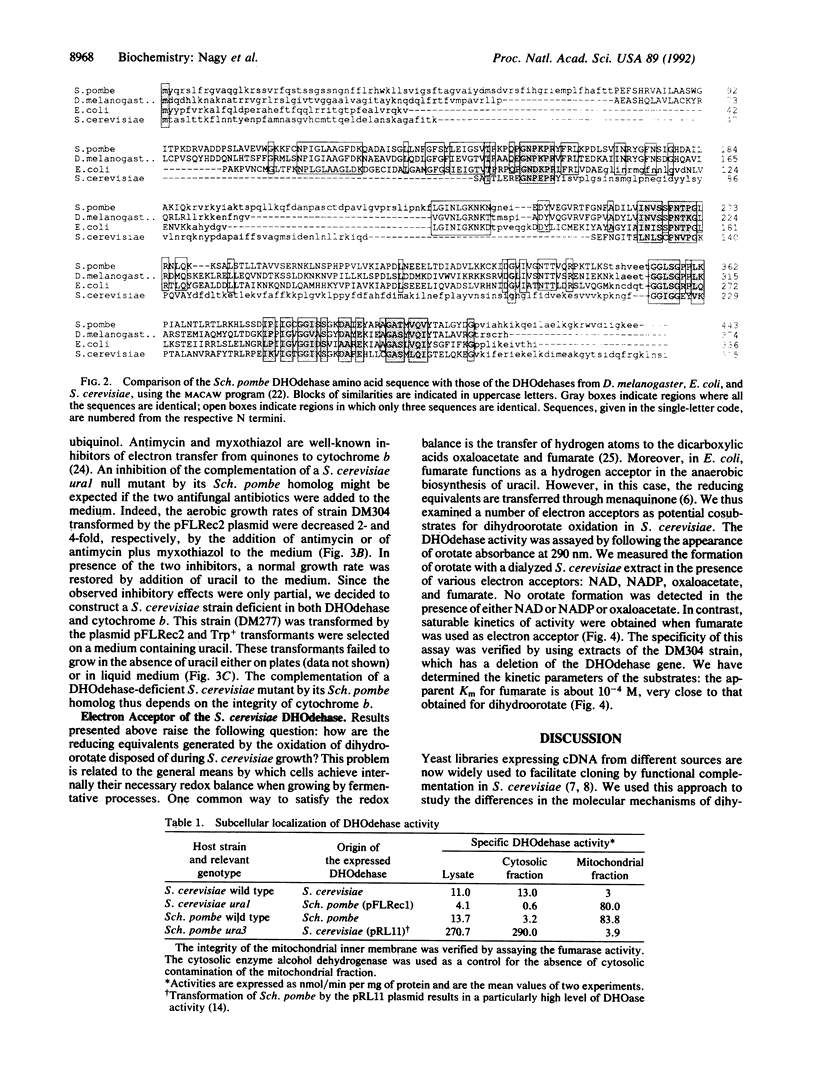
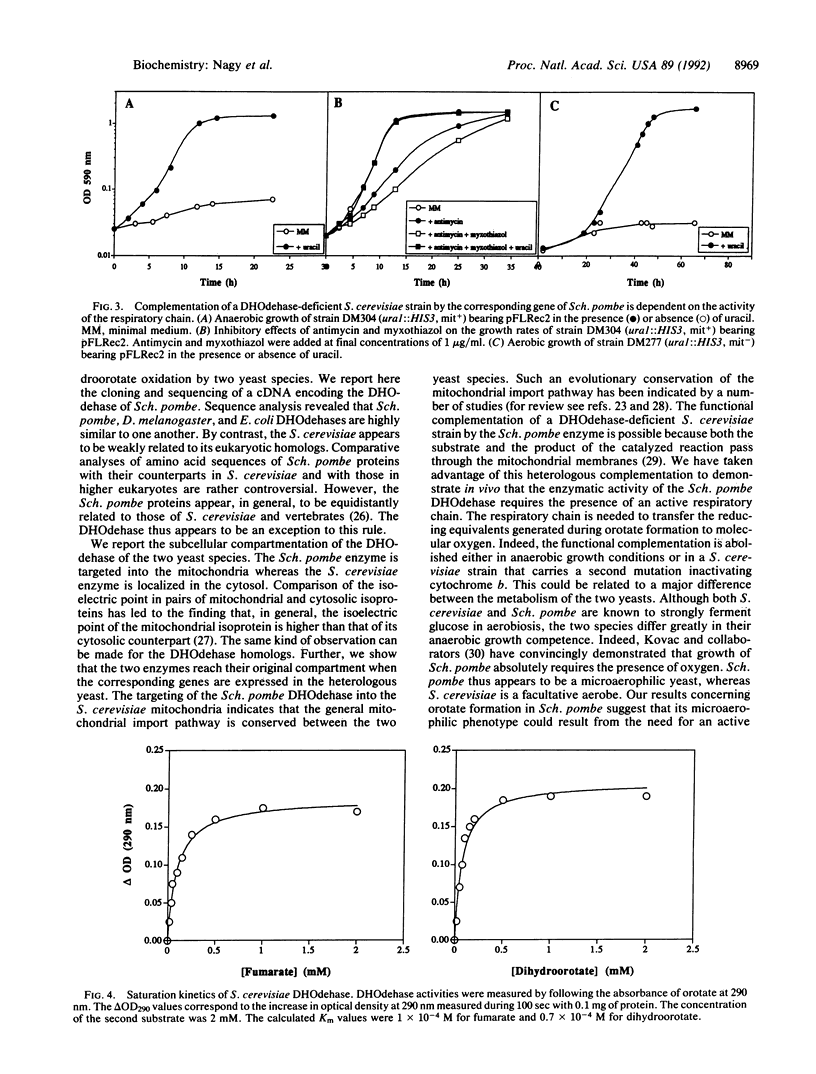
Abstract
A cDNA encoding the dihydroorotate dehydrogenase (DHOdehase; EC 1.3.3.1) of the yeast Schizosaccharomyces pombe was isolated by functional complementation in Saccharomyces cerevisiae. A divergent subcellular compartmentation of the DHOdehase of each yeast was shown. The DHOdehase from Sch. pombe was localized in the mitochondria whereas its homolog from S. cerevisiae was found to be cytosolic. The heterologous expression of the Sch. pombe enzyme in S. cerevisiae allowed us to demonstrate that the Sch. pombe DHOdehase activity requires the integrity of the mitochondrial electron transport chain. Indeed, the presence of a mutation inactivating cytochrome b abolished the complementation of a S. cerevisiae ura1 mutant by the corresponding Sch. pombe gene. By contrast, in vitro studies have revealed that the DHOdehase of S. cerevisiae uses fumarate as terminal electron acceptor. These results are discussed in relation to the anaerobic growth competence of the two yeasts and to the fermentative processes they use.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S., Cox G. B., Gibson F. The anaerobic oxidation of dihydroorotate by Escherichia coli K-12. Biochim Biophys Acta. 1977 Oct 12;462(1):153–160. doi: 10.1016/0005-2728(77)90197-9. [DOI] [PubMed] [Google Scholar]
- Brivet-Chevillotte P., di Rago J. P. Electron-transfer restoration by vitamin K3 in a complex III-deficient mutant of S. cerevisiae and sequence of the corresponding cytochrome b mutation. FEBS Lett. 1989 Sep 11;255(1):5–9. doi: 10.1016/0014-5793(89)81050-6. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Dessen P., Fondrat C., Valencien C., Mugnier C. BISANCE: a French service for access to biomolecular sequence databases. Comput Appl Biosci. 1990 Oct;6(4):355–356. doi: 10.1093/bioinformatics/6.4.355. [DOI] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P., Fox T. D. Nuclear mutations in the petite-negative yeast Schizosaccharomyces pombe allow growth of cells lacking mitochondrial DNA. Genetics. 1992 Jun;131(2):255–260. doi: 10.1093/genetics/131.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Hartmann C., Christen P., Jaussi R. Mitochondrial protein charge. Nature. 1991 Aug 29;352(6338):762–763. doi: 10.1038/352762b0. [DOI] [PubMed] [Google Scholar]
- Hines V., Keys L. D., 3rd, Johnston M. Purification and properties of the bovine liver mitochondrial dihydroorotate dehydrogenase. J Biol Chem. 1986 Aug 25;261(24):11386–11392. [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Kerr C. T., Miller R. W. Dihydroorotate-ubiquinone reductase complex of Escherichia coli B. J Biol Chem. 1968 Jun 10;243(11):2963–2968. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labaille F., Colson A. M., Petit L., Goffeau A. Properties of a mitochondrial suppressor mutation restoring oxidative phosphorylation in a nuclear mutant of the yeast Schizosaccharomyces pombe. J Biol Chem. 1977 Aug 25;252(16):5716–5723. [PubMed] [Google Scholar]
- Larsen J. N., Jensen K. F. Nucleotide sequence of the pyrD gene of Escherichia coli and characterization of the flavoprotein dihydroorotate dehydrogenase. Eur J Biochem. 1985 Aug 15;151(1):59–65. doi: 10.1111/j.1432-1033.1985.tb09068.x. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Losson R., Fuchs R. P., Lacroute F. Yeast promoters URA1 and URA3. Examples of positive control. J Mol Biol. 1985 Sep 5;185(1):65–81. doi: 10.1016/0022-2836(85)90183-4. [DOI] [PubMed] [Google Scholar]
- Miller R. W. A high molecular weight dihydro-orotate dehydrogenase of Neurospora crassa. Purification and properties of the enzyme. Can J Biochem. 1975 Dec;53(12):1288–1300. doi: 10.1139/o75-175. [DOI] [PubMed] [Google Scholar]
- Minet M., Dufour M. E., Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992 May;2(3):417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Pascal R. A., Jr, Le Trang N., Cerami A., Walsh C. Purification and properties of dihydroorotate oxidase from Crithidia fasciculata and Trypanosoma brucei. Biochemistry. 1983 Jan 4;22(1):171–178. doi: 10.1021/bi00270a025. [DOI] [PubMed] [Google Scholar]
- Schuler G. D., Altschul S. F., Lipman D. J. A workbench for multiple alignment construction and analysis. Proteins. 1991;9(3):180–190. doi: 10.1002/prot.340090304. [DOI] [PubMed] [Google Scholar]
- Sigler K., Höfer M. Mechanisms of acid extrusion in yeast. Biochim Biophys Acta. 1991 Dec 12;1071(4):375–391. doi: 10.1016/0304-4157(91)90003-f. [DOI] [PubMed] [Google Scholar]
- Subík J., Kolarov J., Kovác L. Anaerobic growth and formation of respiration-deficient mutants of various species of yeasts. FEBS Lett. 1974 Sep 1;45(1):263–266. doi: 10.1016/0014-5793(74)80858-6. [DOI] [PubMed] [Google Scholar]
- Taylor M. L., Taylor W. H., Eames D. F., Taylor C. D. Biosynthetic dihydroorotate dehydrogenase from Lactobacillus bulgaricus. J Bacteriol. 1971 Mar;105(3):1015–1027. doi: 10.1128/jb.105.3.1015-1027.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Cherest H., Surdin-Kerjan Y. Identification of the structural gene for glucose-6-phosphate dehydrogenase in yeast. Inactivation leads to a nutritional requirement for organic sulfur. EMBO J. 1991 Mar;10(3):547–553. doi: 10.1002/j.1460-2075.1991.tb07981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Jacquemin I., Surdin-Kerjan Y. MET4, a leucine zipper protein, and centromere-binding factor 1 are both required for transcriptional activation of sulfur metabolism in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Apr;12(4):1719–1727. doi: 10.1128/mcb.12.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Surdin-Kerjan Y. An improved strategy for generating a family of unidirectional deletions on large DNA fragments. Genet Anal Tech Appl. 1990 Jun;7(4):87–90. doi: 10.1016/0735-0651(90)90033-c. [DOI] [PubMed] [Google Scholar]
- Tisdale H., Hauber J., Prager G., Turini P., Singer T. P. Studies on succinate dehydrogenase. 15. Isolation, molecular properties, and isoenzymes of fumarate reductase. Eur J Biochem. 1968 May;4(4):472–477. doi: 10.1111/j.1432-1033.1968.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- di Rago J. P., Netter P., Slonimski P. P. Intragenic suppressors reveal long distance interactions between inactivating and reactivating amino acid replacements generating three-dimensional constraints in the structure of mitochondrial cytochrome b. J Biol Chem. 1990 Sep 15;265(26):15750–15757. [PubMed] [Google Scholar]
- von Jagow G., Engel W. D. Complete inhibition of electron transfer from ubiquinol to cytochrome b by the combined action of antimycin and myxothiazol. FEBS Lett. 1981 Dec 21;136(1):19–24. doi: 10.1016/0014-5793(81)81206-9. [DOI] [PubMed] [Google Scholar]




