Abstract
Maternal hypothyroxinemia secondary to iodine deficiency may have neurodevelopmental effects on the specific neurocognitive domain of memory. Associated disruption of thyroid hormone–dependent protein synthesis in the hippocampus has the potential to result in compromised development of the structure with consequential impairments in memory function. Despite links between maternal iodine deficiency during gestation and lactation and abnormal hippocampal development in rat fetuses and pups, there has been little research on the specific function of memory in human infants and young children born to iodine-deficient mothers. Several candidate measures have proven to be sensitive to the effects of gestational iron deficiency on memory function in infants and young children, including habituation and dishabituation, imitation-based tasks, and event-related potentials. Such measures could be used to test the effects of maternal iodine supplementation on the specific neurocognitive domain of memory in infants and young children. Furthermore, progress in understanding the effects of maternal iodine supplementation on neurocognitive development could be accelerated by the development of a nonhuman primate model to complement the rodent model.
Keywords: hippocampus, infant neurodevelopment, iodine status, memory, thyroid hormone
INTRODUCTION
In humans and other animals, thyroid hormone is required for normal physical development and neurodevelopment. In humans, the mother is the sole source of thyroid hormone until the fetal thyroid develops in the second trimester of gestation, and continues to be a supplementary source in late gestation (1). Iodine bound to tyrosine is an intrinsic component of thyroid hormone. Adequate intake of dietary iodine is required for effective production of thyroid hormone, including the primary circulating form, thyroxine. Hypothyroxinemia (below-normal circulating concentrations of thyroxine in the presence of otherwise normal thyroid function) is a more frequent consequence of pregnancy-associated iodine deficiency than either clinical or subclinical hypothyroidism (2).
It is clear that the infants of women with severe iodine deficiency during pregnancy are at risk of cognitive deficits and that this risk is decreased by iodine supplementation (3). In the US population, nearly all pregnancy-associated iodine deficiency is in the mild to moderate range (4). There is less information on the neurodevelopmental effects of these lower levels of iodine deficiency during pregnancy (3). Indirect evidence of negative effects on neurocognitive development comes from a recent meta-analysis of studies in international populations in regions of mild to moderate iodine deficiency. The results indicated improvements in aspects of cognitive function in school-aged children whose mothers received iodine supplementation during pregnancy, with less recovery when supplementation was postnatal (5).
The specific neurocognitive functions that are negatively affected in cases of maternal hypothyroxinemia secondary to iodine deficiency—and thus that may benefit from iodine supplementation—are unknown. Memory is a strong candidate function. The hippocampus, which is involved in learning and memory, is rich in thyroid hormone receptors (6). In rat models, insufficient thyroid hormone during fetal development produces changes in the hippocampus that adversely affect neural growth and development (7). Similar effects in human fetuses could be expected to result in memory impairments (8). In this article we elaborate the basis for this specific concern and make recommendations for measures that could be used to test the potential effects of maternal iodine supplementation on the specific neurocognitive domain of memory in infancy and early childhood.
IMPORTANCE OF THE HIPPOCAMPUS TO MEMORY FUNCTION
The hippocampus is a compound neural structure located in the medial aspect of the temporal lobe. It is critical for the formation of new memories and for retrieval of old memories. The hippocampus plays a central role in the specific form of memory known as explicit or declarative. Declarative memory supports learning and retention of names, dates, places, and concepts (9); it is the type of learning assessed in academic settings, on which professional success depends (10, 11). It also underlies the ability to remember specific events and experiences, including those that are personally relevant and significant (12). The hippocampus is not required for many other forms of memory, for example, motor learning.
The role of the hippocampus in declarative memory function has been established through multiple lines of research. First, patients with lesions to the hippocampus and surrounding structures or with diseases that compromise the structure are impaired on tasks that require new declarative learning, especially when testing takes place after a delay (13). Second, rodents and nonhuman primates with physical or neurochemical lesions to the hippocampus manifest impaired performance on analogous tasks (14, 15). Third, in intact humans, neuroimaging studies that use positron emission tomography and fMRI show activations in the hippocampus during the encoding of new declarative memories and the retrieval of established memories (16).
DEVELOPMENT OF THE HUMAN HIPPOCAMPUS
The hippocampus is made up of 4 differentiated cell fields and the dentate gyrus. The CA1 and CA3 cell fields of the hippocampus mature relatively early in humans (17). The first neurons appear at 36–38 embryonic days. Different subfields of the hippocampus are discernable by the 10th gestational week; by the 32nd gestational week, the cells have migrated to their adult locations. There is some evidence of myelination of the neurons by the 39th gestational week.
The dentate gyrus of the human hippocampus undergoes a longer course of development. As in the cell fields, the first neurons appear at 36–38 embryonic days. Yet, there is still a high rate of cell proliferation between 16 and 22 postnatal weeks. Cell migration occurs as late as 8–11 mo. Within the dentate gyrus, the rise to the peak number of synapses is delayed until 9–12 mo and adult numbers are not reached until 4–5 y of age. Myelination begins in the fifth postnatal month and continues until ≥11 y of age (17).
The long course of development means that the hippocampus is vulnerable to insults such as nutrient deficiencies throughout gestation, as well as postnatally. Moreover, disruption confined to the prenatal period can have a cascading negative impact on later development because the later-developing dentate gyrus receives its inputs through the earlier-developing cell fields. If neuronal signaling from an earlier-developing region is disrupted, the experience on which the later-developing structure depends will be attenuated, with resulting compromise. Thus, an insult to the developing fetus may have long-lasting effects, even on structures that continue to develop postnatally.
RAT MODELS FOR THE EFFECTS OF MATERNAL IODINE DEFICIENCY AND DRUG-INDUCED HYPOTHYROIDISM ON HIPPOCAMPAL DEVELOPMENT
Rats are an appropriate model for understanding the effects of insufficient thyroid hormone during fetal development because the rat hippocampus undergoes protracted development analogous to that of the human hippocampus. For comparison, a rat pup on postnatal day 0 is developmentally equivalent to a human fetus at the start of the third trimester. Viewed the other way around, a newborn human infant is developmentally equivalent to a rat pup on postnatal day 10 (18).
Rat models of developmental hypothyroidism have been used to study the expression of developmentally significant proteins in the hippocampus. For example, one research group found that hypothyroidism produced by a severely iodine-deficient diet or treatment with the antithyroid drug propylthiouracil from gestational day 6 through postnatal day 28 resulted in underexpression or downregulation of doublecortin, a protein involved in neuronal differentiation and migration (7), and of p35, a protein involved in neuronal cell adhesion (19). By contrast, the 180 kDa isoform of neural cell adhesion molecule (NCAM-180),3 which plays a role in synaptic remodeling and long-term potentiation (persistent strengthening of synapses on the basis of recent activity), was found to be overexpressed or upregulated by the same researchers (7). These changes were observed throughout the CA1, CA3, and dentate gyrus regions, both pre- and postnatally. In the rat model, even marginal maternal iodine deficiency can adversely affect protein synthesis important to hippocampal development. Postnatal expression of early growth response 1 (EGR1) protein and brain-derived neurotrophic factor (BDNF) in the hippocampus was observed to be downregulated in the pups of marginally or moderately iodine-deficient rat dams, and the extent of this downregulation was in proportion to the degree of iodine deficiency (20).
Possibly as a result of dysregulated protein synthesis, hypothyroidism produced by gestational through postnatal iodine deficiency or propylthiouracil treatment as described above has been observed to result in structural differences in the hippocampi of rat pups. In one study, granule neuron maturation was reported to be heavily delayed (19). In another, nerve fibers seemed to be more fragile and more frequently broken in addition to being disordered, bundled, and/or fused with one another (7). Maternal hypothyroxinemia secondary to mild iodine deficiency has been found to reduce the immunofluorescence intensity of collapsing response mediator protein 2 (CRMP2) and Tau1, 2 indicators of axonal outgrowth, in rat fetuses on gestational day 19; changes in the expression of CRMP2 and various regulatory proteins (downregulation of some, upregulation of others) support the authors’ conclusion that axonal growth was impaired (21). The investigators found analogous evidence for impaired axonal growth in pups that were continued on a regimen of mild iodine deficiency throughout lactation (21). The same model of maternal hypothyroxinemia secondary to mild iodine deficiency during gestation and lactation has been found to yield decreases in the immunofluorescence of myelin basic protein and the staining of myelin sheaths in the hippocampi of rat pups, evidence of decreased myelination (22). Perhaps as a result of adverse effects on axonal growth and myelination, decreased excitability of hippocampal neurons was observed in the 4- to 6-mo-old offspring of iodine-deficient rat dams, despite having been fed an iodine-sufficient diet since weaning (23). Whether the observed changes contributed to impaired overt behavior is less clear, however. In the face of persistent reductions in excitatory synaptic transmission, the same study reported that maternal iodine deficiency during gestation and lactation did not affect the performance of the offspring on the Morris water maze (a spatial memory task) or trace fear conditioning tasks, both of which are dependent on hippocampal function.
EFFECTS OF CONGENITAL HYPOTHYROIDISM AND MATERNAL HYPOTHYROIDISM ON HUMAN HIPPOCAMPAL STRUCTURE AND MEMORY FUNCTION
In contrast to the sizable body of research on the effects of different degrees of maternal iodine deficiency on hippocampal development in the rat, there are no comparable studies in humans. Given the lack of a reliable biomarker for assessing iodine status in individuals (24), it would be difficult to design and conduct such a study. However, reliable means for ascertaining thyroid status are widely available. A few studies have addressed the effect of either congenital hypothyroidism or maternal hypothyroidism on hippocampal development, most in children and adolescents aged ≥9 y.
Congenital hypothyroidism is an endocrine disorder characterized by insufficient endogenous thyroid hormone production in late gestation (i.e., when the fetal thyroid normally begins to function) and after birth (25). Congenital hypothyroidism has been found to be associated with reduced hippocampal volume in children and adolescents (26). In some reports, memory performance of children and adolescents with congenital hypothyroidism was not found to be different from that of controls (27), whereas in others it was found to be significantly impaired (28, 29).
Children whose mothers were diagnosed with (and treated for) hypothyroidism at some point during pregnancy may have experienced thyroid hormone insufficiency earlier in gestation, before the initiation of treatment. In one longitudinal study, the children (9–15 y of age) of mothers diagnosed with hypothyroidism during pregnancy were found to recall fewer episodic details than control children in the context of an autobiographical memory interview, suggesting hippocampally related memory impairment (29). A follow-up study in a sample drawn from the same study population reported reduced hippocampal volume (as measured by MRI) in children aged 9–12 y whose mothers experienced hypothyroidism during their pregnancies (30). As shown in Table 1, volume reductions were apparent throughout the hippocampus. A similar pattern has been observed in adults with adult-onset hypothyroidism (6). In addition, as shown in Table 1, despite reductions in hippocampal volume, the children performed on par with control children on a number of standardized measures of memory function, even on tasks that involved a delay. Nevertheless, they differed from controls on a parent-report measure of everyday memory behavior, suggesting deficits in memory function not tapped by standardized measures (30).
TABLE 1.
Hippocampal volume and memory task performance in children of mothers with hypothyroidism during pregnancy and children of control mothers1
| Hypothyroid | Control | P | |
| Hippocampal volumes, mm3 | |||
| Total right hippocampus | 1828 ± 264 | 1982 ± 244 | 0.012 |
| Total left hippocampus | 1728 ± 239 | 1862 ± 218 | 0.019 |
| Memory task performance over a delay in hypothyroid and control groups | |||
| CMS dot locations | |||
| Short-delay recall2 | 10.2 ± 2.8 | 10.9 ± 2.9 | NS |
| Long-delay recall2 | 10.4 ± 3.0 | 11.3 ± 2.8 | NS |
| CMS stories: delayed recall2 | 12.0 ± 2.1 | 12.3 ± 2.8 | NS |
| TML word selective reminding, delayed2 | 10.0 ± 2.5 | 10.4 ± 1.5 | NS |
| TML visual selective reminding, delayed2 | 10.3 ± 1.5 | 10.4 ± 1.6 | NS |
| TML face memory, delayed2 | 10.3 ± 1.4 | 10.9 ± 1.3 | NS |
| Rey-Osterrieth, delayed recall3 | 14.5 ± 6.5 | 14.8 ± 5.5 | NS |
| BRIEF working memory3 | 17.7 ± 5.9 | 14.4 ± 4.9 | 0.04 |
| EMQ total3 | 49.9 ± 18.2 | 66.2 ± 30.4 | 0.04 |
Values are means ± SDs. Data are from reference 30. BRIEF, Behavioral Rating Inventory of Executive Function; CMS, Children’s Memory Scale; EMQ, Everyday Memory Questionnaire; TML, Test of Memory and Learning.
Presented as scale scores.
Presented as raw scores.
The finding by Willoughby et al. (30) of a dissociation between the outcomes for hippocampal volume (reduced) and performance on standardized measures of memory (largely unaffected) is surprising in light of the results of neuroimaging and behavioral studies in healthy children. In such studies, lower hippocampal volume (which has typically been observed in younger relative to older children) was found to be associated with lower levels of performance on behavioral measures of memory (31), as well as with lower levels of activation of the hippocampus during memory encoding and retrieval (32). A major difference between the approaches is the use of standardized memory measures in the study by Willoughby and coworkers in contrast to the use of potentially more sensitive experimental measures in neuroimaging studies of typical development.
SENSITIVE MEASURES OF MEMORY FUNCTION IN INFANCY AND EARLY CHILDHOOD
The literature on the development of memory is replete with measures that are highly sensitive to age-related and group differences in memory function (33). Here we focus on measures for infants and young children, populations most at risk of memory deficits that are a direct result of maternal iodine deficiency during pregnancy. There are 3 broad categories of memory measures applicable to infants and children too young to be tested with the verbal means typically used with older children (including standardized tests).
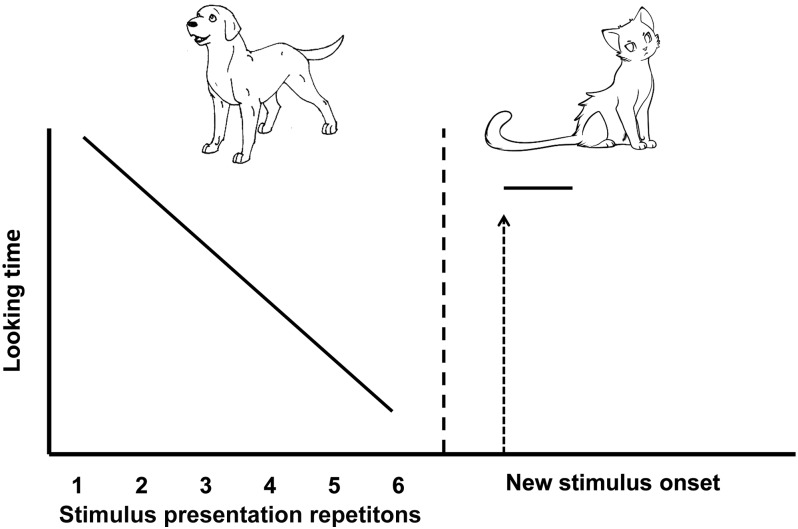
The first category of memory measures applicable to infants and young children involves assessment of looking or listening. It is well documented that with increasing familiarity with a stimulus, infants (as well as older children and adults) begin to prefer to look or listen to a novel stimulus. This tendency is exploited in measures of visual and auditory habituation and dishabituation and the related task of paired comparison. As depicted in Figure 1, in these tasks, a visual (or auditory) stimulus is presented repeatedly until looking (or listening) time decreases to a pre-established level. The familiar stimulus then is replaced with a novel stimulus (or is presented next to a novel stimulus). Memory of the original stimulus is inferred by differential looking (or listening) to the familiar and novel stimuli. The task can be used almost from birth. Measures of memory derived from looking-time tasks in infancy correlate with assessments of intelligence quotient in children as old as 11 y of age (34).
FIGURE 1.
Schematic illustration of stimuli and pattern of looking in a habituation and dishabituation paradigm. Looking time decreases as the original stimulus (dog) is repeated and recovers with the presentation of a new stimulus (cat).
The second category of memory measures for infants and young children involves the use of demonstration and imitation. Infants readily attempt to imitate behaviors that they find interesting. This tendency is exploited in imitation-based tasks in which props are used by an adult to demonstrate an interesting action or sequence of actions (35). Either immediately or after a delay (ranging from a few minutes to months), infants are given the props and permitted to imitate. Differences in performance in the postdemonstration phase relative to a predemonstration baseline are taken as evidence that the infant remembered the actions of the model. This task has been adapted for inclusion in the NIH Toolbox Picture Sequence Memory Task (11, 36). The imitation-based version of the task can be used as early as the second half of the first year of life and well into the preschool years. Measures of imitation-based task performance in the second year of life correlate with standardized assessments of memory at age 6 y (37).
The third category of memory measures for infants and young children involves the use of event-related potentials (ERPs), which are scalp-recorded electrical signals associated with postsynaptic potentials. ERPs are time-locked to a visual or auditory stimulus (e.g., mother’s voice or face compared with a stranger’s voice or face, or props used in an imitation-based task). The electrical signal is amplified and averaged over many trials. Typical measures are as follows: 1) mean amplitude of a response to a stimulus, which is reflective of the size of the population of neurons firing in synchrony to the stimulus, and 2) latency to peak amplitude of a response to a stimulus. Differences in amplitude and/or latency of response to stimuli in 2 classes (e.g., mother’s voice compared with a stranger’s voice) or more are indicative of differentiation of the stimuli. ERPs can be recorded even in newborns (38). They can be used alone or in conjunction with measures of looking or listening to differentiate familiar from novel stimuli (39).
APPLICATION OF SENSITIVE MEASURES OF MEMORY FUNCTION TO GESTATIONAL IRON DEFICIENCY
Poorly controlled maternal diabetes during pregnancy causes intrauterine hypoxia, which can lead to increased fetal erythropoiesis and consequent depletion of fetal iron stores (40). Tasks from all 3 of the broad categories of memory measures described above have been used to assess potential memory deficits in the infants and young children of women with poorly controlled diabetes (as evidenced by elevated blood glucose) during pregnancy. These measures have proven to be sensitive to differences in memory above and beyond the variance explained by the Bayley Scales of Infant Development; they have also proven to be sensitive to memory deficits in infants born before term, infants adopted from institutional care, and infants neglected by their caregivers (8).
To show the utility of the above-described measures as sensitive tests of memory function, we summarize reported longitudinal assessment results for infants of diabetic mothers (IDMs) and control infants at 3 time points in the first year of life. First, 10–12 d after birth, each infant listened to her or his mother’s voice or a stranger’s voice uttering the word “baby” over and over again. ERPs were recorded for each stimulus category. Control infants and IDMs responded comparably to the sound of the stranger’s voice. However, control infants differentiated the stranger’s voice from the mother’s voice, whereas IDMs did not (41). Second, at 8 mo of age, infants were tested for visual recognition of an object they previously had felt with their hands but had never seen (cross-modal recognition). Control infants differentiated between familiar and novel stimuli in their behavior and in their ERP responses. In contrast, IDMs did not differentiate the 2 classes of stimuli (42). Third, at 12 mo of age, infants were tested for imitation of a novel sequence of action immediately and after a 10-min delay. The immediate imitation performance of the 2 groups did not differ. Over the delay, the control infants retained their memories such that delayed performance did not differ from immediate performance. IDMs evidenced significantly lower memory after the delay relative to immediate performance and relative to controls (43). In summary, in each of 3 tests of memory function during the first year of life, IDMs (born iron deficient but supplemented at birth) had lower levels of memory performance relative to control infants. At birth and at 8 mo of age, infants who experienced prenatal iron deficiency failed to show evidence of memory for a stimulus; at 12 mo of age, they showed immediate memory but significant forgetting over a 10-min delay.
SUGGESTED APPLICATION OF SENSITIVE MEASURES OF MEMORY FUNCTION FOR INVESTIGATING NEURODEVELOPMENTAL OUTCOMES OF IODINE SUPPLEMENTATION IN PREGNANT WOMEN
The successful detection of memory function impairments in IDMs during the first year of life shows the promise of applying the same sensitive measures to test the potential effects of maternal iodine supplementation on memory function in infants and young children. We recommend pilot testing a battery of tasks modeled after those used in the case of iron deficiency, with tests shortly after birth to assess initial status and over the course of the first year or longer to assess persistence of effects. In the hours and days after birth, ERPs are optimal for assessment because they make no performance demands on the infants. Indeed, ERPs to sounds can be recorded while infants sleep (44). Auditory stimuli are especially well suited to tests in neonates because they bypass the less well-developed visual system.
As infants gain more control over their neck and hands and become able to sit upright and bring objects to their eyes for inspection, tests that use visual stimuli and matches between stimuli inspected visually and haptically (i.e., perceived through touch) become developmentally appropriate. Ideally, the test battery would incorporate both familiar and novel stimuli, thereby enabling testing of the recognition of information that should already have been encoded by the infant (e.g., the mother’s face or a favorite toy) as well as the recognition of information that must be newly encoded at the time of testing (e.g., a novel toy). Combining behavioral and ERP measures maximizes the sensitivity of the battery. As infants approach the end of the first year of life and beyond, they can be tested with tasks that allow them to use their better developed and controlled motor skills to actively manipulate objects. In the case of imitation-based tasks, inclusion of both immediate and delayed tests affords the opportunity to measure encoding processes (tests of immediate imitation) as well as long-term recall processes (tests of imitation after a delay). Delays of even 10 min are highly sensitive to memory impairment in infants (43) as well as in adults (13). Imitation-based tasks can be used throughout the preschool years (37, 45), after which time the transition can be made to the analogous task in the NIH Toolbox, the Toolbox Picture Sequence Memory Task (11, 36).
CONCLUSIONS
Research in children and adolescents with congenital hypothyroidism or whose mothers were hypothyroid during pregnancy indicates that impairment of memory function is a possible outcome of maternal iodine deficiency. Because, at present, there is no rapid, reliable means for assessing the iodine status of individuals, it is not currently feasible to identify individual women who are iodine-deficient in pregnancy and study the memory function of their offspring. However, memory function testing in infants and young children as an outcome measure of maternal iodine supplementation could be undertaken. The literature on cognitive development in infancy and early childhood features several candidate measures that have proven to be sensitive to memory function in the infants and young children of diabetic mothers (born iron deficient but supplemented at birth). We recommend that these tasks be pilot tested as a means to study the specific neurocognitive domain of memory in infants and young children whose mothers received iodine supplementation while pregnant.
In the absence of a rapid, reliable means for assessing the iodine status of individuals, the development of a nonhuman primate model should be considered as a means of testing the effect of maternal iodine deficiency on the neurocognitive domain of memory. To date, the primary model of gestational iodine deficiency is the rat. This model is limited by virtue of the fact that much of rat hippocampal development takes place postnatally, such that the newborn rat is developmentally equivalent to a human fetus in the fourth to fifth gestational month (18). Hippocampal development in nonhuman and human primates is more closely aligned. Moreover, the larger memory system of nonhuman primates (including connections with the prefrontal cortex) is more analogous to that of humans (14). As such, manipulations of the timing, severity, and duration of maternal iodine deficiency in nonhuman primate models could be especially revealing of its effect on memory. The dual approach of applying tests of the specific neurocognitive domain of memory, early in development, in both human and nonhuman primates is an especially promising avenue for investigating the effects of maternal iodine deficiency and supplementation on children’s hippocampal structure and function.
Acknowledgments
We thank Gay Goodman, Iodine Initiative Consultant to the NIH Office of Dietary Supplements, for contributions made in the course of providing expert scientific and technical review.
The authors’ responsibilities were as follows—PJB; had primary responsibility for the final content; and both authors: conducted the literature reviews, wrote the manuscript collaboratively, and read and approved the final manuscript. The authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: BDNF, brain-derived neurotrophic factor; CRMP2, collapsing response mediator protein 2; EGR1, early growth response 1; ERP, event-related potential; IDM, infant of diabetic mother; NCAM-180, the 180 kDa isoform of neural cell adhesion molecule.
REFERENCES
- 1.de Escobar GM, Obregón MJ, Escobar del Rey F. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab 2004;18:225–48. [DOI] [PubMed] [Google Scholar]
- 2.Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 2000;85:3975–87. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann MB. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol 2012;26(Suppl 1):108–17. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 2011;21:419–27. [DOI] [PubMed] [Google Scholar]
- 5.Taylor PN, Okosieme OE, Dayan CM, Lazarus JH. Therapy of endocrine disease: impact of iodine supplementation in mild-to-moderate iodine deficiency: systematic review and meta-analysis. Eur J Endocrinol 2014;170:R1–15. [DOI] [PubMed] [Google Scholar]
- 6.Cooke GE, Mullally S, Correia N, O’Mara S, Gibney J. Hippocampal volume is decreased in adults with hypothyroidism. Thyroid 2014;24:433–40. [DOI] [PubMed] [Google Scholar]
- 7.Gong J, Liu W, Dong J, Wang Y, Xu H, Wei W, Zhong J, Xi Q, Chen J. Developmental iodine deficiency and hypothyroidism impair neural development in rat hippocampus: involvement of doublecortin and NCAM-180. BMC Neurosci 2010;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BauerPJ. Declarative memory in infancy: an introduction to typical and atypical development. In: Bauer PJ, editor. Advances in child development and behavior. Varieties of early experience: implications for the development of declarative memory in infancy. Vol. 38. London: Elsevier; 2010. p. 3–28. [DOI] [PubMed]
- 9.Squire LR, Knowlton B, Mussen G. The structure and organization of memory. Annu Rev Psychol 1993;44:453–95. [DOI] [PubMed] [Google Scholar]
- 10.Bauer PJ. Neurodevelopmental changes in infancy and beyond: implications for learning and memory In: Barbarin OA, Wasik BH, editors. Handbook of child development and early education: research to practice. New York: The Guilford Press; 2009. p. 78–102. [Google Scholar]
- 11.Bauer PJ, Dikmen SS, Heaton RK, Mungas D, Slotkin J, Beaumont JL. NIH Toolbox Cognition Battery (CB): measuring episodic memory. In: Zelazo PD, Bauer PJ, editors. National Institutes of Health Toolbox—Cognition Battery (NIH Toolbox CB): validation for children between 3 and 15 years. Monogr Soc Res Child Dev 2013;78(4, Serial No. 309). p. 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer PJ. A complementary processes account of the development of childhood amnesia and a personal past. Psychol Rev 2015;122:204–31. [DOI] [PubMed] [Google Scholar]
- 13.Reed JM, Squire LR. Retrograde amnesia for facts and events: findings from four new cases. J Neurosci 1998;18:3943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bachevalier J. The development of memory from a neurocognitive and comparative perspective Bauer PJ, Fivush R, editors. The Wiley-Blackwell handbook on the development of children’s memory. West Sussex (United Kingdom): Wiley-Blackwell; 2014. p. 109–25. [Google Scholar]
- 15.Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- 16.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 2006;44:2189–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seress L, Abrahám H. Pre- and postnatal morphological development of the human hippocampal formation Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2nd ed. Cambridge (MA): MIT Press; 2008. p. 187–212. [Google Scholar]
- 18.Bernal J. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 2007;3:249–59. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Wang Y, Xu H, Dong J, Wei W, Wang Y, Shan Z, Teng W, Xi Q, Chen J. Developmental iodine deficiency delays the maturation of newborn granule neurons associated with downregulation of p35 in postnatal rat hippocampus. Environ Toxicol 2014;29:847–55. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Zhang L, Li J, Shan Z, Teng W. Maternal marginal iodine deficiency affects the expression of relative proteins during brain development in rat offspring. J Endocrinol 2013;217:21–9. [DOI] [PubMed] [Google Scholar]
- 21.Wei W, Wang Y, Wang Y, Dong J, Min H, Song B, Teng W, Xi Q, Chen J. Developmental hypothyroxinaemia induced by maternal mild iodine deficiency delays hippocampal axonal growth in the rat offspring. J Neuroendocrinol 2013;25:852–62. [DOI] [PubMed] [Google Scholar]
- 22.Wei W, Wang Y, Dong J, Wang Y, Min H, Song B, Shan Z, Teng W, Xi Q, Chen J. Hypothyroxinemia induced by maternal mild iodine deficiency impairs hippocampal myelinated growth in lactational rats. Environ Toxicol 2015;30:1264–74. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert ME, Hedge JM, Valentín-Blasini L, Blount BC, Kannan K, Tietge J, Zoeller RT, Crofton KM, Jarrett JM, Fisher JW. An animal model of marginal iodine deficiency during development: the thyroid axis and neurodevelopmental outcome. Toxicol Sci 2013;132:177–95. [DOI] [PubMed] [Google Scholar]
- 24.Pearce EN, Caldwell KL. Urinary iodine, thyroid function, and thyroglobulin as biomarkers of iodine status. Am J Clin Nutr 2016;104(Suppl):898S–901S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rovet JF. The role of thyroid hormones for brain development and cognitive function. Endocr Dev 2014;26:26–43. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler SM, Willoughby KA, McAndrews MP, Rovet J. Hippocampal size and memory functioning in children and adolescents with congenital hypothyroidism. J Clin Endocrinol Metab 2011;96:E1427–34. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler SM, McAndrews MP, Sheard ED, Rovet J. Visuospatial associative memory and hippocampal functioning in congenital hypothyroidism. J Int Neuropsychol Soc 2012;18:49–56. [DOI] [PubMed] [Google Scholar]
- 28.Song SI, Daneman D, Rovet J. The influence of etiology and treatment factors on intellectual outcome in congenital hypothyroidism. J Dev Behav Pediatr 2001;22:376–84. [DOI] [PubMed] [Google Scholar]
- 29.Willoughby KA, McAndrews MP, Rovet J. Effects of early thyroid hormone deficiency on children’s autobiographical memory performance. J Int Neuropsychol Soc 2013;19:419–29. [DOI] [PubMed] [Google Scholar]
- 30.Willoughby KA, McAndrews M, Rovet J. Effects of maternal hypothyroidism on offspring hippocampus and memory. Thyroid 2014;24:576–84. [DOI] [PubMed] [Google Scholar]
- 31.DeMaster D, Pathman T, Lee JK, Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cereb Cortex 2014;24:3036–45. [DOI] [PubMed] [Google Scholar]
- 32.DeMaster D, Pathman T, Ghetti S. Development of memory for spatial context: hippocampal and cortical contributions. Neuropsychologia 2013;51:2415–26. [DOI] [PubMed] [Google Scholar]
- 33.Bauer PJ. Memory. In: Zelazo PD, editor. Oxford handbook of developmental psychology. Vol. 1: Body and mind. New York: Oxford University Press; 2013. p. 505–41. [Google Scholar]
- 34.Rose SA, Feldman JF, Jankowski JJ. Memory in at-risk populations In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell handbook on the development of children’s memory. West Sussex (United Kingdom): Wiley-Blackwell; 2014. p. 996–1016. [Google Scholar]
- 35.Bauer PJ, Wenner JA, Dropik PL, Wewerka SS. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monogr Soc Res Child Dev 2000;65:i–vi. [PubMed] [Google Scholar]
- 36.Bauer PJ, Zelazo PD. National Institutes of Health toolbox for the assessment of neurological and behavioral function: a tool for developmental science. Child Dev Perspect 2014;8:119–24. [Google Scholar]
- 37.Riggins T, Cheatham C, Stark E, Bauer PJ. Elicited imitation performance at 20 months predicts memory abilities in school age children. J Cogn Dev 2013;14:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.deRegnier RA, Wewerka SS, Georgieff MK, Mattia F, Nelson CA. Influences of post-conceptional age and postnatal experience on the development of auditory recognition memory in the newborn infant. Dev Psychobiol 2002;41:216–25. [DOI] [PubMed] [Google Scholar]
- 39.Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Dev Sci 2000;3:234–46. [Google Scholar]
- 40.Verner AM, Manderson J, Lappin TR, McCance DR, Halliday HL, Sweet DG. Influence of maternal diabetes mellitus on fetal iron status. Arch Dis Child Fetal Neonatal Ed 2007;92:F399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deregnier RA, Nelson CA, Thomas K, Wewerka SS, Georgieff MK. Neurophysiologic evaluation of auditory recognition memory in healthy newborn infants and infants of diabetic mothers. J Pediatr 2000;137:777–84. [DOI] [PubMed] [Google Scholar]
- 42.Nelson CA, Wewerka SS, Borscheid AJ, deRegnier R-A, Georgieff MK. Electrophysiologic evidence of impaired cross-modal recognition memory in 8-month-old infants of diabetic mothers. J Pediatr 2003;142:575–82. [DOI] [PubMed] [Google Scholar]
- 43.DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol 2005;47:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suppiej A, Mento G, Zanardo V, Franzoi M, Battistella PA, Ermani M, Bisiacchi PS. Auditory processing during sleep in preterm infants: an event related potential study. Early Hum Dev 2010;86:807–12. [DOI] [PubMed] [Google Scholar]
- 45.Bauer PJ, Larkina M, Doydum AO. Explaining variance in long-term recall in 3- and 4-year-old children: the importance of post-encoding processes. J Exp Child Psychol 2012;113:195–210. [DOI] [PubMed] [Google Scholar]