Management of acute leukemia has seen a trend toward outpatient treatment strategies to reduce the negative effects of prolonged hospitalization.1 Outpatient treatment requires frequent hospital visits with ongoing transfusion support, symptom management, and infection surveillance. Physical activity and psychosocial support have been applied as rehabilitative strategies to prevent functional decline and manage treatment-related symptoms predominantly in oncology patients following adjuvant chemotherapy.2 There is a need for clinical-based comprehensive rehabilitation strategies initiated early, i.e. prior to stem cell transplantation, that address the specific needs and challenges, i.e. physical deterioration and treatment-related symptoms, of patients with acute leukemia during treatment.3,4
We conducted a randomized controlled trial (PACE-AL; clinicaltrials.gov identifier: 01404520)5 to investigate the effect of a 12-week multimodal intervention of aerobic, strength and relaxation exercise, nutrition support, pedometer and health counseling in patients with acute leukemia during consolidation treatment. It was hypothesized that the multimodal intervention would minimize physical and functional debilitation, increase physical activity levels, reduce the symptom burden, i.e. fatigue, and improve health-related quality of life (HRQOL) and psychological wellbeing.
Patients aged 18 years and over with a diagnosis of acute leukemia were consecutively recruited from two Copenhagen University hospitals from July 2011 to January 2014; patients in complete remission after completing induction treatment were eligible. Exclusion criteria were: severe or unstable psychological, cardiorespiratory, neurological or musculoskeletal disease, secondary active malignancy, and abnormal electrocardiogram. Patients were randomized to an intervention (IG) or control group (CG) and stratified for age (<45>years), sex, and treatment facility.
The IG group received standard care and the 12-week multimodal intervention during consolidation treatment. Each session was 1 hour +/− 10 min. between 10 am and 12 pm, three days a week, and took place during the patient’s outpatient visit. A safety precaution procedure was instituted due to the risks associated with pancytopenia, i.e. participation if platelets were 15×109/L or over.5 The supervised session consisted of stationary cycling for 20–25 min with six work intervals to reach 80% of the maximal heart rate, six dynamic resistance exercises using hand weights in 2 sets of 12 repetitions, guided relaxation, and nutrition support (16–19 g protein and 1600–2000 kj carbohydrate) within 30 min of exercise. Patients were fitted with a pedometer (Omron Walking Style Pro) at the first exercise session. Counseling sessions (CS) of 30–60 min were conducted at baseline, six and 12 weeks. CS were goal-oriented, schematized and carried out individually to create a partnership with the patient to secure adherence to the intervention and to increase the knowledge, skills and confidence needed to promote positive health behavior. The CG received standard care that did not include supervised physical activity and/or health counseling. Patients were not asked to refrain from physical activity.
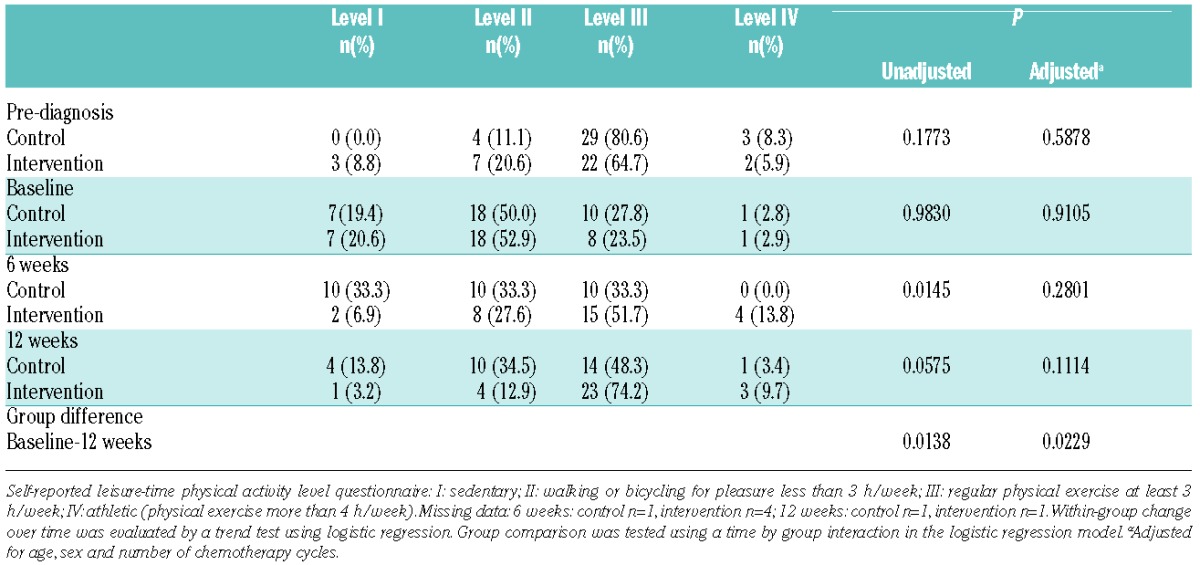
Of 94 eligible patients, 70 (mean 53.1 years) were randomly assigned to the IG (n=34) or CG (n=36). During the study period, two IG patients dropped out; one due to emotional reasons, and one died from disease progression (2.9% attrition). In the CG, 5 patients withdrew due to lack of motivation and one developed medical complications (8.6% attrition). In all, 62 of 70 patients (88.6%) completed study requirements. There were no statistically significant differences between groups on demographic and medical baseline data; however, we supplemented the analyses described in the protocol with analyses adjusted for age, sex, and number of chemotherapy cycles (Tables 1–3). Changes in the primary outcome 6-minute walk distance (6MWD) differed significantly across groups in favor of the IG [85.5 m (51.2–119.8); P<0.0001; effect size 1.00]. The secondary outcomes (physical capacity) were substantial (P<0.0001; effect sizes 1 or above) (Table 1). Self-reported leisure-time physical activity levels increased significantly (Table 2). Sedentary behavior reported at baseline of 19.4% and 20.6% in the CG and IG, respectively, decreased to 13.8% and 3.2% at 12 weeks.
Table 1.
Effect on physical capacity and functional performance (n=70).
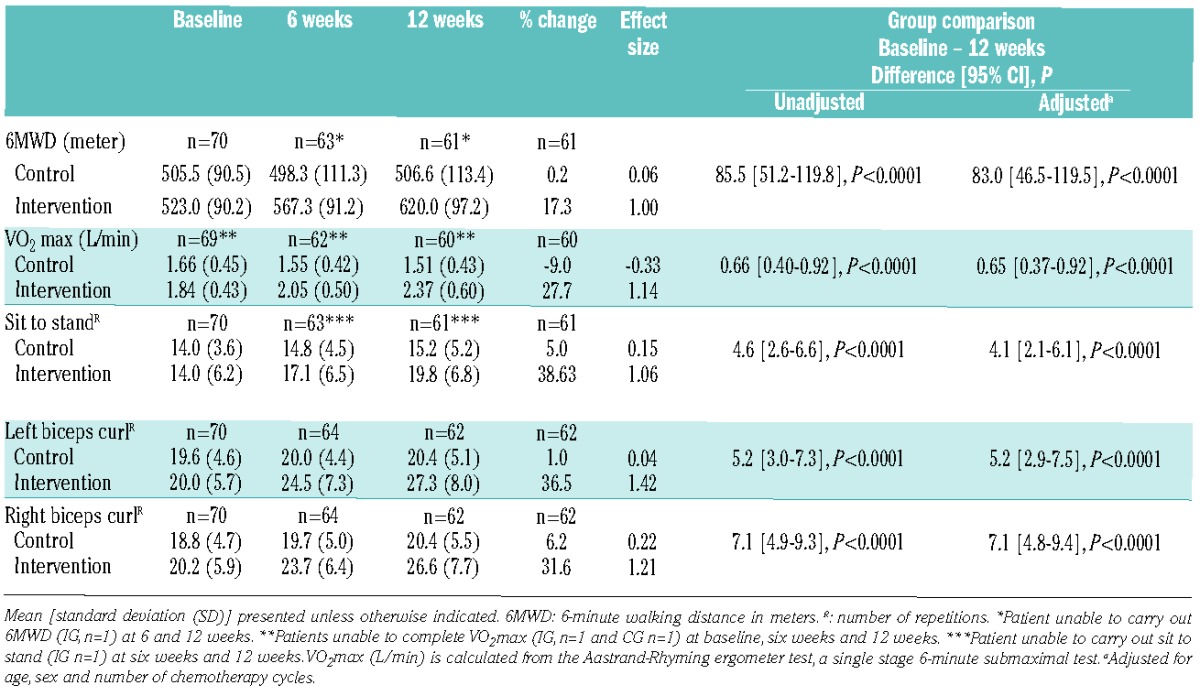
Table 3.
Patient-reported outcomes for health-related quality of life and psychological wellbeing.
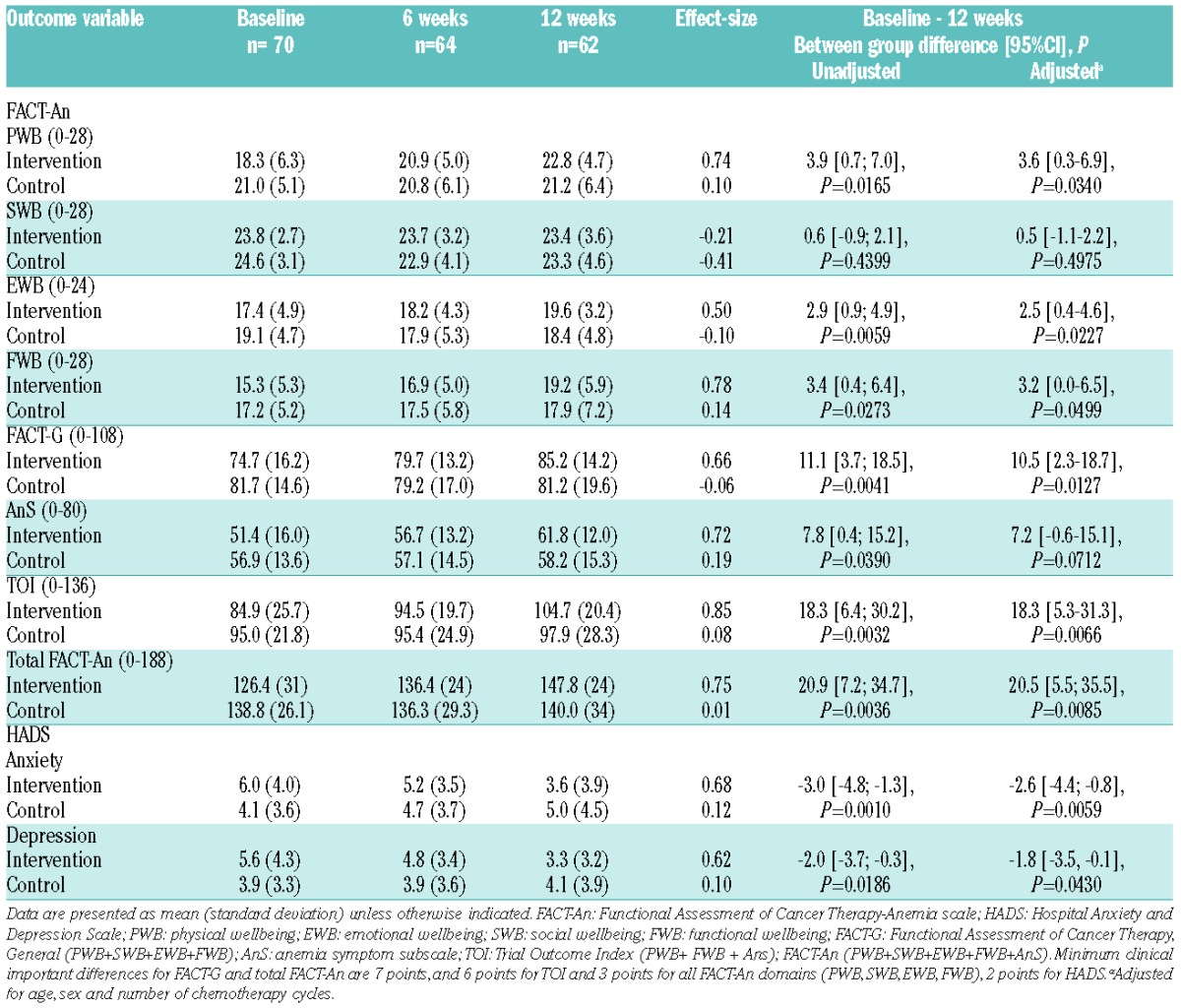
Table 2.
Effect on leisure-time physical activity level (I–IV) pre-diagnosis, baseline, 6 weeks and 12 weeks.
The Functional Assessment of Cancer Therapy-Anemia scale (FACT-An) showed significant intervention effects for the domains’ functional, physical and emotional wellbeing. Total scores for FACT-G, fatigue subscale (AnS), Trial Outcome Index and FACT-An were statistically and clinically significant. Anxiety and depression were significantly reduced on the Hospital Anxiety and Depression Scale (HADS) (Table 1). There were significant between-group differences on SF36 physical health component scale (P=0.0198) and EORTC QLQ-C30 domains’ global health (P=0.0069), emotional functioning (P=0.0258) and nausea and vomiting (P=0.0081); however, fatigue did not reach between-group significance. A significant correlation was found between 6MWD and VO2max (r=0.5277; P=<0.0001) and between changes in 6MWD and FACT-An (r=0.2694; P=0.0467) and anxiety (r=−0.35891; P=0.0045).
Intervention group adherence to supervised sessions (aerobic, strength, relaxation, nutrition) was 70.8%; mean 25.5 sessions (range 8.0–36.0), CS 95.9% and pedometer participation was 83.6%, mean 10.0 weeks (range 3–12). Non-adherence was due to elevated temperature and hospitalization, treatment-related symptoms and outpatient scheduling and travel constraints.
The daily mean pedometer steps and aerobic steps from baseline to 12 weeks increased by 21.3% and 14.1%, respectively. IG, compared to the CG, had fewer means days where CRP level was over 10 (11 vs. 14), days with neutropenia (25 vs. 28), and RBC and platelet transfusions (7 vs. 8; 6 vs. 7), although these differences were not significant. Eight IG patients (23.5%) experienced non-serious adverse events: sport-related (n=5), cardiorespiratory (n=5), dizziness (n= 3), gastrointestinal (n= 3), pain/discomfort (n=2) and bruising (n=1).
The intervention was effective in increasing outcomes to healthy reference levels for the objective physical assessments. The CG maintained walk-distance and incrementally increased lower body strength; however, they did not reach those values of the healthy population. The IG increased 6MWD by 17.3%; in line with exercise studies during induction (15.5%)6 and consolidation (11.1%).7 According to Prichard et al., 6MWD predicts aerobic capacity8 and, in our study, we found a significant correlation between 6MWD and aerobic capacity. The IG increased aerobic capacity by 27.7%, while the CG reduced it by 9.0%. The intervention may have facilitated return to pre-diagnosis levels. Upper body strength at baseline was an average of normal values and may indicate that induction had a less negative effect on upper body strength. This is in contrast to results that found upper body fitness in patients with AML to be below healthy values, with less improvement over time.9 The current multimodal intervention showed greater physical/functional effects than demonstrated in exercise-only studies carried out in patients with AML during induction treatment.6,10–12 Dietary support directly after exercise has been shown to speed recovery by rebuilding muscle tissue13 and may be considered a relevant component in achieving recovery during treatment.
An increase in walk distance was statistically associated with better HRQOL, suggesting that physical and functional capacity may generate HRQOL and psychological benefits. Significant and clinically important improvements were found for global health, emotional, physical and functional wellbeing, and nausea and vomiting. Fatigue was found to be significant on the FACT-An subscale, though this did not reach between-group significance on EORTC QLQ-C30, which other exercise studies have demonstrated may be attributed to the sample size. Gastrointestinal benefits have been demonstrated in exercise studies of hematologic patients during chemotherapy.4,7 In-hospital exercise studies of 3–6 weeks have been shown to reduce depression in older acute myeloid leukemia patients,14 increase vitality, and reduce symptom burden and depression.7,10,11 Psychosocial interventions such as relaxation have been shown to have a positive effect on emotional wellbeing applied alone, and a greater effect when combined with exercise. Counseling in combination with exercise in cancer survivors has been found to have a positive effect on lifestyle behavior and QOL.15 The pedometer was applied as motivation for independent physical activity. The IG became more physically active, returning to pre-diagnosis levels despite the fact that the majority of patients received two cycles of chemotherapy (67.7%) while experiencing symptoms, i.e. fatigue and complex medical issues. Our results indicate that physically inactive behavior can be positively modulated during chemotherapy; however, the daily improvements achieved did not meet national recommendations.
The strengths of this study include recruitment of a well-defined population, and a sample with comparable between-group demographic and medical characteristics. Aspects of treatment at both hospitals were standardized which decreased variations in medical outcomes. The mean sample age was 54 years, which may pose a question of applicability for an older population. The program was supervised, structured and uniform in modality, intensity, frequency, duration and progression; however, due to the multimodality of the intervention, we cannot conclude on the mechanistic effect of individual components. In general, statistical uncertainty related to the secondary outcomes must be considered.
In conclusion, we found a 12-week supervised multimodal intervention produced clinical benefits in patients with acute leukemia undergoing outpatient consolidation treatment with significant effects on physical and functional capacity measures and on physical activity levels. Significant and clinically important improvements on HRQOL and reduced symptoms of fatigue, nausea/vomiting, anxiety and depression, highlight the importance of the role of exercise and counseling in patients with acute leukemia integrated in the clinical setting. Multimodal approaches can activate and provide safety for patients with low levels of physical activity during intensive and demanding treatment trajectories. The intervention took advantage of the opportunity presented by the patients’ hospital visits and was offered while the patients were waiting to be seen. The study confirms that exercise and counseling can counteract physical debilitation and improve HRQOL in a cancer population with frequent outpatient visits, severe pancytopenia, complex symptom burden, and uncertain prognosis. The multimodal intervention integrated into the clinical management of acute leukemia may optimize the treatment and care pathway by reducing treatment-related physical and psychological symptoms and enable better treatment toleration, and in this way can ease the patients’ transition from illness to the resumption of everyday activities.
Acknowledgments
The authors present this paper on behalf of the PACE-AL clinical study groups at Copenhagen University Hospital, Rigshospitalet and Herlev Hospital, and acknowledge the patients and health-care professionals for their participation.
Footnotes
Funding: the research was supported by The Center for Integrated Rehabilitation of Cancer Patients (CIRE), a center established and supported by The Danish Cancer Society and The Novo Nordic Foundation, The University Hospitals’ Centre for Health Research (UCSF), The Lundbeck Foundation, The Novo Nordic Foundation for Clinical Nursing Research and The Danish Cancer Society.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Møller T, Nielsen OJ, Welinder P, et al. Safe and feasible outpatient treatment following induction and consolidation chemotherapy for patients with acute leukaemia. Eur J Haematol. 2010;84(4):316–322. [DOI] [PubMed] [Google Scholar]
- 2.Stubblefield MD, Hubbard G, Cheville A, et al. Current perspectives and emerging issues on cancer rehabilitation. Cancer. 2013;119 Suppl 11:2170–2178. [DOI] [PubMed] [Google Scholar]
- 3.Paul KL. Rehabilitation and exercise considerations in hematologic malignancies. Am J Phys Med Rehabil. 2011;90(5 Suppl 1):S88–94. [DOI] [PubMed] [Google Scholar]
- 4.Jarden M, Baadsgaard MT, Hovgaard DJ, et al. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Tansplant. 2009;43(9):725–737. [DOI] [PubMed] [Google Scholar]
- 5.Jarden M, Møller T, Kjeldsen L, et al. Patient Activation through Counseling and Exercise - Acute Leukemia (PACE-AL): a randomized controlled trial. BMC Cancer. 2013;13:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alibhai SMH, O’Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012;36(10):1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarden M, Adamsen L, Kjeldsen L, et al. The emerging role of exercise and health counseling in patients with acute leukemia undergoing chemotherapy during outpatient management. Leuk Res. 2013;37(2):155–161. [DOI] [PubMed] [Google Scholar]
- 8.Prichard RA, Juul M, Gazibarich G, et al. Six-minute walk distance predicts VO₂ (max) in patients supported with continuous flow left ventricular assist devices. Int J Artif Organs. 2014;37(7):539–545. [DOI] [PubMed] [Google Scholar]
- 9.Alibhai SMH, Breunis H, Timilshina N, et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol. 2015;6(4):262–271. [DOI] [PubMed] [Google Scholar]
- 10.Chang P-H, Lai Y-H, Shun S-C, et al. Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage. 2008;35(5):524–534. [DOI] [PubMed] [Google Scholar]
- 11.Battaglini CL, Hackney AC, Garcia R, et al. The effects of an exercise program in leukemia patients. Integr Cancer Ther. 2009;8(2):130–138. [DOI] [PubMed] [Google Scholar]
- 12.Elter T, Stipanov M, Heuser E, et al. Is physical exercise possible in patients with critical cytopenia undergoing intensive chemotherapy for acute leukaemia or aggressive lymphoma? Int J Hematol. 2009;90(2):199–204. [DOI] [PubMed] [Google Scholar]
- 13.Beelen M, Burke LM, Gibala MJ, et al. Nutritional strategies to promote postexercise recovery. Int J Sport Nutr Exerc Metab. 2010;20(6):515–532. [DOI] [PubMed] [Google Scholar]
- 14.Klepin HD, Danhauer SC, Tooze JA, et al. Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. J Geriatr Oncol. 2011;2(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract. 2002;10(4):208–215. [DOI] [PubMed] [Google Scholar]


