Abstract
Background: Prevalences of iodine inadequacy and excess are usually evaluated by comparing the population distribution of urinary iodine concentration (UIC) in spot samples with established UIC cutoffs. To our knowledge, until now, dietary intake data have not been assessed for this purpose.
Objective: Our objective was to compare 2 methods for evaluating the prevalence of iodine inadequacy and excess in sex- and life stage–specific subgroups of the US population: one that uses UIC cutoffs, and one that uses iodine intake cutoffs.
Design: By using the iodine concentrations of foods measured in the US Food and Drug Administration’s Total Diet Study (TDS), dietary intake data from the NHANES 2003–2010, and a file that maps each NHANES food to a TDS food with similar ingredients, we estimated each NHANES participant’s iodine intake from each NHANES food as the mean iodine concentration of the corresponding TDS food in samples gathered over the same 2-y period. We calculated prevalences of iodine inadequacy and excess in each sex- and life stage–specific subgroup by both the UIC cutoff method and the iodine intake cutoff method—using the UIC values and dietary intakes reported for NHANES participants who provided both types of data—and compared the prevalences across methods.
Results: We found lower prevalences of iodine inadequacy across all sex- and life stage–specific subgroups with the iodine intake cutoff method than with the UIC cutoff method; for pregnant females, the respective prevalences were 5.0% and 37.9%. For children aged ≤8 y, the prevalence of excessive iodine intake was high by either method.
Conclusions: The consideration of dietary iodine intake from all sources may provide a more complete understanding of population prevalences of iodine inadequacy and excess and thus better inform dietary guidance than consideration of UIC alone. Methods of adjusting UIC for within-person variation are needed to improve the accuracy of prevalence assessments based on UIC.
Keywords: dietary surveys, food content, iodine, Total Diet Study, urinary iodine, variability
INTRODUCTION
Prevalences of iodine inadequacy and excess are usually evaluated by comparing the population distribution of urinary iodine concentrations (UICs)9 in spot samples with established cutoffs for median UIC concentrations in sex- and life stage–specific population subgroups, such as those developed by the WHO (1). To our knowledge, until now, the use of dietary intake data for this purpose has not been assessed.
Because UIC mostly reflects recent iodine intake, large day-to-day variability in iodine intake is reflected in large day-to-day variability in UIC (2). For that reason, as discussed elsewhere in this supplement issue, UIC measured in spot samples or single 24-h collections is not a reliable measure of the iodine status of individuals (3). In addition, UIC is not informative as to specific dietary sources of iodine or the amounts obtained from each source; thus, it is not helpful for formulating dietary guidance.
In contrast, a reliable means of assessing dietary intake could be used to monitor the iodine status of individuals over time and to inform the interpretation of UIC in individuals. However, there are also limitations to the use of dietary survey data for assessing iodine status. One issue is that food intakes may be under- or overreported (4). In addition, the iodine concentration of many foods is poorly characterized and its variability is often high. A discussion of the causes of variability in the iodine concentration of foods (5) and a statistical assessment of that variability (6) are presented elsewhere in this supplement issue.
Three sets of Dietary Reference Intakes for iodine have been developed for children and adults by the Food and Nutrition Board of the Institute of Medicine: Estimated Average Requirements (EARs), Recommended Dietary Allowances (RDAs), and Tolerable Upper Intake Levels (ULs) (7). The RDA is intended for use primarily as a goal for an individual’s usual intake, whereas the EAR is used to assess the population prevalence of inadequate intakes.
In the present study, our first aim was to develop a method for using dietary intake data from NHANES and data on the iodine concentration of foods from the US Food and Drug Administration’s Total Diet Study (TDS) to estimate total usual daily iodine intakes. Our second aim was to compute the prevalences of iodine inadequacy and excess across sex- and life stage–specific subgroups of NHANES participants by comparing the estimated total usual daily iodine intakes for each subgroup with subgroup-specific EARs and ULs. Our third aim was to identify and use EAR-like and UL-like UIC cutoffs to calculate subgroup-specific prevalences of iodine inadequacy and excess among NHANES participants who provided both UIC data and dietary intake data. Our final aim was to compare prevalences of iodine inadequacy and excess calculated by using iodine intake cutoffs with those estimated by using UIC cutoffs.
METHODS
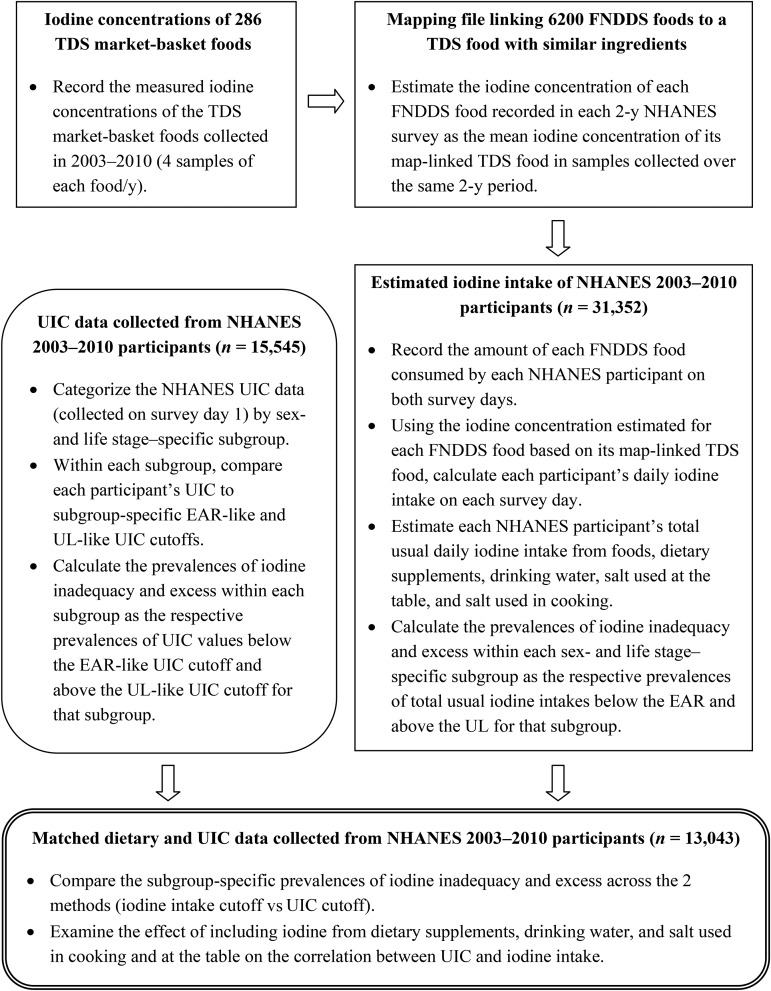
We used SAS version 9.3 (SAS Institute) for all statistical analyses unless otherwise noted. A flowchart summarizing the study protocol is shown in Figure 1.
FIGURE 1.
Flowchart of the study protocol. EAR, Estimated Average Requirement; FNDDS, Food and Nutrient Database for Dietary Studies; TDS, Total Diet Study; UIC, urinary iodine concentration; UL, Tolerable Upper Intake Level.
NHANES data
NHANES, an annual survey of the civilian, noninstitutionalized US population conducted by the CDC’s National Center for Health Statistics, collects dietary intake data for all participants ≥1 y old on 2 survey days and measures UIC in nontimed spot urine samples from participants ≥6 y old on the first survey day (8). In the present study, we examined the dietary intake and UIC data of NHANES participants for the years 2003−2004, 2005−2006, 2007−2008, and 2009−2010. During this time period, the CDC measured UIC by using variations on an inductively coupled plasma mass spectrometry method (9, 10).
Dietary intake data
NHANES collects data on the foods, plain drinking water, and beverages consumed from midnight to midnight of the previous day and the types and amounts of dietary supplements consumed in the previous 30 d. We included in our study all NHANES participants aged ≥1 y with complete 24-h dietary data for both survey days (n = 31,352) and analyzed the dietary iodine intake data for each day separately.
UIC data
The CDC measured UIC in spot samples from approximately one-third of the 2003−2004, 2005−2006, and 2009−2010 participants aged ≥6 y and all of the 2007−2008 participants aged ≥6 y. All of the NHANES participants with urinary iodine data (n = 15,545) were included in our study.
Dietary iodine evaluation
We estimated iodine intake from food, drinking water, dietary supplements, salt used in cooking, and salt used at the table.
Iodine from drinking water
On the basis of the reported amount of total plain drinking water consumed by NHANES participants on each dietary survey day, we calculated iodine intake from drinking water by assuming an iodine concentration of 9.2 μg/L, the median of values (ranging from 0.1 to 25 μg/L) measured at various US locations in available studies (11, 12).
Iodine from dietary supplements
We calculated the daily iodine intake from dietary supplements on the basis of the fractional number of days they were used, the number of servings per day, and the iodine content per serving as listed on the product label. Dietary supplements containing kelp are very high in iodine. We excluded kelp-containing supplements when calculating the iodine intakes of participants who reported the use of such supplements (n = 39) to avoid biasing the summary statistics toward high values inconsistent with the overall distributions of the iodine intake from supplements and the total iodine intake.
Iodine from salt used at the table
Table salt contains 387.6 mg Na/g (13); if the salt is iodized, the iodine concentration is nominally 45 μg/g (14). First, we estimated the daily intake of sodium from salt used at the table, as described below. Next, we estimated the daily intake of table salt (in g) as the sodium intake (in mg) divided by 387.6. Under the assumption that ∼70% of table salt sold in the United States is iodized (14), we estimated the daily intake of iodine from table salt (in μg) as the table salt intake (in g) multiplied by 45 × 0.7. For NHANES participants who indicated the use of “ordinary salt” (i.e., table salt), we assigned a default intake of 580 mg sodium from salt used at the table to all ages on the basis of the mean value for adults reported in a 1991 study (15). We are not aware of a later study in adults or any similar study in children. For participants who indicated the use of “lite salt,” we assigned a default table salt sodium intake of one-half that value (290 mg). For participants whose response was “salt substitute,” “other,” or “don’t use,” we assigned a default table salt sodium intake of zero. If the frequency of salt use was “very often,” “occasionally,” or “rarely,” we multiplied the default table salt sodium intake by 1, 0.5, or 0.25, respectively. A similar approach has been used for estimating total sodium consumption (16).
Iodine from foods
The Food and Drug Administration’s TDS measures the concentrations of iodine and other nutrients in a “market basket” of 286 foods collected 4 times/y, once from each of 4 geographic regions (17, 18). The foods recorded by NHANES participants are drawn from the ∼6200 foods in the USDA’s Food and Nutrient Database for Dietary Studies (FNDDS). The FNDDS provides data on the concentrations of 65 nutrients and food components, but iodine is not among them (19). To account for the iodine content of foods recorded by NHANES participants, we used a file that maps each FNDDS food to a TDS food on the basis of the similarity of their ingredients (JH Spungen, unpublished data, 2014). Because there are far fewer TDS foods than FNDDS foods, multiple FNDDS foods are mapped to a single TDS food. For example, 15 natural cheeses listed in the FNDDS are mapped to natural cheddar cheese in the TDS. With the use of the TDS foods as surrogates for the FNDDS foods, we estimated the iodine concentration of each food recorded by NHANES participants as the mean iodine concentration of the corresponding TDS food in samples collected over the same 2-y period as the NHANES data.
Iodine from salt in prepared foods
The TDS kitchens use only noniodized salt. To account for the use of iodized salt in home food preparation, first we estimated the amount of salt used in preparing 47 TDS foods on the basis of recipes for similar foods in the FNDDS (20). We then calculated iodine intake from salt used in cooking as described above for table salt.
Total usual daily iodine intakes
We estimated total usual daily iodine intakes using the “usual intake” method developed at Iowa State University (21). By taking the inter- and intraindividual variability in food consumption into account, the Iowa State University method diminishes the impact of over- and underreporting.
Correlation of UIC with sources of dietary iodine
We examined the relation between UIC and various combinations of dietary iodine sources by calculating the Pearson correlation coefficient at a significance criterion of P < 0.05.
Prevalence of inadequate and excessive intakes
We calculated prevalences of iodine inadequacy and excess from urinary data using SAS version 9.3 and from iodine intake data using Personal Computer Software for Intake Distribution Estimation (PC-SIDE version 1.1, 2003; Department of Statistics and Center for Agricultural and Rural Development, Iowa State University).
Iodine intake cutoff method
Table 1 shows subgroup-specific EARs, RDAs, and ULs developed by the Food and Nutrition Board of the Institute of Medicine. To avoid overestimating the prevalence of iodine inadequacy in a population, it is crucial to use the EAR and not the RDA as the cutoff (22, 23). We estimated the prevalence of iodine inadequacy as the prevalence of total usual iodine intakes below the subgroup-specific EAR. Similarly, we estimated the prevalence of excessive iodine intake as the prevalence of total usual iodine intakes above the subgroup-specific UL.
TABLE 1.
Calculated UIC cutoffs for sex- and life stage–specific subgroups based on Dietary Reference Intakes for iodine1
| Dietary Reference Intakes, μg/d |
UIC cutoffs, μg/L |
|||||
| EAR | RDA | UL | EAR-like2 | RDA-like3 | UL-like4 | |
| Children | ||||||
| 1–3 y | 65 | 90 | 200 | NC | NC | NC |
| 4–5 y | 65 | 90 | 300 | NC | NC | NC |
| 6–8 y | 65 | 90 | 300 | 72 | 100 | 300 |
| 9–13 y | 73 | 120 | 600 | 61 | 100 | 300 |
| Teens and adults aged ≥14 y, excluding pregnant females | ||||||
| 14–18 y | 95 | 150 | 900 | 63 | 100 | 300 |
| ≥19 y | 95 | 150 | 1100 | 63 | 100 | 300 |
| Pregnant females | ||||||
| 14–18 y | 160 | 220 | 900 | 109 | 150 | 500 |
| 19–50 y | 160 | 220 | 1100 | 109 | 150 | 500 |
Urinary data were available only for survey participants ≥6 y of age; for that reason, we did not develop UIC cutoffs for children <6 y of age. The EARs, RDAs, and ULs are Dietary Reference Intakes developed by the Food and Nutrition Board of the Institute of Medicine (7). EAR, Estimated Average Requirement; RDA, Recommended Dietary Allowance; NC, not calculated; UIC, urinary iodine concentration; UL, Tolerable Upper Intake Level.
EAR-like UIC cutoffs were calculated as follows: RDA-like UIC cutoff (μg/L) × EAR (μg/d)/RDA (μg/d).
RDA-like UIC cutoffs were set at the lowest WHO guideline median UIC cutoffs for adequate iodine intake: 150 μg/L for pregnant females and 100 μg/L for other population subgroups, including lactating females and children ≥6 y of age (1).
UL-like UIC cutoffs were set at the WHO guideline median UIC cutoffs for excessive iodine intake: 500 μg/L for pregnant females and 300 μg/L for other population subgroups, excluding lactating females and including children ≥6 y of age (1).
UIC cutoff method
Because UIC is typically used to assess the iodine adequacy of populations, we set the RDA-like UIC cutoffs to the lowest WHO guideline median UIC cutoffs for adequate intake: 150 μg/L for pregnant females and 100 μg/L for other population subgroups, including lactating females and children ≥6 y of age (1). Similarly, we set the UL-like UIC cutoffs to the WHO guideline median UIC cutoffs for excessive intake: 500 μg/L for pregnant females and 300 μg/L for other population subgroups, excluding lactating females and including children ≥6 y of age (1). There is no established WHO UIC cutoff guideline for excessive iodine intake in lactating females.
We calculated EAR-like UIC cutoffs as follows (24):
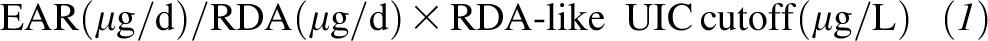 |
We estimated the subgroup-specific prevalence of iodine inadequacy as the prevalence of UIC values below the relevant EAR-like UIC cutoff. Similarly, we estimated the subgroup-specific prevalence of excessive iodine intake as the prevalence of UIC values above the relevant UL-like UIC cutoff. The UIC cutoffs for each subgroup are shown in Table 1.
RESULTS
Distributions of iodine intake and UIC
Table 2 reports mean and median values of total usual iodine intake and UIC across sex- and life stage–specific subgroups for NHANES participants who reported data for both measures on survey day 1 (the “matched” population, n = 13,043). Percentiles of the distributions of total usual iodine intake and UIC in these subgroups are shown in Supplemental Tables 1 and 2, respectively. Percentiles of the distribution of total usual iodine intake in subgroups of the larger, intersecting population of NHANES participants who provided dietary intake data on both survey days (the “intake” population, n = 31,352) are shown in Supplemental Table 3. Unlike the matched population, the intake population includes children as young as 1 y old.
TABLE 2.
Mean and median estimated usual iodine intakes and UICs in sex- and life stage–specific subgroups of the “matched” population1
| Estimated usual iodine intake, μg/d |
UIC, μg/L |
||||
| n | Mean ± SEM | Median ± SE | Mean ± SEM | Median ± SE | |
| Males | |||||
| 6–8 y | 405 | 346 ± 7 | 325 ± 8 | 327 ± 29 | 262 ± 20 |
| 9–13 y | 747 | 313 ± 4 | 303 ± 6 | 292 ± 16 | 210 ± 15 |
| 14–18 y | 817 | 386 ± 6 | 362 ± 9 | 254 ± 10 | 185 ± 9 |
| 19–30 y | 833 | 397 ± 5 | 376 ± 8 | 212 ± 13 | 153 ± 7 |
| 31–50 y | 1397 | 372 ± 4 | 350 ± 6 | 211 ± 9 | 149 ± 6 |
| 51–70 y | 1327 | 371 ± 4 | 351 ± 5 | 276 ± 23 | 175 ± 7 |
| ≥71 y | 768 | 344 ± 4 | 330 ± 6 | 491 ± 66 | 213 ± 6 |
| Nonpregnant females | |||||
| 6–8 y | 427 | 317 ± 5 | 307 ± 8 | 277 ± 16 | 217 ± 8 |
| 9–13 y | 775 | 301 ± 4 | 289 ± 6 | 271 ± 14 | 193 ± 15 |
| 14–18 y | 811 | 265 ± 4 | 251 ± 6 | 275 ± 56 | 147 ± 7 |
| 19–30 y | 827 | 278 ± 4 | 262 ± 6 | 213 ± 22 | 134 ± 8 |
| 31–50 y | 1504 | 289 ± 3 | 675 ± 4 | 423 ± 235 | 122 ± 5 |
| 51–70 y | 1405 | 308 ± 4 | 287 ± 5 | 540 ± 303 | 144 ± 7 |
| ≥71 y | 744 | 287 ± 4 | 272 ± 5 | 576 ± 148 | 183 ± 13 |
| Pregnant females | |||||
| 14–50 y | 256 | 332 ± 8 | 315 ± 12 | 194 ± 18 | 138 ± 18 |
The “matched” population consisted of the participants in NHANES 2003–2010 who provided both UIC data and dietary intake data. The estimated usual iodine intake is based on iodine from foods, plain drinking water, dietary supplements, salt used in cooking, and salt used at the table. The iodine concentration of drinking water was assumed to be 9.2 μg/L. UIC, urinary iodine concentration.
Prevalence of iodine inadequacy
Table 3 presents, for the matched population, a comparison of the prevalence of iodine inadequacy calculated on the basis of total usual iodine intake with that calculated on the basis of UIC. In every sex- and life stage–specific subgroup, the prevalence of iodine inadequacy on the basis of total usual iodine intake was markedly lower than that based on UIC. Based on iodine intake, both boys and girls aged 6–8 y and 9–13 y had a 0.0% prevalence of iodine inadequacy; the corresponding prevalences based on UIC were 5.8% and 7.9% in boys and 11.2% and 12.5% in girls, respectively. The highest calculated prevalence of inadequacy based on iodine intake, 5.0%, was for pregnant females; the corresponding prevalence based on UIC was 37.9%.
TABLE 3.
Prevalence rates of iodine inadequacy and iodine excess in sex- and life stage–specific subgroups of the “matched” population based on UIC or estimated total usual iodine intake1
| Prevalence rates based on estimated total usual iodine intake,2 % |
Prevalence rates based on UIC,3 % |
|||
| Rate of inadequacy ± SE | Rate of excess ± SE | Rate of inadequacy ± SE | Rate of excess ± SE | |
| Males | ||||
| 6–8 y | 0.0 ± NA | 59.8 ± 0.0 | 5.8 ± 1.5 | 41.7 ± 4.0 |
| 9–13 y | 0.0 ± NA | 0.9 ± 0.0 | 7.9 ± 1.5 | 32.6 ± 3.1 |
| 14–18 y | 0.3 ± 0.0 | 0.9 ± 0.0 | 11.9 ± 1.7 | 26.5 ± 1.8 |
| 19–30 y | 0.0 ± 0.0 | 0.1 ± 0.0 | 11.8 ± 1.4 | 18.4 ± 1.7 |
| 31–50 y | 0.1 ± 0.0 | 0.2 ± 0.0 | 14.7 ± 1.2 | 18.5 ± 1.3 |
| 51–70 y | 0.3 ± 0.0 | 0.1 ± 0.0 | 13.5 ± 1.3 | 24.4 ± 1.5 |
| ≥71 y | 0.4 ± 0.0 | 0.0 ± NA | 5.9 ± 1.0 | 31.3 ± 1.9 |
| Nonpregnant females | ||||
| 6–8 y | 0.0 ± NA | 53.0 ± 0.0 | 11.2 ± 2.0 | 35.1 ± 2.6 |
| 9–13 y | 0.0 ± 0.0 | 0.8 ± 0.0 | 12.5 ± 1.6 | 31.7 ± 2.2 |
| 14–18 y | 1.9 ± 0.0 | 0.0 ± NA | 17.2 ± 2.2 | 20.0 ± 1.9 |
| 19–30 y | 1.4 ± 0.0 | 0.0 ± NA | 22.4 ± 2.2 | 15.0 ± 1.4 |
| 31–50 y | 0.6 ± 0.0 | 0.0 ± NA | 22.9 ± 1.3 | 14.7 ± 1.1 |
| 51–70 y | 1.9 ± 0.0 | 0.0 ± 0.0 | 16.9 ± 1.1 | 17.0 ± 1.4 |
| ≥71 y | 1.3 ± 0.0 | 0.0 ± NA | 11.2 ± 1.5 | 24.4 ± 2.1 |
| Pregnant females | ||||
| 14–50 y | 5.0 ± 0.0 | 0.0 ± 0.0 | 37.9 ± 5.6 | 5.9 ± 2.1 |
The “matched” population consisted of the 13,043 participants in NHANES 2003–2010 who provided both UIC data and dietary intake data. EAR, Estimated Average Requirement; NA, not available (the SE cannot be computed); UIC, urinary iodine concentration; UL, Tolerable Upper Intake Level.
The rate of iodine inadequacy is calculated as the percentage of the subgroup with estimated iodine intake below the EAR; the rate of iodine excess is calculated as the percentage of the subgroup with estimated iodine intake above the UL. The estimated total usual iodine intake is based on iodine from foods, plain drinking water, dietary supplements, salt used in cooking, and salt used at the table. The iodine concentration of drinking water was assumed to be 9.2 μg/L.
The rate of iodine inadequacy is calculated as the percentage of the subgroup with UIC below the EAR-like cutoff; the rate of iodine excess is calculated as the percentage of the subgroup with UIC above the UL-like cutoff.
Calculated prevalences of iodine inadequacy in the intake population across 4 iodine intake scenarios of varying completeness are shown in Table 4 for females and in Supplemental Table 4 for males. The scenarios included the following: 1) foods and dietary supplements, 2) the latter plus drinking water, 3) the latter plus salt used in cooking, and 4) the latter plus salt used at the table (identified as total usual iodine intake). When salt used in cooking and at the table was included in the calculation (scenario 4 compared with scenario 2), the calculated prevalence of iodine inadequacy decreased from 11.9% to 3.7% in pregnant females and from 6.3% to 1.2%, from 7.9% to 1.2%, and from 6.3% to 0.8% in the 3 subgroups of nonpregnant females aged 14–50 y.
TABLE 4.
Prevalence of iodine inadequacy by age and pregnancy status in female members of the “intake” population, based on estimated usual iodine intake from various sources1
| Prevalence of iodine inadequacy,2 % |
||||
| Iodine from foods and DS | Iodine from foods, DS, and DW | Iodine from foods, DS, DW, and SC | Iodine from foods, DS, DW, SC, and ST | |
| Nonpregnant | ||||
| 1–3 y | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 |
| 4–8 y | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± NA | 0.0 ± NA |
| 9–13 y | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.2 ± 0.1 | 0.0 ± NA |
| 14–18 y | 7.7 ± 1.6 | 6.3 ± 1.4 | 3.4 ± 1.0 | 1.2 ± 0.5 |
| 19–30 y | 10.5 ± 1.7 | 7.9 ± 1.5 | 3.2 ± 1.0 | 1.2 ± 0.5 |
| 31–50 y | 8.6 ± 1.1 | 6.3 ± 1.0 | 2.3 ± 0.6 | 0.8 ± 0.3 |
| 51–70 y | 9.8 ± 1.0 | 7.8 ± 0.9 | 3.4 ± 0.6 | 1.7 ± 0.4 |
| ≥71 y | 9.0 ± 1.2 | 7.6 ± 1.1 | 3.7 ± 0.8 | 2.3 ± 0.6 |
| Pregnant | ||||
| 14–50 y | 14.6 ± 2.6 | 11.9 ± 2.4 | 5.9 ± 1.8 | 3.7 ± 1.4 |
The “intake” population consisted of the 31,352 participants in NHANES 2003–2010 who provided dietary intake data on both survey days. The iodine concentration of DW was assumed to be 9.2 μg/L. DS, dietary supplements; DW, drinking water; NA, not available (the SE cannot be computed); SC, salt used in cooking; ST, salt used at the table.
Values are percentages ± SEs of individuals in each age group with iodine intakes below the Estimated Average Requirement for each iodine intake scenario shown.
Prevalence of iodine excess
Table 3 also presents, for the matched population, a comparison of the prevalence of iodine excess calculated on the basis of total usual iodine intake with that calculated on the basis of UIC. The prevalences of iodine excess based on iodine intake in boys and girls aged 6–8 y were 59.8% and 53.0%, respectively; the corresponding prevalences based on UIC were somewhat lower: 41.7% and 35.1%, respectively. For all other subgroups, the calculated prevalences of iodine excess based on iodine intake were in the range of 0.0–0.9%, values that are markedly lower than those based on UIC (5.9–32.6%).
The calculated prevalences of iodine excess in the intake population across the same 4 iodine intake scenarios defined above are shown in Table 5 for females and in Supplemental Table 5 for males. The largest prevalences of iodine excess were found in children <6 y of age. On the basis of total usual iodine intake (scenario 4), prevalences of ≥82% and ≥49% were calculated for children of either sex aged 1–3 y and 4–8 y, respectively.
TABLE 5.
Prevalence of excessive iodine intake by age and pregnancy status in female members of the “intake” population, based on estimated usual iodine intake from various sources1
| Prevalence of excessive iodine intake,2 % |
||||
| Iodine from foods and DS | Iodine from foods, DS, and DW | Iodine from foods, DS, DW, and SC | Iodine from foods, DS, DW, SC, and ST | |
| Nonpregnant | ||||
| 1–3 y | 78.3 ± 1.8 | 79.2 ± 1.8 | 82.9 ± 1.8 | 84.2 ± 1.7 |
| 4–8 y | 36.3 ± 1.9 | 38.0 ± 1.9 | 43.7 ± 1.8 | 49.2 ± 1.7 |
| 9–13 y | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.6 ± 0.3 | 0.9 ± 0.4 |
| 14–18 y | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA |
| 19–30 y | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA |
| 31–50 y | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA |
| 51–70 y | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.2 |
| ≥71 y | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA | 0.0 ± NA |
| Pregnant | ||||
| 14–50 y | 0.0 ± NA | 0.0 ± NA | 0.1 ± 0.1 | 0.2 ± 0.2 |
The “intake” population consisted of the 31,352 participants in NHANES 2003–2010 who provided dietary intake data on both survey days. The iodine concentration of DW was assumed to be 9.2 μg/L. DS, dietary supplements; DW, drinking water; NA, not available (the SE cannot be computed); SC, salt used in cooking; ST, salt used at the table.
Values are percentages ± SEs of individuals in each age group with iodine intake above the Tolerable Upper Intake Level for each iodine intake scenario shown.
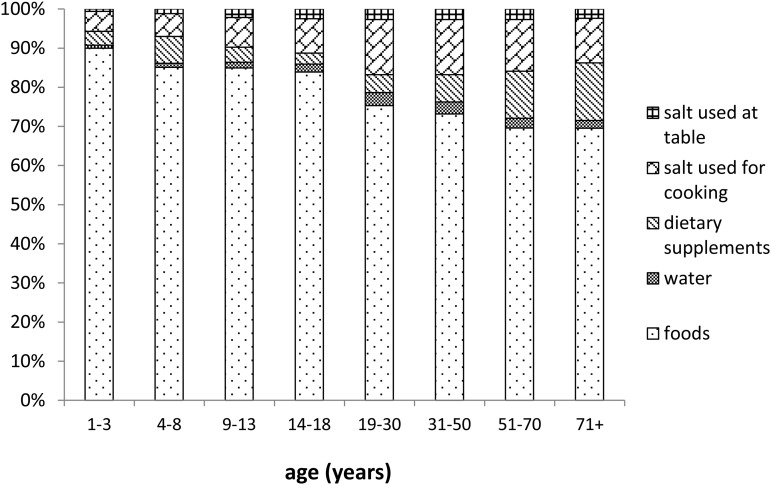
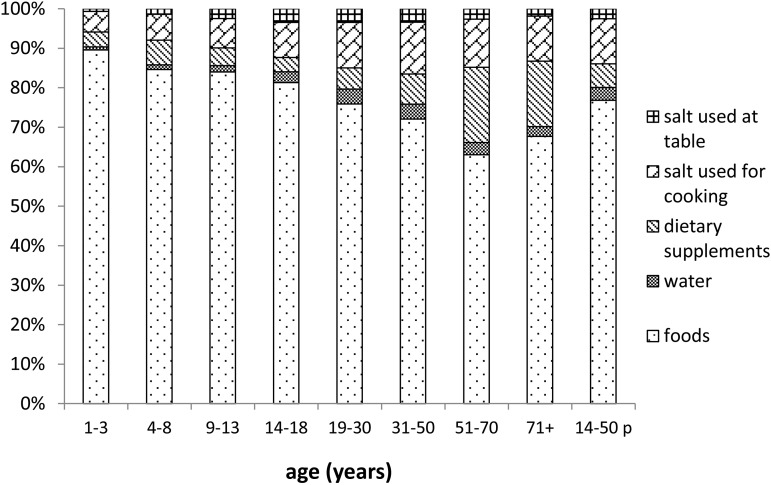
The relative contributions of foods, drinking water, dietary supplements, salt used in cooking, and salt used at the table to the calculated iodine intake of the intake population are shown in Figure 2 for males and Figure 3 for females. Consistent with the data presented in Supplemental Table 4, the major sources of iodine intake were foods, dietary supplements, and salt used in cooking.
FIGURE 2.
Proportions of mean iodine intake contributed by 5 dietary sources, calculated for male participants in NHANES 2003–2010 with dietary intake data for both survey days.
FIGURE 3.
Proportions of mean iodine intake contributed by 5 dietary sources, calculated for female participants in NHANES 2003–2010 with dietary intake data for both survey days. The right-most column (labeled “p”) presents data for pregnant females; the other columns exclude data from pregnant females.
Correlations between UIC and various combinations of dietary iodine sources are shown in Table 6. Participants with UICs >1000 μg/L (n = 106) were excluded from the correlation analyses to avoid biasing the results toward nonrepresentative data pairs at the extreme high end of the UIC distribution. All combinations of dietary iodine sources tested were significantly correlated with UIC (P < 0.0001). However, the correlations were not strong and there was little or no change across the various combinations of dietary sources. In nonpregnant females aged 14–50 y, r ranged from 0.12 to 0.15, and in pregnant females aged 14–50 y, r ranged from 0.36 to 0.40.
TABLE 6.
Correlation between UIC and various measures of dietary iodine intake in sex- and life stage–specific subgroups of the “matched” population1
| Correlation between UIC and iodine intake2 from |
||||||
| n | Foods and DW | Foods and DS | Foods, DW, and DS | Foods, DW, DS, and SC | Foods, DW, DS, SC, and ST | |
| Age | ||||||
| 6–18 y | 3875 | 0.23 | 0.25 | 0.25 | 0.24 | 0.24 |
| ≥19 y | 8726 | 0.16 | 0.21 | 0.20 | 0.19 | 0.19 |
| Sex | ||||||
| Male | 6057 | 0.20 | 0.23 | 0.22 | 0.21 | 0.21 |
| Female3 | 6294 | 0.15 | 0.19 | 0.18 | 0.17 | 0.17 |
| Pregnancy status | ||||||
| Nonpregnant, 14–50 y | 3073 | 0.12 | 0.15 | 0.14 | 0.13 | 0.13 |
| Pregnant, 14–50 y | 250 | 0.36 | 0.40 | 0.39 | 0.40 | 0.40 |
The “matched” population consisted of the 13,043 participants in NHANES 2003–2010 who provided both UIC data and dietary intake data. Analysis was based on dietary intake data gathered at the in-person interview on survey day 1, the same day that urine was collected for analysis. Kelp-containing DS were excluded when calculating the iodine intakes of participants who reported their use (n = 39) to avoid biasing the summary statistics toward high values inconsistent with the overall distributions. Participants with UICs >1000 μg/L (n = 106) were excluded from the correlation analyses to avoid biasing the results toward nonrepresentative data pairs at the extreme high end of the UIC distribution. The iodine concentration of DW was assumed to be 9.2 μg/L, the median of values reported in several studies. P < 0.0001 for all comparisons. DS, dietary supplements; DW, drinking water; SC, salt used in cooking; ST, salt used at the table; UIC, urinary iodine concentration.
Pearson correlation coefficient, P < 0.05.
Excluding pregnant females.
DISCUSSION
According to our analysis of NHANES UIC data for 2003–2010, the median UICs of all sex- and life stage–specific subgroups of the US population aged ≥6 y other than pregnant females exceeded the WHO’s cutoff of 100 μg/L for iodine adequacy (1). Our findings are consistent with other analyses of NHANES data on the adequacy and stability of iodine status in the general US population since 2000 (25–27). In addition, according to our analysis, the median UIC of pregnant females (∼140 μg/L) was slightly below the WHO criterion for adequacy, 150 μg/L. Other studies reported median UICs of 125–145 μg/L for pregnant females surveyed by NHANES between 2001 and 2010 (28, 29).
A previous study found that salt used in cooking was not significantly associated with an increase in median UIC among females aged 15–44 y (28). Consistent with that result, we found that the correlation between UIC and iodine intake did not improve by accounting for iodized salt used in cooking and at the table. The observed lack of improvement in the correlation might be explained in part by our inability to account for variability in the iodine content of iodized salt, which depends on temperature, humidity, and length of storage; concentrations ranging from 15 to 80 mg/kg have been reported (2, 14). In addition, our method of calculating the iodine intake attributable to table salt had the effect of inflating the amount consumed by NHANES participants who used noniodized salt at the table and, at the same time, depressing (by ∼30%) the amount consumed by NHANES participants who used iodized salt. In addition, because salt intake (measured as urinary excretion of sodium) increased slightly in US adults from the years 1988–1991 to 2010 (30), our reliance on 1991 data are more likely to have underestimated adult use of salt at the table than to have overestimated such use. Finally, our reliance on a value obtained in adults may have overestimated or underestimated children’s use of salt at the table.
Our estimates of iodine intake have several limitations. First, the thousands of foods potentially consumed by NHANES participants do not necessarily provide the same iodine content as the 286 TDS foods to which they were matched. Thus, our mapping strategy may have over- or underestimated iodine intake from the foods consumed by NHANES participants. In addition, for foods with highly variable iodine concentrations, our use of the mean iodine concentration rather than the median may have overestimated iodine content, thereby inflating the estimated iodine intake of individuals who reported consuming those foods (31). Another potential limitation in assessing iodine intake by using 24-h dietary recalls is that saltwater fish and seafood, both important sources of dietary iodine, tend to be consumed episodically in the Western diet (32). Episodic intake might be missed by two 24-h recalls, resulting in underestimation of some participants’ intakes. Less often, intake might be overestimated for a participant who rarely consumes fish or seafood but does so coincident with the survey. Another concern is that by excluding kelp-containing supplements when calculating the iodine intakes of the 39 NHANES participants who reported using such supplements, we may have greatly underestimated their iodine intake from supplements as well as their total iodine intake.
In general, food intake data are subject to both under- and overreporting of the consumption of foods with high iodine content, which can result in both under- and overestimation of the prevalence of iodine inadequacy and excess. However, the impact of individual reporting errors was reduced by our adjustment of individual intake distributions for intra- and interindividual variation.
In all of the subgroups, the estimated prevalence of iodine inadequacy based on total usual dietary iodine intake was substantially lower than that based on UIC. One explanation for this discrepancy concerns the timing of urine collection. In NHANES, UIC was measured in single spot urine samples that were nontimed (i.e., collection times varied). If sampling times tended to precede the meal with the highest iodine intake, this would have biased the UIC toward values that underestimate 24-h intake. In one study, UIC measured in timed spot urine samples showed a good correlation with median 24-h urinary iodine excretion (33). In addition to the uniformity produced by timing the spot urines, the participants’ iodine intakes in that study may have been more homogeneous than those of the general US population because dietary screening was performed before enrollment (33, 34). Another study found that because of large day-to-day variability in dietary iodine intake, ≥10 d of spot urine sampling were needed to reach 20% precision and to develop a reliable marker of individual iodine status in women (35). Substantial within-person variability in iodine excretion between two 24-h urine collections 4–11 d apart (25% for blacks and 17% for other races) and among timed spot urines (23–34%) has been reported (34).
Another important reason why UIC predicted a higher percentage of the population with “inadequate” iodine status than the percentage predicted by total usual dietary iodine intake is that the intake data were adjusted for within-person variability whereas the UIC data were not. Basing the prevalence of inadequacy on unadjusted UIC leads to overestimation because the distribution of single UIC values measured in single spot samples is broader than the distribution of usual UIC (7, 23). One study found that when the UIC was adjusted for population-specific intra- and interindividual variability, the percentage of women with values <63 μg/L decreased from 41% to 7% (24). Other studies have also suggested that the wide variability in the iodine content of some foods and their frequency of consumption may contribute to the magnitude of the intraindividual variation in UICs observed in single spot urine samples. Under some circumstances, the day-to-day variation in UICs could be 30–40% (24, 35, 36). In one study, when ≥2 spot urine samples were collected, thereby allowing a variability adjustment to be made to the UIC, the distribution tightened; this, in turn, reduced the estimated percentage of the population with UICs <50 μg/L from 33% to 19% and those with UICs ≥100 μg/L from 21% to 17% (37). In a population with suboptimal iodine status and diets less varied than the typical Western diet, variability adjustment of the UIC likewise decreased the prevalences of both iodine inadequacy and iodine excess (38).
The calculated prevalence of iodine excess was very high for children <9 y of age, whether based on UIC or iodine intake, leading to questions about the validity of both the ULs and the UL-like UIC cutoffs for young children. In all subgroups composed of individuals aged ≥9 y, including pregnant females, the estimated prevalence of iodine excess based on total usual dietary iodine intake was considerably lower than that based on UIC. Following on the discussion above, overestimation of iodine excess may also be a consequence of the use of unadjusted UIC values measured in single spot samples. Furthermore, EAR-like and UL-like UIC cutoffs should be validated against a more reliable biomarker of individual iodine status than UIC (24), assuming that such a biomarker becomes available.
It has been noted by others that collecting multiple measures from a subject at the individual level decreases intraindividual variability and may allow more meaningful analysis of associations between diet-related risk factors and various biomarkers of risk (39). By estimating the prevalences of iodine inadequacy and excess among subjects who provided both UIC and dietary recall data on a single day, we sought to provide a more accurate assessment than what could be provided by either UIC data or iodine intake data alone.
The fact that the correlation between UIC and iodine intake is moderately weak for pregnant females and weaker still for all other subgroups indicates that the 2 assessment methods do not always identify the same individuals with either low or high intakes. It is likely that neither a single spot urine sample nor a single 24-h dietary recall is sufficient to screen individuals for iodine inadequacy and excess; repeated measurements would be needed for this purpose. However, if properly adjusted for within-person variability, either method should be appropriate for estimating the population prevalences of inadequacy and excess.
Acknowledgments
We acknowledge the contributions to the manuscript made by Gay Goodman, Iodine Initiative Consultant to the NIH Office of Dietary Supplements, in the course of providing expert scientific and technical review.
The authors’ responsibilities were as follows—JHS and ALC: provided essential materials; WYJ: analyzed the data; WYJ, PRT, and SPM: wrote the manuscript; WYJ: had primary responsibility for the final content; and all authors: designed the research and read and approved the manuscript. The authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: EAR, Estimated Average Requirement; FNDDS, Food and Nutrient Database for Dietary Studies; RDA, Recommended Dietary Allowance; TDS, Total Diet Study; UIC, urinary iodine concentration; UL, Tolerable Upper Intake Level.
REFERENCES
- 1.WHO. Vitamin and Mineral Nutrition Information System: urinary iodine concentrations for determining iodine status in populations. Geneva (Switzerland): WHO; 2013. [cited 2015 Mar 12]. Available from: http://apps.who.int/iris/bitstream/10665/85972/1/WHO_NMH_NHD_EPG_13.1_eng.pdf.
- 2.Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, Raiten DJ. Biomarkers of nutrition for development—iodine review. J Nutr 2014;144(Suppl):1322S–42S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce EN, Caldwell KL. Urinary iodine, thyroid function, and thyroglobulin as biomarkers of iodine status. Am J Clin Nutr 2016;104(Suppl):898S–901S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee RD, Nieman DC. Nutritional assessment. Dubuque (IA): William C Brown Communications; 1993. [Google Scholar]
- 5.Trumbo PR. FDA regulations regarding iodine addition to foods and labeling of foods containing added iodine. Am J Clin Nutr 2016;104(Suppl):864S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carriquiry AL, Spungen JH, Murphy SP, Pehrsson PR, Dwyer JT, Juan WY, Wirtz MS. Variation in the iodine concentrations of foods: considerations for dietary assessment. Am J Clin Nutr 2016;104(Suppl):877S–87S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed] [Google Scholar]
- 8.National Center for Health Statistics; CDC. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 2013;1(56). [cited 2015 Mar 12]. Available from: http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. [PubMed]
- 9.Caldwell KL, Maxwell CB, Makhmudov A, Pino S, Braverman LE, Jones RL, Hollowell JG. Use of inductively coupled plasma mass spectrometry to measure urinary iodine in NHANES 2000: comparison with previous method. Clin Chem 2003;49:1019–21. [DOI] [PubMed] [Google Scholar]
- 10.Environmental Health, CDC. Laboratory procedure manual. Iodine and mercury in urine: NHANES 2009–2010. [cited 2015 Mar 12]. Available from: http://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/UIOUHG_F_met_iodine_mercury.pdf.
- 11.Snyder SA, Vanderford BJ, Rexing DJ. Trace analysis of bromate, chlorate, iodate, and perchlorate in natural and bottled waters. Environ Sci Technol 2005;39:4586–93. [DOI] [PubMed] [Google Scholar]
- 12.Dorman JW, Steinberg SM. Analysis of iodide and iodate in Lake Mead, Nevada using a headspace derivatization gas chromatography-mass spectrometry. Environ Monit Assess 2010;161:229–36. [DOI] [PubMed] [Google Scholar]
- 13.National Agricultural Library, Agricultural Research Service, USDA. National Nutrient Database for Standard Reference, release 27: basic report: 02047, salt, table. Version current 1 August 2014 [cited 2014 Nov 12]. Available from: http://ndb.nal.usda.gov/ndb/foods/show/277?qlookup=table+salt&fg=&format=&man=&lfacet=&max=25&new=1.
- 14.Dasgupta PK, Liu Y, Dyke JV. Iodine nutrition: iodine content of iodized salt in the United States. Environ Sci Technol 2008;42:1315–23. [DOI] [PubMed] [Google Scholar]
- 15.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr 1991;10:383–93. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics, CDC. Health Indicators Warehouse: sodium consumption (milligrams) [cited 2014 Jul 22]. Available from: http://www.healthindicators.gov/Indicators/Sodium-consumption-milligrams_1222/Profile.
- 17.Egan SK, Bolger PM, Carrington CD. Update of US FDA’s Total Diet Study food list and diets. J Expo Sci Environ Epidemiol 2007;17:573–82. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Total Diet Study: study design. 2009. Version current 10 April 2013 [cited 2014 Oct 15]. Available from: http://www.fda.gov/Food/FoodScienceResearch/TotalDietStudy/ucm184232.htm.
- 19.Agricultural Research Service, USDA. Food and Nutrient Database for Dietary Studies. 2014. Version current 4 December 2014 [cited 2016 Feb 12]. Available from: http://www.ars.usda.gov/services/docs.htm?docID=12089.
- 20.Montville JB, Ahuja JKC, Martin CL, Heendeniya KY, Omolewa-Tomobi G, Steinfeldt LC, Anand J, Adler ME, LaComb RP, Moshfegh A. USDA Food and Nutrient Database for Dietary Studies (FNDDS), 5.0. Procedia Food Sci 2013;2:99–112. [Google Scholar]
- 21.Nusser SM, Carriquiry AL, Dodd KW, Fuller WA. A semiparametric transformation approach to estimating usual daily intake distributions. J Am Stat Assoc 1996;91:1440–9. [Google Scholar]
- 22.Murphy SP, Barr SI, Poos MI. Using the new Dietary Reference Intakes to assess diets: a map to the maze. Nutr Rev 2002;60:267–75. [DOI] [PubMed] [Google Scholar]
- 23.Murphy SP, Guenther PM, Kretsch MJ. Using the Dietary Reference Intakes to assess intakes of groups: pitfalls to avoid. J Am Diet Assoc 2006;106:1550–3. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 2012;70:553–70. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell KL, Jones R, Hollowell JG. Urinary iodine concentration: United States National Health and Nutrition Examination Survey, 2001–2002. Thyroid 2005;15:692–9. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell KL, Makhmudov A, Ely E, Jones RL, Wang RY. Iodine status of the U.S. population, National Health and Nutrition Examination Survey, 2005–2006 and 2007–2008. Thyroid 2011;21:419–27. [DOI] [PubMed] [Google Scholar]
- 27.Caldwell KL, Miller GA, Wang RY, Jain RB, Jones RL. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003–2004. Thyroid 2008;18:1207–14. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell KL, Pan Y, Mortensen ME, Makhmudov A, Merrill L, Moye J. Iodine status in pregnant women in the National Children’s Study and in U.S. women (15–44 years), National Health and Nutrition Examination Survey 2005–2010. Thyroid 2013;23:927–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan Y, Caldwell KL, Li Y, Caudill SP, Mortensen ME, Makhmudov A, Jones RL. Smoothed urinary iodine percentiles for the US population and pregnant women: National Health and Nutrition Examination Survey, 2001–2010. Eur Thyroid J 2013;2:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeiffer CM, Hughes JP, Cogswell ME, Burt VL, Lacher DA, Lavoie DJ, Rabinowitz DJ, Johnson CL, Pirkle JL. Urine sodium excretion increased slightly among U.S. adults between 1988 and 2010. J Nutr 2014;144:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennington JA, Schoen SA. Contributions of food groups to estimated intakes of nutritional elements: results from the FDA total diet studies, 1982–1991. Int J Vitam Nutr Res 1996;66:342–9. [PubMed] [Google Scholar]
- 32.Kipnis V, Midthune D, Buckman DW, Dodd KW, Guenther PM, Krebs-Smith SM, Subar AF, Tooze JA, Carroll RJ, Freedman LS. Modeling data with excess zeros and measurement errors: application to evaluating relationships between episodically consumed food and health outcomes. Biometrics 2009;65:1003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrine CG, Cogswell ME, Swanson CA, Sullivan KM, Chen TC, Carriquiry AL, Dodd KW, Caldwell KL, Wang CY. Comparison of population iodine estimates from 24-hour urine and timed-spot urine samples. Thyroid 2014;24:748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, et al. Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr 2013;143:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011;141:2049–54. [DOI] [PubMed] [Google Scholar]
- 36.Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Andersen S, Rasmussen LB, Ovesen L, Jörgensen T. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 2009;19:1281–6. [DOI] [PubMed] [Google Scholar]
- 37.Charlton KE, Batterham MJ, Buchanan LM, Mackerras D. Intraindividual variation in urinary iodine concentrations: effect of adjustment on population distribution using two and three repeated spot urine collections. BMJ Open 2014;4:e003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackerras DE, Singh GR, Eastman CJ. Iodine status of Aboriginal teenagers in the Darwin region before mandatory iodine fortification of bread. Med J Aust 2011;194:126–30. [DOI] [PubMed] [Google Scholar]
- 39.Sempos CT, Johnson NE, Smith EL, Gilligan C. Effects of intraindividual and interindividual variation in repeated dietary records. Am J Epidemiol 1985;121:120–30. [DOI] [PubMed] [Google Scholar]