to a MM microenvironment on MM progression are significantly different. While exosomes derived from normal-BMSCs inhibited tumor growth, exosomes derived from MM-BMSCs induced tumor growth.4 It was also shown that normal or MM-derived exosomes differentially affect MM cell homing and growth in vivo.15 Altogether, these studies warrant the need for a better understanding of the role of the malignant BM microenvironment in MM.
The current models used to study stroma in MM rely on a series of stromal cell lines (HS-5, HS-21, HS-23, and HS-27) derived from normal subjects.16 Alternatively, stromal cells from MM patients are frequently used where mononuclear cells from BM samples are obtained after density gradient centrifugation using Ficoll-Paque and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with serum. After a few days in culture, non-adherent cells are removed, and BMSCs are selected by their adherence to the plastic plates.9 The use of the primary stromal cells derived from different patients can result in non-reproducible results due to each laboratory having their own technique and methodology; there is a lack of cell line authentication and the heterogeneity between patients is enormous.17 There is therefore an urgent need to develop a MM-derived stromal cell line which will provide the effects of a malignant environment while simultaneously improving the reproducibility of data derived from studies using primary stromal cells.
In this study, we established and characterized a myeloma-derived stromal cell line named Myeloma Stromal Puente-1 (MSP-1) (Figure 1A); we started with 10 BM biopsies, after the initial month in culture we were left with 3 cell lines, and only one (MSP-1) was proliferative after 2 months in culture. We further investigated the differences between this cell line and two cell lines derived from the BM of healthy subjects (HS-5 and HS-27A). We further studied the performance of the MSP-1 cell line in classic 2D cultures and in a more physiologically relevant 3D culture system that we developed to accurately recapitulate the complex biology of BM microenvironment in MM.13
Figure 1.
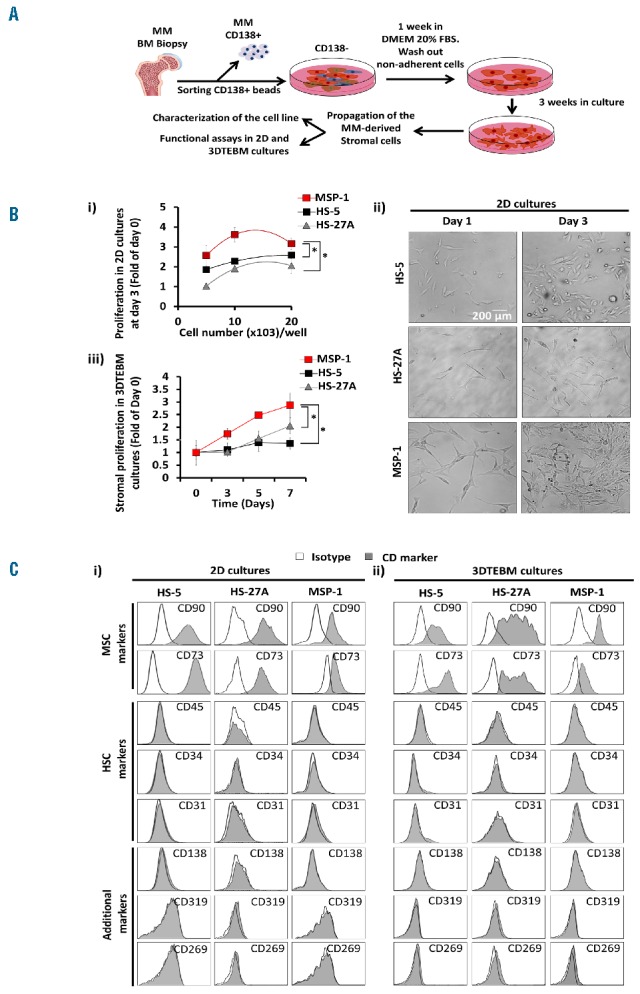
Establishment and characterization of myeloma-derived stromal cell line in 2D and 3DTEBM cultures. (A) Design of strategy for establishment of myeloma-derived stromal cell line. Primary CD138- cells were isolated from BM aspirate of MM patient 1 (female, had 9% plasma cells with progressive disease; gene expression analysis: Trisomy 1q21, Monosomy 13, 13q Deletion; treatment history prior to banking: Thalidomide, Revlimid, Dexamethasone, Ibrutinib, ASCT, and Velcade) from the Siteman Cancer Center, Washington University in Saint Louis, by CD138-magnetic-bead sorting and bone marrow aspirates were subjected to Ficoll-Paque gradient centrifugation. Informed consent was obtained from all patients with an approval from the Washington University Medical School IRB committee and in accord with the Declaration of Helsinki. Primary CD138- cells were cultured in DMEM supplemented with 20% fetal bovine serum, non-adherent cells were washed out, then stromal cells were maintained on DMEM culture for 3 weeks and monitored for the development of spindle-shaped cells. Establish myeloma-derived stromal cell line named Myeloma Stromal Puente-1 (MSP-1) was initially characterized and then frozen. Cultures were tested for mycoplasma and the results were negative, and the name of the cell line was checked against the International Cell Line Authentication Committee (ICLAC). B) Effect of stromal cell density (HS-5, HS-27A and MSP-1) (5 × 103 – 20 × 103 cells/well) on i) proliferation in 2D cultures by flow cytometry analysis, ii) representative images of 2D cultures at day 1 and 3; effect of time of culture on stromal (HS-5, Hs-27A and MSP-1), and iii) proliferation in 3DTEBM cultures measured by flow cytometry analysis. Scale bar= 200 μm, (*) P< 0.05. Morphological observations of cells in 2D were performed with an inverted microscope (Zeiss Axiovert 35 and camera Canon EOS rebel T3). C) Flow cytometry representative histogram of expression of mesenchymal stromal markers (MSC) markers (CD90, CD73), hematopoietic stem cell markers (HSC) (CD45, CD34), MM markers (CD138, CD269/BCMA, CD319/SLAMF7) and endothelial markers (CD31) (grey) and respective isotype controls (white) in stromal cells maintained in i) 2D cultures and ii) 3DTEBM cultures. Experiments were performed in triplicates (Figure 1Bii, Figure 1C) or quintuplicates (Figure 1Bi, Figure 1Biii) and each experiment was repeated 3 times. Results are shown as mean ± standard deviation and analyzed using two-way ANOVA for statistical significance, and were considered significantly different for P value less than 0.05.
Stromal cell lines (HS-5, HS-27A and MSP-1) (5 – 20×103 cell/well) were cultured in 2D or in relevant three-dimensional tissue engineered bone marrow (3DTEBM) cultures as previously described.13 To investigate the stromal cell behavior and influence on MM proliferation in 2D and 3DTEBM cultures, MM and stromal cells (1×106 cells/ml) were pre-labeled with Invitrogen cell tracers DiO (10 μg/ml) and DiD (10 μg/ml) for 1 hour, respectively. 3DTEBM cultures were developed through cross-linking of fibrinogen, as previously described.13,18,19 Cell proliferation assays were performed by digestion of 3DTEBM cultures with type I collagenase (25 mg/ml for 2 – 3 hours at 37oC), and classic 2D cultures were washed with PBS and removed by pipetting or trypsinization. For all flow cytometry analyses, internal standard control cells labeled with Calcein violet were added, a minimum of 5,000 control events were acquired using MACSQuant Analyzer (Miltenyi Biotec) and the data were analyzed using FlowJo program v10 (Ashland, OR). MM or stromal cells were identified by gating cells with a high DiO or DiD signal, respectively. The effect of cell density (2D cultures) and culture time (3DTEBM cultures) on growth rate was measured by flow cytometry. It was shown that in 2D cultures MSP-1 had a higher expansion rate at different cell densities than HS-5 and HS-27A (Figure 1Bi). Additionally, adherent MSP-1 cells had a multipolar morphology while HS-5 and HS-27A had a fibroblast-like bipolar morphology (Figure 1Bii). Several models have illustrated the superior ability of 3D cultures to recreate the BM microenvironment and promote MM cell growth compared to classic 2D cultures.13,20,21 We have recently shown that a novel 3DTEBM model based on fibrinogen naturally found in the BM supernatant of MM patients recapitulated the pathophysiological environment of MM, recreated the interactions of MM cells with their microenvironment more accurately, allowed the progression of primary patient samples and showed significantly higher resistance to proteasome inhibitors (PIs) compared to other 3D culture models.13
We characterize the behavior of MSP-1 compared to HS-5 and HS-27A in these 3DTEBM cultures. Stromal proliferation was found to be significantly higher for MSP-1 than HS-5 and HS-27A in 3DTEBM cultures after 7 days (Figure 1Biii). Specific cell surface markers were used to confirm stromal origin of the cells.22 The MSP-1 cell line, similar to HS-5 and HS-27A, expressed CD90 and CD73 and lacked the expression of hematopoietic stem cell (CD45 and CD34), endothelial (CD31), and myeloma (CD138, CD269/BCMA and CD319/SLAMF7) markers in 2D cultures (Figure 1Ci). Thereafter, we confirmed that MSP-1, HS-5 and HS-27A, when cultured in the 3DTEBM, maintained their expression profile of stromal markers (as in 2D cultures) (Figure 1Cii).
The role of BM microenvironment in mediating proliferation, cell trafficking and homing, and resistance to therapy in myeloma is well characterized.2,3,9,10 We further demonstrated the effect of MSP-1 on MM proliferation, migration, adhesion, and drug resistance in 2D cultures and 3DTEBM cultures. MM1s proliferation increased modestly (15–20%) in co-culture with MSP-1, HS-5 and HS-27A in 2D cultures compared to MM proliferation alone, and no effect was observed on the cell line H929 (Figure 2Ai). However, while in MM1s and H929 proliferation there was not a significant difference in co-culture with HS-27A and HS-5 in 3DTEBM cultures, MSP-1 co-culture had significantly greater increases (1.75–2.25-fold) than MM proliferation alone (Figure 2Aii). Therefore, 3DTEBM cultures with MSP-1 promoted superior growth compared to 3D co-cultures with HS-5 or HS-27A. This is proof of the concept that different stromal cell lines have different effects on the proliferation of MM, and the patient-derived MSP-1 cell line promoted higher MM cell proliferation. Confocal imaging confirmed that mono-cultures of MSP-1 stromal (red) and MM cells (green) grew through the scaffold over time by forming sheetlike structures and by increasing density all over the 3DTEBM culture, respectively. When MM and MSP-1 cells were co-cultured, the distribution remained the same but the MM cells showed enhanced cell density with more tumor bulk (Figure 2B).
Figure 2.
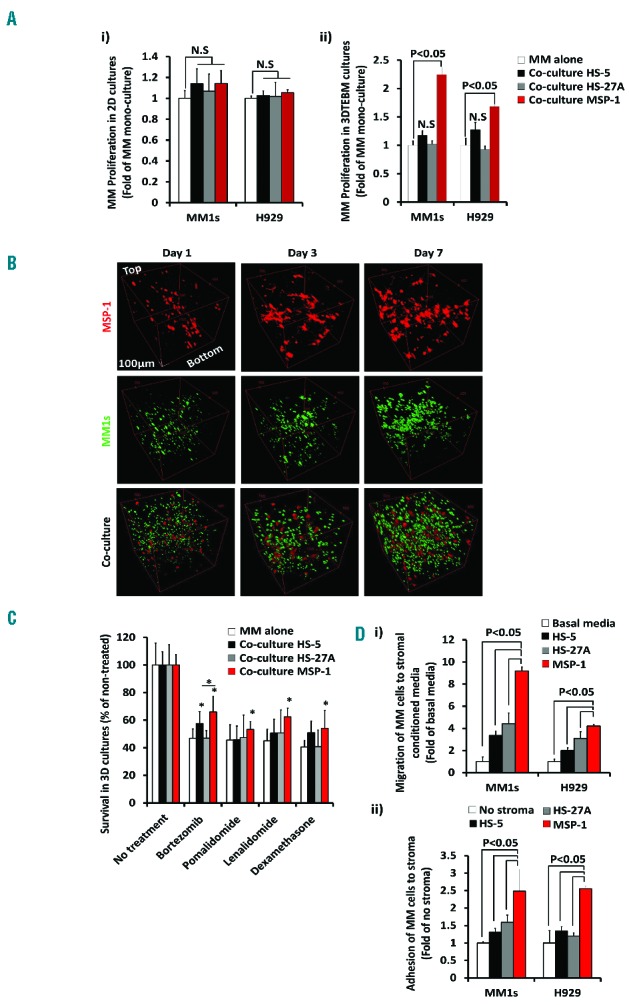
Effect of myeloma-derived stromal cell line on MM proliferation, migration, adhesion, and drug resistance. A) Effect of stromal cells (HS-5, HS-27A or MSP-1) on MM proliferation after 3 days in culture in i) 2D cultures or ii) 3DTEBM cultures measured by flow cytometry analysis normalized to MM cultured alone. B) Confocal microscopy images of mono-culture of MSP-1-DiD (red) (up), MM1s-GFP (green) (intermediate), or co-culture of both cells (down) in 3DTEBM after 1(left), 3 (middle) and 7 days (right), shown from Z-Stack rotated view; Scale bar= 100 μm. The 3DTEBM cultures were imaged using a FV1000 confocal microscope with an XLUMPLFLN 20XW/1.0 immersion objective lens (Olympus, PA, USA). Z-stack images of approximately 1 mm thickness were taken of each sample at 2 μm step sizes. Each frame consisted of a 520 × 520 pixel image, taken at a rate of 1 μs/pixel. C) The effect of bortezomib (30 nM), pomalidomide (60 μM), lenalidomide (60 μM), and dexamethasone (6 μM treatment) on MM cell survival cultured alone or in co-cultured with stromal cells (HS-5, HS-27A and MSP-1) for 48h in 3DTEBM cultures, normalized to no treatment, (*) P<0.05. D) i) The effect of stromal conditioned media on MM cell chemotaxis analyzed by flow cytometry and shown as a percentage of migrated cells normalized to cells which migrated toward non-conditioned basal media, ii) or effect of stromal cells on MM adhesion analyzed by flow cytometry and shown as a percentage of adhered cells normalized to cells which adhered to no stroma. For migration assays, MM cells were plated in the upper chamber of a Transwell migration plate (Costar, Corning) and were allowed to transmigrate into the lower chamber containing conditioned media of stromal cells according to manufacturer’s instructions. After 4hrs of incubation, the cells which migrated to the lower chamber were counted using flow cytometry. For adhesion assays, monolayer of stromal cells was plated overnight; MM cells were pre-labeled with calcein AM, then co-cultured and let to adhere to stroma or control (no stroma) for 1 hour. Non-adherent cells were washed with PBS, and adherent cells were measured by detecting the fluorescent intensity signal using fluorescent reader (Ex/Em = 485/520 nm). Experiments were performed in triplicates (Figure 2B) or quintuplicates (Figure 2A, C and D) and each experiment was repeated 3 times. Results are shown as mean ± standard deviation and analyzed using student t-test for statistical significance, and were considered significantly different for P value less than 0.05.
MSP-1, in contrast to HS-5 or HS-27A, induced a higher resistance to different contemporary therapies including proteasome inhibitors (bortezomib), immunomodulatory drugs (lenalidomide and pomalidomide) and steroid drugs (dexamethasone) than MM mono-culture alone. The drug concentrations chosen reflect 50% killing (IC50) of MM cells in the 3D mono-culture model. In the case of co-culture with HS-27A, no increase in drug resistance was appreciated, and with HS-5 a modest (non-significant) increase in drug resistance was found compared to mono-culture, with the exception of HS-5 and bortezomib treatment (significant). Co-cultures with MSP-1 induced significantly more resistance by killing only 20 – 40% of the MM cells (Figure 2C). Once again, these results demonstrate the importance of BM microenvironment aspects on drug resistance including interactions with stroma-derived from MM patients and a pathophysiological relevant 3D structure.13 Finally, MSP-1 was shown to significantly enhance migration of MM cells towards MSP-1-conditioned media compared to media from HS-5, HS-27A and control media (basal migration) (Figure 2Di). In addition, MSP-1 was shown to significantly increase adhesion of MM cells to MSP-1 compared to HS-5, HS-27A (Figure 2Dii) or no stroma (control), due to the differences in the culture milieu of myeloma-derived stroma versus healthy stroma.4,23
In conclusion, we have established and characterized the myeloma-derived stromal cell line MSP-1. This cell line was found to have a multi-polar morphology, proliferate better than normal stromal cells (HS-5 and HS-27A), and express stromal cell markers in 2D cultures as well as in 3DTEBM cultures made from BM supernatants of MM patients. Moreover, we have confirmed the effect of MSP-1 interactions in the BM microenvironment on MM and found that MSP-1 affected proliferation, adhesion, migration and drug resistance in a more profound manner than HS-5 and HS-27A, especially when cultured in the 3DTEBM.
Further research will need to focus on the development of additional stromal cell lines derived from MM patients with diverse genetic backgrounds since we understand that the limitation of the specific genetic background of the MSP-1 cell line will not represent the heterogeneity between MM patients.17
Footnotes
Funding: this research was partially supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and the National Cancer Institute of the NIH under Award Number U54CA199092.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14(9):2519–2526. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Mitsiades C, Tonon G, et al. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–598. [DOI] [PubMed] [Google Scholar]
- 3.Hazlehurst LA, Dalton WS. Mechanisms associated with cell adhesion mediated drug resistance (CAM-DR) in hematopoietic malignancies. Cancer Metastasis Rev. 2001;20(1–2):43–50. [DOI] [PubMed] [Google Scholar]
- 4.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17(6):1175–1182. [DOI] [PubMed] [Google Scholar]
- 6.Mitsiades CS, McMillin DW, Klippel S, et al. The role of the bone marrow microenvironment in the pathophysiology of myeloma and its significance in the development of more effective therapies. Hematol Oncol Clin North Am. 2007;21(6):1007–1034. [DOI] [PubMed] [Google Scholar]
- 7.Caligaris-Cappio F, Bergui L, Gregoretti M, et al. Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 1991;77(12):2688–2693. [PubMed] [Google Scholar]
- 8.Hideshima T, Chauhan D, Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1(7):539–544. [PubMed] [Google Scholar]
- 9.Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azab AK, Hu J, Quang P, et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood. 2012;119(24):5782–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gazitt Y, Akay C. Mobilization of myeloma cells involves SDF-1/CXCR4 signaling and downregulation of VLA-4. Stem Cells. 2004;22(1):65–73. [DOI] [PubMed] [Google Scholar]
- 12.Roccaro AM, Sacco A, Purschke WG, et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014;9(1):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Puente P, Muz B, Gilson RC, et al. 3D tissue-engineered bone marrow as a novel model to study pathophysiology and drug resistance in multiple myeloma. Biomaterials. 2015;73:70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidmaier R, Baumann P, Simsek M, et al. The HMG-CoA reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of Rho protein and activation of Rho kinase. Blood. 2004;104(6):1825–1832. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi G, Munshi NC. Pathogenesis beyond the cancer clone(s) in multiple myeloma. Blood. 2015;125(20):3049–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85(4):997–1005. [PubMed] [Google Scholar]
- 17.Broyl A, Hose D, Lokhorst H, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010;116(4):2543–2553. [DOI] [PubMed] [Google Scholar]
- 18.de la Puente P, Ludena D, Lopez M, et al. Differentiation within autologous fibrin scaffolds of porcine dermal cells with the mesenchymal stem cell phenotype. Exp Cell Res. 2013;319(3):144–152. [DOI] [PubMed] [Google Scholar]
- 19.de la Puente P, Ludena D, Fernandez A, et al. Autologous fibrin scaffolds cultured dermal fibroblasts and enriched with encapsulated bFGF for tissue engineering. J Biomed Mater Res A. 2011;99(4):648–654. [DOI] [PubMed] [Google Scholar]
- 20.Kirshner J, Thulien KJ, Martin LD, et al. A unique three-dimensional model for evaluating the impact of therapy on multiple myeloma. Blood. 2008;112(7):2935–2945. [DOI] [PubMed] [Google Scholar]
- 21.Calimeri T, Battista E, Conforti F, et al. A unique three-dimensional SCID-polymeric scaffold (SCID-synth-hu) model for in vivo expansion of human primary multiple myeloma cells. Leukemia. 2011;25(4):707–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boxall SA, Jones E. Markers for Characterization of Bone Marrow Multipotential Stromal Cells. Stem Cells Int. 2012;2012:975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burness ML, Sipkins DA. The stem cell niche in health and malignancy. Semin Cancer Biol. 2010;20(2):107–115. [DOI] [PubMed] [Google Scholar]


