Abstract
The US Food and Drug Administration (FDA) and the Nutrient Data Laboratory (NDL) of the USDA Agricultural Research Service have worked independently on determining the iodine content of foods and dietary supplements and are now harmonizing their efforts. The objective of the current article is to describe the harmonization plan and the results of initial iodine analyses accomplished under that plan. For many years, the FDA’s Total Diet Study (TDS) has measured iodine concentrations in selected foods collected in 4 regions of the country each year. For more than a decade, the NDL has collected and analyzed foods as part of the National Food and Nutrient Analysis Program; iodine analysis is now being added to the program. The NDL recently qualified a commercial laboratory to conduct iodine analysis of foods by an inductively coupled plasma mass spectrometry (ICP-MS) method. Co-analysis of a set of samples by the commercial laboratory using the ICP-MS method and by the FDA laboratory using its standard colorimetric method yielded comparable results. The FDA recently reviewed historical TDS data for trends in the iodine content of selected foods, and the NDL analyzed samples of a limited subset of those foods for iodine. The FDA and the NDL are working to combine their data on iodine in foods and to produce an online database that can be used for estimating iodine intake from foods in the US population. In addition, the NDL continues to analyze dietary supplements for iodine and, in collaboration with the NIH Office of Dietary Supplements, to publish the data online in the Dietary Supplement Ingredient Database. The goal is to provide, through these 2 harmonized databases and the continuing TDS focus on iodine, improved tools for estimating iodine intake in population studies.
Keywords: DSID, FDA, NHANES, Total Diet Study, USDA
INTRODUCTION
Iodine is an essential component of thyroid hormone, which regulates metabolism and is required for normal development, including neurodevelopment (1). Because of iodine’s critical role in development, it is especially important to ensure the iodine sufficiency of pregnant women, other women of reproductive age, infants, and young children. In recent years, there have been reports that suggested that some pregnant women in the United States may not be receiving adequate dietary iodine (2). On the other hand, there is evidence that some proportion of children in the United States and other iodine-sufficient areas may be consuming excess iodine (3) and that excessive iodine intake is associated with increased rates of thyroid autoimmunity and other forms of thyroid dysfunction (4).
People obtain iodine from the diet and from dietary supplements. Iodine is naturally present in foods that are grown or raised in marine environments (e.g., fish and seaweed). Iodine is also present in food crops in concentrations that depend on the iodine content of the soils in which the crops are grown (5). Other foods have small amounts of iodine from natural sources but contain larger amounts due to direct fortification (e.g., salt, infant formulas, and meal replacements) or adventitious sources. Iodine-supplemented animal feeds are potentially the main contributors to the iodine content of milk (6, 7) and eggs (8). In addition, iodine can be introduced into milk directly or indirectly from the iodophors used as teat dips and general cleaning agents (6, 7). However introduced to dairy cows and laying hens, the iodine content of milk and eggs is reflected in the iodine concentrations of foods made with these products. Seaweed and seaweed-based food additives contribute iodine to a wide variety of foods (9–11); patients following a low-iodine diet in preparation for radioactive iodine treatment are advised not to consume foods containing alginates, agar-agar, carrageenan, and other seaweed-based thickeners (12). Iodates have been used as dough conditioners in some commercially produced baked goods for >50 y (13) but are currently being phased out (T Moore, AIB International, personal communication, 2014). Commercially processed foods may also contain erythrosine (FD&C Red no. 3), an organoiodine color additive.
To the extent that pregnant women rely on supplements to provide adequate iodine, it is worth noting that prenatal supplements sold in the United States do not universally contain iodine. The 2001−2006 NHANES found that 77% of pregnant women surveyed were taking prenatal vitamin supplements but only 20% were taking prenatal vitamin supplements that contained iodine (14).
Although, on average, both men and women were meeting the Recommended Dietary Allowance for iodine as recently as the 1990s (15), the population’s iodine intake was found to be quite variable and generally lower than in previous decades, possibly because people were making fewer foods in the home (thus using less iodized salt in food preparation) and getting more of their foods from commercial vendors that do not routinely use iodized salt (16). The increased consumer use of salts or salt substitutes that are not iodized may also have contributed to the decline.
Urinary iodine concentration (UIC)7 measured in spot urine samples is the most commonly used surrogate for total iodine intake (17). Although spot urine UIC is useful for estimating the average iodine status of a population, the low and high ends of the distribution must be interpreted with caution to avoid overestimating the respective prevalences of iodine deficiency and iodine excess. Day-to-day intraindividual variability in iodine intake (and, correspondingly, UIC) can be pronounced; a recent study in women found that 10 urine collections are needed to provide a reasonable estimate of the iodine status of individuals (18). It is clear that adjustment for intraindividual variation narrows the population distribution of spot urine UIC, particularly at the upper end (19); modeling the relation between population UIC and iodine intake may offer further improvement (20). In part to provide iodine intake data for use in interpreting UIC measured in spot samples, it is important to develop efficient tools to monitor and assess the population’s chronic dietary exposure to iodine. Progress in evaluating the iodine status of population subgroups relies on continuing progress in the assessment of iodine in foods and supplements sold in the United States and the dissemination of this information to the public.
The US Food and Drug Administration (FDA) and the Nutrient Data Laboratory (NDL) of the USDA Agricultural Research Service have worked independently to determine the iodine content of selected foods and are now harmonizing their efforts. For many years, the FDA’s Total Diet Study (TDS) has measured iodine concentrations in selected foods collected in 4 regions of the country each year. These foods are chosen on a generic basis, without information on brand names. For more than a decade, the NDL has been collecting and analyzing foods as part of the National Food and Nutrient Analysis Program (NFNAP), gathering full information on location and brand.
For many years, iodine in foods was not included in the annually released USDA Nutrient Database for Standard Reference (SR), the official federal food-composition database. The introduction of inductively coupled mass spectrometry (ICP-MS) for measuring iodine in foods has made the analysis less complicated. As a result, iodine analysis is now being added to the NFNAP. Another source of data on the iodine content of foods is the Dietary Supplement Ingredient Database (DSID), which is produced jointly by the NDL and the NIH Office of Dietary Supplements (ODS). The DSID reports the results of iodine analysis for iodine-containing "multivitamin/mineral supplements" (MVMs) (21), which are defined in the DSID as dietary supplements with ≥3 vitamins, with or without minerals. In addition, the NDL is collaborating with the FDA and the NIH ODS on an iodine research initiative (17). As part of this initiative, the NDL has evaluated a new analytical method which it will be using to measure the iodine content of foods that are expected to be important contributors of iodine to the US diet. The goal is to produce a USDA database that includes both the USDA’s NDL data and the FDA’s TDS data on the iodine content of foods. The present article describes the initial harmonization of sample collection and iodine analysis.
METHODS
The FDA’s TDS program
The FDA initiated the TDS program in 1961 to address concerns about the effects of nuclear testing on concentrations of radionuclides in food, but the program quickly broadened to include other uses (22). In the current program, the TDS collects retail samples of 280 specific foods that are identified as being of importance in the American diet (23). Samples are collected each quarter from 1 of 4 geographic regions of the United States (the North Central region in fall, the Western region in winter, the Southern region in spring, and the Northeastern region in summer). In addition to using the analytical results to estimate population exposures to contaminants in the diet, the FDA uses the TDS results to track trends in the concentrations of contaminants and nutrients in foods. Iodine, although an essential nutrient, would be considered a contaminant if found at unexpectedly high concentrations resulting from iodine additives used in foods or feed.
The foods collected in each sampling period are referred to collectively as a “market basket.” For each quarterly market basket, the TDS purchases foods in each of 3 cities within the region and sends them to the FDA’s Kansas City District Laboratory. A nearby institutional kitchen prepares the foods, takes samples, and makes composites of the prepared food samples from the 3 cities. The FDA’s Kansas City District Laboratory analyzes the food composites to determine concentrations of mineral nutrients, toxic elements, and pesticides and sends samples to the FDA’s Arkansas Regional Laboratory for analysis of industrial chemicals and to the FDA’s Winchester Engineering and Analytical Center (in Massachusetts) for analysis of radionuclides.
Iodine was included in the TDS program from 1973 to 1991, dropped from the program due to shifting priorities, and added back to the program in 2003. The method in use for iodine analysis of TDS foods from 2003 through 2015 entails ternary acid digestion to release iodide, after which UV-visible spectrophotometry is used to follow iodide catalysis of the Ce(IV)-As(III) reaction (FDA KAN-LABORATORY-MET.95, available from the FDA Kansas City District Laboratory, Lenexa, KS). The limit of quantitation (LOQ) for this colorimetric method is 30−60 μg/100 g (24).
TDS data on iodine in foods collected in 1982−1991 were published by Pennington et al. (25). TDS data on iodine in foods collected in 2003−2011 are available online (26).
The USDA’s NFNAP
The NDL maintains the SR, which covers ∼8600 foods in the US diet and lists up to 150 different nutrients in each food. Nearly 20 y ago, the USDA implemented the NFNAP with the goal of obtaining, for inclusion in the SR, nationally representative estimates of the nutritional composition of foods commonly consumed in the United States (27). The food sampling design is statistically determined and is revised with each census (28).
The NDL recently qualified a commercial laboratory to conduct ICP-MS analysis of iodine in foods upon demonstration of accurate ICP-MS analysis of certified reference materials from the National Institute of Standards and Technology. Using the method of Sullivan and Zywicki (29), samples are prepared by dissolution with potassium hydroxide in either an oven or open-vessel microwave system, stabilized with sodium thiosulfate in an ammonium solution, diluted, and analyzed using ICP-MS with a praseodymium ionization internal standard. The LOQ of this method is 10 μg/100 g, which is lower than the LOQ of the colorimetric method used by the FDA’s TDS program.
We report here provisional, previously unpublished results from the initial 2 phases of the NDL’s analysis of foods for iodine. In the first phase, the NDL arranged for divided samples of 12 foods and 3 control materials to be analyzed for iodine by the FDA’s TDS laboratory (colorimetric method) and the prequalified commercial laboratory (ICP-MS method). These foods included fish, pizza, American cheese, baked products, salami, eggs, and fast-food sandwiches. In the next phase, NDL staff reviewed the most recent report of the TDS and identified ∼60 iodine-containing foods also represented in the NFNAP. They arranged for ∼160 samples of these foods to be shipped from the Food Analysis Laboratory Control Center at Virginia Tech for iodine analysis by the same commercial laboratory used in the first phase. In some cases, multiple types of a given food were sampled. For example, macaroni and cheese samples were obtained from several restaurants and a sample from a prepared box mix.
The NDL-ODS dietary supplement program
To more accurately estimate intakes from the consumption of dietary supplements, the NDL worked cooperatively with the NIH’s ODS and other federal entities to launch the DSID project in 2004 and released databases in 2009, 2012, and 2015 (21, 30–32). Analytical data in the DSID are generated in parallel with the ongoing NFNAP study mentioned above. The DSID currently provides nationally representative analytical composition data for vitamins and minerals (including iodine) for adult and children’s MVMs. National estimates for the ingredient content of prenatal nonprescription MVMs were released in DSID-3 in 2015 (21).
Supporting the DSID effort is the Dietary Supplement Label Database, an ODS-sponsored compilation of almost 40,000 different products sold as dietary supplements in the United States (33). A recent search of the Dietary Supplement Label Database found 2228 products on the market with iodine listed in the Supplemental Facts label. The label information in this database is a valuable resource for identifying representative products for analysis.
We report here results from 3 studies representative of the US marketplace (adult MVMs, children’s MVMs, and nonprescription prenatal MVMs) (21). The 3 studies systematically compared labeled and analytical values of the iodine content of the vitamins and minerals listed on the label’s Supplement Facts panel and use regression analysis to calculate predicted mean values and measures of variability. Multiple lots of products from retail mass market, retail natural health, and direct channels were purchased nationally through the use of statistically based sampling plans (109 adult MVMs, 66 children’s MVMs, and 71 nonprescription prenatal MVMs). Samples were sent to qualified laboratories for analysis of the vitamins and minerals, including iodine. The methods of analysis evolved over the course of the 3 studies and are discussed in Results.
RESULTS
Iodine analyses conducted under the TDS program
Mean iodine concentrations in TDS foods analyzed in 2003−2011 ranged from nondetectable (in approximately one-third of tested foods) to 141 μg/100 g in white cake with icing. In Table 1, the 25 foods with the highest mean iodine concentrations in 2003−2011 TDS market baskets are compared with comparable foods in 1982−1984 TDS market baskets. Foods with a 2003−2011 mean iodine concentration far in excess of the median included commercially prepared cakes (white and chocolate), sherbet, meal-replacement beverages, breads, bagels, popsicles, and sugar cookies. From 1982−1991 to 2003−2011, the mean concentration of iodine in chocolate cake increased by >300% (from 23 to 78 μg/100 g), and the ratio of the median to the mean increased from 2.1 to 5.6. Mean and median concentrations of iodine in milks increased by ∼100% over the same time period.
TABLE 1.
Iodine concentrations in selected foods sampled under the FDA’s TDS1
| Iodine, μg/100 g |
||||
| TDS 2003−2011 |
TDS 1982−1991 |
|||
| Food description | Mean ± SD | Median | Mean ± SD | Median |
| Cake, white with icing, commercial | 141 ± 265 | 49 | — | — |
| Sherbet, fruit-flavored | 133 ± 300 | 16 | — | — |
| Meal replacement, liquid RTD, any flavor | 122 ± 157 | 31 | — | — |
| Cake, chocolate with icing, commercial | 78 ± 226 | 14 | 23 ± 27 | 11 |
| Bread, white, enriched | 74 ± 151 | 3 | 91 ± 84 | 73 |
| Cheese, Swiss, natural | 69 ± 57 | 66 | — | — |
| Candy bar, milk chocolate, plain | 67 ± 14 | 67 | 43 ± 15 | 44 |
| Ice cream, light, vanilla | 62 ± 15 | 63 | 30 ± 12 | 28 |
| Eggs, scrambled | 58 ± 42 | 47 | 42 ± 34 | 35 |
| Fish sticks or patty, frozen, oven-cooked | 53 ± 19 | 53 | 63 ± 53 | 52 |
| Cheese, American, processed | 51 ± 10 | 50 | 46 ± 71 | 35 |
| Eggs, boiled | 51 ± 40 | 38 | 48 ± 39 | 39 |
| Ice cream, regular, vanilla | 51 ± 14 | 49 | — | — |
| Cheese, cheddar, natural (sharp/mild) | 49 ± 16 | 50 | 43 ± 38 | 36 |
| Bagel, plain, toasted | 48 ± 101 | 4 | — | — |
| Milk shake, chocolate, fast-food | 48 ± 13 | 46 | 53 ± 89 | 22 |
| Popsicle, fruit-flavored | 47 ± 91 | 0 | — | — |
| Sugar cookies | 46 ± 148 | 5 | — | — |
| Cream cheese | 43 ± 7 | 42 | — | — |
| Milk, skim, fluid | 42 ± 12 | 42 | 21 ± 10 | 20 |
| Milk, chocolate, low-fat, fluid | 41 ± 8 | 42 | 24 ± 14 | 22 |
| Milk, low-fat (2%), fluid | 41 ± 5 | 41 | 23 ± 9 | 24 |
| Milk, whole, fluid | 41 ± 8 | 42 | 20 ± 8 | 21 |
| Yogurt, low-fat, fruit-flavored | 41 ± 9 | 42 | 20 ± 18 | 19 |
Data are from TDS 1982−1991 (25) and TDS 2003−2011 (26) and are presented in decreasing order of the mean values in the latter. Samples were analyzed by the FDA’s colorimetric method in use during the years 1982−1991 (39) or 2003−2011 (24). Iodine concentrations below the laboratory’s limit of detection (3–6 μg/100 g) were assumed to be zero. Summary statistics are shown for the composite samples of each food analyzed in TDS 1982−1991 (n = 37) and TDS 2003−2011 (n = 33). FDA, Food and Drug Administration; RTD, ready-to-drink; TDS, Total Diet Study.
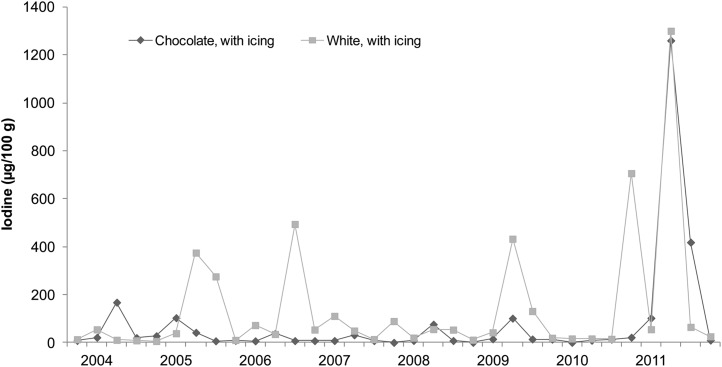
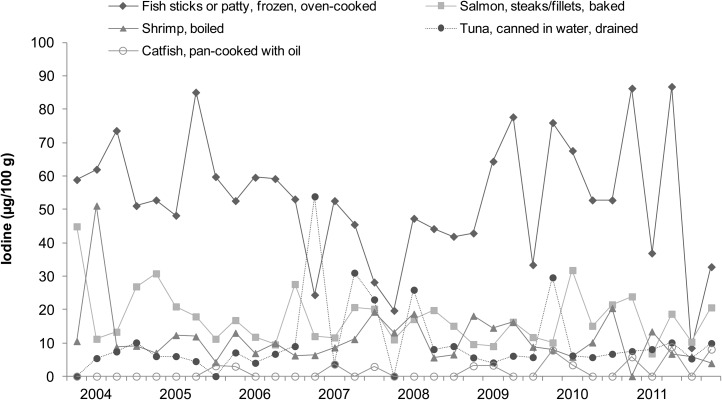
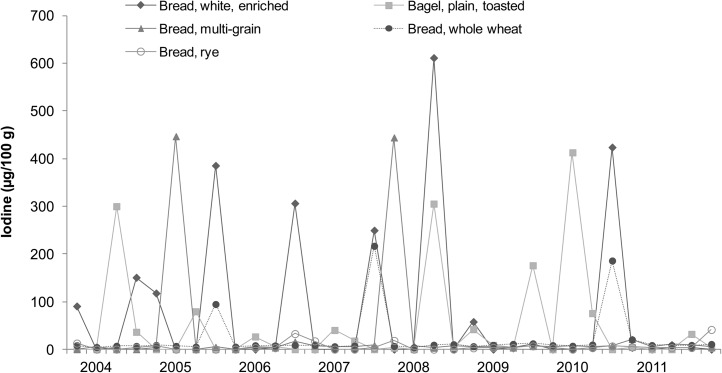
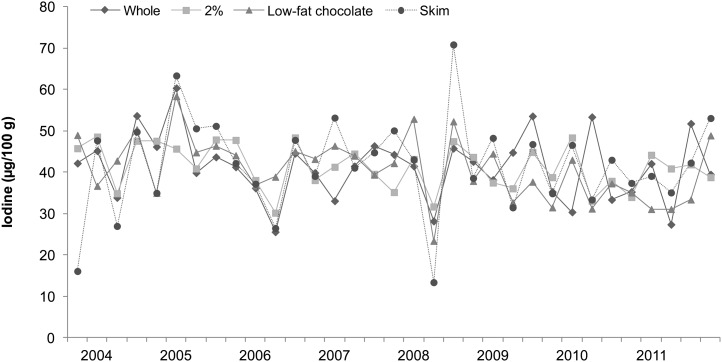
Iodine concentrations of cakes, breads, milks, and seafood (broad categories of foods with relatively high iodine content) analyzed in the 2003−2011 TDS are shown in Figures 1–4, respectively. Each of the 33 data points in these figures represents the iodine concentration of a single quarterly sample; each quarterly sample was a composite of 3 subsamples collected in different cities. The figures reveal much iodine concentration variability in cakes and breads, with unusually high values (>200 μg/100 g) in several samples. In dairy products, iodine concentrations were fairly consistent. Fish sticks contained more iodine than salmon, shrimp, or tuna in all but 2 market baskets. Iodine was present at moderate concentrations in all marine seafood species sampled, but catfish (presumably of the freshwater variety because saltwater catfish are not usually sold as food) contained little iodine.
FIGURE 1.
Iodine concentrations in cakes: US Food and Drug Administration’s Total Diet Study, third quarter 2003–fourth quarter 2011. Each data point represents the iodine concentration in a single quarterly sample; each quarterly sample is a composite of subsamples from 3 cities (26).
FIGURE 4.
Iodine concentrations in seafood products: US Food and Drug Administration’s Total Diet Study, third quarter 2003–fourth quarter 2011. Each data point represents the iodine concentration in a single quarterly sample; each quarterly sample is a composite of subsamples from 3 cities (26).
FIGURE 2.
Iodine concentrations in breads: US Food and Drug Administration’s Total Diet Study, third quarter 2003–fourth quarter 2011. Each data point represents the iodine concentration in a single quarterly sample; each quarterly sample is a composite of subsamples from 3 cities (26).
FIGURE 3.
Iodine concentrations in milks: US Food and Drug Administration’s Total Diet Study, third quarter 2003–fourth quarter 2011. Each data point represents the iodine concentration in a single quarterly sample; each quarterly sample is a composite of subsamples from 3 cities (26).
Iodine analyses conducted under the NFNAP
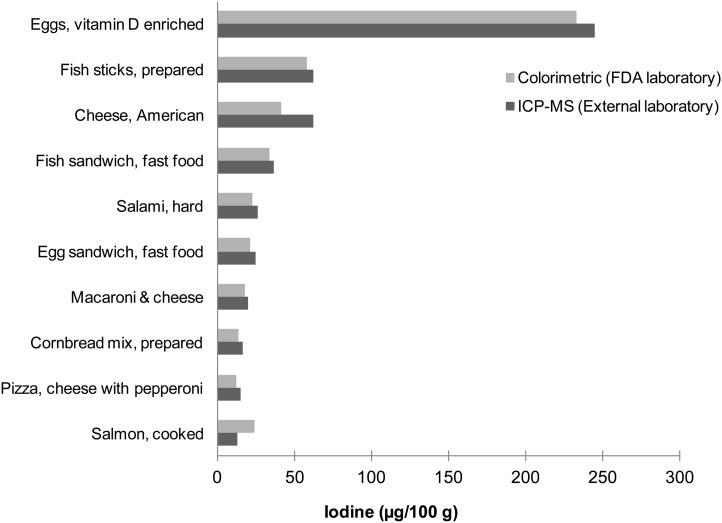
Figure 5 shows the iodine concentrations in 10 of the 12 foods co-analyzed by the FDA’s TDS laboratory and the commercial laboratory prequalified by the USDA’s NDL. Interlaboratory differences in reported iodine concentrations, averaging ∼11%, showed reasonably good agreement, except for salmon and American cheese; possible explanations for the larger differences observed with these 2 foods are being explored. The 2 foods with iodine concentrations below the laboratories’ LOQs are not shown.
FIGURE 5.
Comparison of iodine analysis by ICP-MS (29) and a colorimetric method (24) in 10 foods. A single sample of each food (n = 1) was analyzed by both methods. The ICP-MS analysis was conducted by a commercial laboratory prequalified by the USDA’s Nutrient Data Laboratory. The colorimetric analysis was conducted by the FDA. Foods are displayed in order of their iodine content as measured by the ICP-MS method. FDA, Food and Drug Administration; ICP-MS, inductively coupled plasma mass spectrometry.
Table 2 shows mean iodine concentrations for seafood samples analyzed by the USDA’s prequalified commercial laboratory as part of the NFNAP analysis of 60 foods. There were large differences in iodine content within each of the 3 seafood categories sampled (fin fish, crustaceans, and mollusks). The haddock sample had the highest iodine concentration of any of the fin fish tested, whereas in the halibut sample the iodine concentration was below the LOQ. Among the crustaceans sampled, lobster had far more iodine than either blue crab or shrimp. Among the mollusks sampled, oyster had the highest iodine concentration; in scallops, iodine was below the LOQ. Although the data were limited (2–5 samples/seafood) and additional analyses would be useful, the results provide preliminary evidence of the relative distribution of iodine concentrations in the seafood categories sampled.
TABLE 2.
Iodine concentrations in seafoods sampled under the USDA’s National Food and Nutrient Analysis Program1
| Iodine, μg/100 g |
||
| Seafood description | Mean ± SD | n |
| Crustaceans | ||
| Lobster, prepared | 185 ± 44 | 3 |
| Blue crab, prepared | 38 ± 12 | 3 |
| Shrimp, prepared | 24 ± 8 | 4 |
| Mollusks | ||
| Oyster, prepared | 109 ± 26 | 3 |
| Clams, canned | 66 ± 9 | 4 |
| Scallops, raw | <10 ± NC | 4 |
| Fin fish (raw) | ||
| Haddock | 227 ± 88 | 3 |
| Cod | 94 ± 36 | 4 |
| Pollock | 44 ± 9 | 3 |
| Swordfish | 20 ± 4 | 4 |
| Tuna | 18 ± 6 | 4 |
| Salmon | 14 ± 3 | 4 |
| Rockfish | 14 ± 5 | 3 |
| Flounder | 12 ± NC | 2 |
| Ocean perch | 11 ± 1 | 4 |
| Halibut | <10 ± NC | 5 |
Data are presented in decreasing order of mean iodine concentration within each of the 3 seafood categories. Samples were analyzed using inductively coupled plasma mass spectrometry (29). The SD was calculated for foods with n > 2 samples. Values less than the limit of quantitation (10 μg/100 g) are entered as <10. NC, not calculated.
The iodine concentrations of eggs and dairy products (yogurts, milks, and cheese) reported in the current survey were similar to those measured in earlier TDS market baskets, except for 2 egg samples in which the values were very high (>200 μg/100 g) (data not shown).Table 3 shows the results of analyzing 3 pizza samples: 2 of one brand of fast-food pepperoni pizza and 1 of a second brand. The 3 samples were from different US locations. One of the samples was very high in iodine (191 μg/100 g), whereas the other 2 (including the same brand from a different location) had moderate iodine concentrations.
TABLE 3.
Iodine concentrations in pepperoni pizza sampled under the USDA’s National Food and Nutrient Analysis Program1
| Pizza description | Iodine, μg/100 g |
| Brand B, location 2 | 191 |
| Brand B, location 1 | 15 |
| Brand A | 12 |
Samples were analyzed using inductively coupled plasma mass spectrometry (29). Each reported concentration represents the analysis of a single sample (n = 1).
Iodine analyses conducted under the USDA-ODS supplement program
Adult MVMs
In the adult MVM study, conducted in 2006−2008, 63.3% of the products (n = 69) listed iodine on the label. Labeled values were 10−150 μg/serving, with 59 of the products labeled at 150 μg.
Iodine was analyzed by a thiosulfate titration method (34). To evaluate its accuracy and precision, National Institute of Standards and Technology Standard Reference Material (SRM) 3280, an MVM matrix with certified concentrations of iodine, was analyzed alongside food samples in most batches. Iodine results for the SRM showed that the laboratory mean value was within 1% of the certified value [relative SD (RSD) = 6.1%; n = 21], which is an acceptable result. Based on regression analysis, the predicted mean iodine content of adult MVMs labeled at 150 μg/serving is 26.2% above the labeled value (mean = 189, SEM = 3.1).
Children’s MVMs
In the children’s MVM study, conducted in 2008−2010, 59.1% of the products (n = 39) listed iodine on the label. Labeled values were 30−150 μg/serving on the basis of the serving size for children aged ≥4 y. The most common labeled value was 150 μg (n = 20).
Iodine was analyzed by both the thiosulfate titration method and an ICP-MS method. Each method was evaluated by performing analytical measurement of the iodine content of SRM 3280 and comparing those results to the material's certified value for iodine; both methods were found to be acceptable. Comparing the titration method, iodine concentrations averaged 0.9% above the certified mean (RSD = 9.7%; n = 16); with the ICP-MS method, iodine concentrations averaged 6.7% below the certified mean (RSD = 5.7%; n = 16). Based on regression analysis, the difference between labeled and analytical values in children's MVMs varied across the range of labeled values. For those labeled at 150 μg/serving, the predicted mean iodine content is 24.9% above the labeled value (mean = 187, SEM = 8.6).
Nonprescription prenatal MVMs
In the nonprescription prenatal MVM study, conducted in 2009−2011, 77.5% of the products (n = 55) listed iodine on the label. Labeled concentrations were 10−300 μg/serving, with 33 products labeled at 150 μg.
Iodine was analyzed by both the thiosulfate titration method and an ICP-MS method. Both methods provided acceptable results for SRM 3280, with the ICP-MS method yielding more consistent results and the titration method yielding a mean value that was closer to the certified value. For the titration method, the laboratory mean was 1.9% below the certified value (RSD = 6.9%; n = 9); for the ICP-MS method, the laboratory mean was 4.2% below the certified value (RSD = 4.1%; n = 14). Based on regression analysis, the predicted mean iodine content of nonprescription prenatal MVMs labeled at 150 μg/serving is 25.9% above the labeled value (mean = 189, SEM = 7.0).
DISCUSSION
In the present article, we focused on the iodine content of foods and dietary supplements and did not evaluate iodine intakes or the prevalences of iodine inadequacy or excess. Elsewhere in this supplement issue, Juan et al. (35) use food consumption data from NHANES and iodine concentration data from the TDS to estimate usual iodine intakes and prevalence rates of iodine inadequacy and excess in sex- and life stage–specific subgroups of the US population.
Iodine in foods
For foods with right-skewed distributions of iodine concentrations (in which the mean is considerably higher than the median because of outliers with unusually high values), the mean may not be a useful summary statistic and thus should be interpreted with caution. As shown by Carriquiry et al. (36) in this supplement issue, the summary statistic chosen to represent the iodine concentration of foods greatly influences the predicted prevalence rates of iodine inadequacy and excess. The right-skewed distributions reported by the TDS for a number of commercially prepared foods (including some cakes, breads, pizzas, other baked goods, sherbets, meal-replacement beverages, and popsicles) likely reflect variability in the commercial use of iodate dough conditioners, milk products, eggs, seaweed-based gums, and Red no. 3. Although iodized salt is another potential source of iodine in breads, pizzas, and other prepared foods, it is not commonly used in commercial products. Although some commercially prepared breads still contain high concentrations of iodine, the current industry trend away from the commercial use of iodates as dough conditioners is evidenced by the low iodine concentrations measured in most samples.
Increases in the iodine content of milks in 2003−2011 compared with 1982−1991 are likely due to increased use of iodine-fortified feeds and teat-cleaning iodophors. The relatively small variation in iodine in milks is consistent with minor regional and/or seasonal differences in feed supplementation and iodophor use.
Because seaweed-derived thickening agents, iodates, and other iodine-containing additives contribute iodine to a wide variety of processed foods, such foods tend to have more iodine than related nonprocessed foods. In the case of fish sticks, which have higher iodine concentrations then the nonprocessed seafoods sampled, the additional iodine may be attributable to batter ingredients (including milk, eggs, and iodate-containing flour) and iodine-containing food additives.
The very high iodine concentrations (>200 μg/100 g) observed in a few egg samples in both the NFNAP study reported here and the first TDS market basket in 2006 (25) may reflect excessive iodine supplementation in laying hens. To gain additional information, the NDL is planning to conduct iodine analysis in a nationwide sampling of eggs.
Iodine in supplements
On the basis of DSID results for MVMs sold in the United States, iodine concentrations average 25% above labeled values. A DSID pilot study of prescription prenatal MVMs is currently planned; information gained in this study will complement the information obtained from the 2009−2011 national study of nonprescription prenatal MVMs sold via mass market, natural health, and direct marketing channels. According to data from NHANES, the majority of prenatal MVMs consumed are prescription products (37). It should be noted that prescription prenatal products, although sold only through pharmacies, are not regulated as pharmaceuticals. The iodine concentrations of both prescription and nonprescription prenatal MVMs should be considered when evaluating the iodine supplied to pregnant women.
Collaboration between the FDA and the USDA
The TDS sampling plan, foods list, and analyte list are all currently under review, and changes to the program are likely. Nevertheless, it is probable that iodine analysis will remain an important focus of the TDS in upcoming years. The FDA is collaborating with the NDL on iodine analysis and other areas of mutual interest. This will include synchronizing the sampling and analysis of select foods. The NDL has proposed, funding permitting, to analyze more foods for iodine under the NFNAP. Among the candidates for analysis are seaweed and seaweed extracts as ingredients, additional types of seafood, processed foods with iodine-containing ingredients, commercially processed mixed dishes containing egg and/or dairy ingredients, other foods containing eggs and/or dairy products, and retail salts.
Data development by the USDA’s NDL
With regard to collecting food-composition data on a nutrient not currently in the SR, the NDL often begins by presenting the data in a Special Interest Database (SID). SIDs, which usually contain data on a nutrient’s concentration in 300−500 foods, are peer-reviewed and then released on the NDL website (21). The iodine SID under development will include analytical data from the TDS and the NFNAP. These data will be evaluated using a rigorous data-quality evaluation system developed by the NDL specifically for evaluating the NFNAP (38). The scientific literature will also be reviewed for iodine data on foods that are currently consumed, and if the analytical methodology and quality-control information meet the NDL standards, these data will also be included in the iodine SID. We anticipate that when sufficient data are available, iodine will be considered for addition to the SR.
NDL nutrient data are used to support the Food and Nutrient Database for Dietary Studies; in turn, this supports “What We Eat in America”—NHANES (30), which estimates nutrient intakes for the US population. Likewise, the iodine SID and the DSID databases will be available for future reporting on iodine in foods and supplements.
Accurate and timely information on the iodine content of foods and supplements is essential for the accurate estimation of iodine intakes by the US population. Working together, the FDA and the USDA will provide the scientific community with comprehensive, high-quality data on iodine concentrations in foods to support estimation of US population iodine intakes and related research efforts.
Acknowledgments
We thank Pavel Gusev of the Nutrient Data Laboratory for his assistance with writing and research into the background information. We also thank 3 scientists whose data relevant to iodine were useful to this work: Jaime Gahche of the National Center for Health Statistics; Leila Saldanha, consultant to the NIH ODS; and Regan Bailey of the NIH ODS. In addition, we thank Gay Goodman, Iodine Initiative Consultant to the NIH ODS, for expert technical editing of the manuscript and associated contributions.
The authors’ responsibilities were as follows—KYP, MSW, KWA, JTD, and CAS: designed the research; KYP, MSW, and KWA: conducted the research; KYP, JHS, MSW, and KWA: analyzed the data; PRP, KYP, JHS, and KWA: wrote the manuscript; PRP: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors reported no conflicts of interest related to the study.
Footnotes
Abbreviations used: DSID, Dietary Supplement Ingredient Database; FDA, US Food and Drug Administration; ICP-MS, inductively coupled plasma mass spectrometry; LOQ, limit of quantitation; MVM, multivitamin/mineral supplement; NDL, Nutrient Data Laboratory; NFNAP, National Food and Nutrient Analysis Program; ODS, Office of Dietary Supplements; RSD, relative standard deviation; SID, Special Interest Database; SR, National Nutrient Database for Standard Reference; SRM, Standard Reference Material; TDS, Total Diet Study; UIC, urinary iodine concentration.
REFERENCES
- 1.Delange F. The role of iodine in brain development. Proc Nutr Soc 2000;59:75–9. [DOI] [PubMed] [Google Scholar]
- 2.Pearce EN. Iodine in pregnancy: is salt iodization enough? J Clin Endocrinol Metab 2008;93:2466–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann MB. Iodine deficiency and excess in children: worldwide status in 2013. Endocr Pract 2013;19:839–46. [DOI] [PubMed] [Google Scholar]
- 4.Chung HR. Iodine and thyroid function. Ann Pediatr Endocrinol Metab 2014;19:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead DC. The distribution and transformations of iodine in the environment. Environ Int 1984;10:321–39. [Google Scholar]
- 6.Castro SI, Berthiaume R, Robichaud A, Lacasse P. Effects of iodine intake and teat-dipping practices on milk iodine concentrations in dairy cows. J Dairy Sci 2012;95:213–20. [DOI] [PubMed] [Google Scholar]
- 7.Flachowsky G, Franke K, Meyer U, Leiterer M, Schone F. Influencing factors on iodine content of cow milk. Eur J Nutr 2014;53:351–65. [DOI] [PubMed] [Google Scholar]
- 8.Röttger AS, Halle I, Wagner H, Breves G, Dänicke S, Flachowsky G. The effects of iodine level and source on iodine carry-over in eggs and body tissues of laying hens. Arch Anim Nutr 2012;66:385–401. [DOI] [PubMed] [Google Scholar]
- 9.Mabeau S, Fleurence J. Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci Technol 1993;4:103–7. [Google Scholar]
- 10.Teas J, Pino S, Critchley A, Braverman LE. Variability of iodine content in common commercially available edible seaweeds. Thyroid 2004;14:836–41. [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Chan A, Xiao Y, Lin V, Ho YY. Iodine content in commonly consumed food in Hong Kong and its changes due to cooking. Food Addit Contam Part B Surveill 2013;6:24–9. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health. Low-iodine diet: preparing to receive radioactive iodine. Patient education. Version current January 2014 [cited 2014 Nov 14]. Available from: http://www.cc.nih.gov/ccc/patient_education/pepubs/lo_io_diet.pdf.
- 13.Pittman JA Jr, Dailey GE III, Beschi RJ. Changing normal values for thyroidal radioiodine uptake. N Engl J Med 1969;280:1431–4. [DOI] [PubMed] [Google Scholar]
- 14.Gregory CO, Serdula MK, Sullivan KM. Use of supplements with and without iodine in women of childbearing age in the United States. Thyroid 2009;19:1019–20. [DOI] [PubMed] [Google Scholar]
- 15.Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US Food and Drug Administration’s Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol 2008;18:571–80. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta PK, Lui Y, Dyke JV. Iodine nutrition: iodine content of iodized salt in the United States. Environ Sci Technol 2008;42:1315–23. [DOI] [PubMed] [Google Scholar]
- 17.Swanson CA, Zimmerman MB, Skeaff S, Pearce E, Dwyer JT, Trumbo PR, Zehaluk C, Andrews KW, Carriquiry A, Caldwell KL, et al. . NIH workshop summary: shaping the development of an iodine research initiative for the U.S. J Nutr 2012;142(Suppl):1175S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.König F, Andersson M, Hotz K, Aeberli I, Zimmermann MB. Ten repeat collections for urinary iodine from spot samples or 24-hour samples are needed to reliably estimate individual iodine status in women. J Nutr 2011;141:2049–54. [DOI] [PubMed] [Google Scholar]
- 19.Charlton KE, Batterham MJ, Buchanan LM, Mackerras D. Intraindividual variation in urinary iodine concentrations: effect of adjustment on population distribution using two and three repeated spot urine collections. BMJ Open 2014;4:e003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 2012;70:553–70. [DOI] [PubMed] [Google Scholar]
- 21.Nutrient Data Laboratory; Agricultural Research Service, USDA; Office of Dietary Supplements; National Institutes of Health. Dietary Supplement Ingredient Database (DSID) 3.0. Version current March 2015 [cited 2015 Aug 19]. Available from: http://dietarysupplementdatabase.usda.nih.gov.
- 22.Pennington JAT, Gunderson EL. History of the Food and Drug Administration’s Total Diet Study – 1961 to 1987. J Assoc Off Anal Chem 1987;70:772–82. [PubMed] [Google Scholar]
- 23.Egan SK, Bolger PM, Carrington CD. Update of US FDA’s Total Diet Study food list and diets. J Expo Sci Environ Epidemiol 2007;17:573–82. [DOI] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration. Determination of iodine in foods. Laboratory procedure number KAN-LAB-MET.95, version 3.2. Lenexa (MO): FDA Kansas City District Office; 2007.
- 25.Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of core foods of the US food supply, 1982–1991. III. Copper, manganese, selenium, and iodine. J Food Compost Anal 1995;8:171–217. [Google Scholar]
- 26.US Food and Drug Administration. Total Diet Study statistics on element results—2006–2011. Version current 27 March 2014 [cited 2014 Jul 15]. Available from: http://www.fda.gov/downloads/Food/FoodScienceResearch/TotalDietStudy/UCM184301.pdf.
- 27.Haytowitz DB, Pehrsson PR, Holden JM. The National Food and Nutrient Analysis Program: a decade of progress. J Food Compost Anal 2008;21:S94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perry CR, Pehrsson PR, Holden J. A revised sampling plan for obtaining food products for nutrient analysis. Proceedings of the American Statistical Association, Section on Survey Research Methods. Alexandria (VA): 2003 p. 3270–7. [cited Aug 19]. Available from: http://www.amstat.org/sections/srms/proceedings/y2003/files/jsm2003-000826.pdf.
- 29.Sullivan D, Zywicki R. Determination of total iodine in foods and dietary supplements using inductively coupled plasma-mass spectrometry. J AOAC Int 2012;95:195–202. [DOI] [PubMed] [Google Scholar]
- 30.Coates PM. Dietary supplements and health: the research agenda. Novartis Found Symp 2007;282:202–7, discussion 207–18. [DOI] [PubMed] [Google Scholar]
- 31.Dwyer J, Picciano MF, Raiten DJ. Estimation of usual intakes: What We Eat in America—NHANES. J Nutr 2003;133(Suppl):609S–23S. [DOI] [PubMed] [Google Scholar]
- 32.Dwyer J, Picciano MF, Raiten DJ. Food and dietary supplement databases for What We Eat in America—NHANES. J Nutr 2003;133(Suppl):624S–34S. [DOI] [PubMed] [Google Scholar]
- 33.Office of Dietary Supplements, National Institutes of Health; National Library of Medicine, NIH. Dietary Supplements Label Database. Version current July 2014 [cited 2014 Nov 14]. Available from: http://dsld.nlm.nih.gov.
- 34.AOAC International. Official methods of analysis of AOAC International. 19th ed. Official methods 935.14, 932.21 (modified). Gaithersburg (MD): AOAC International; 2012. [Google Scholar]
- 35.Juan WY, Trumbo PR, Spungen JH, Dwyer JT, Carriquiry AL, Zimmerman TP, Swanson CA, Murphy SP. Comparison of 2 methods for estimating the prevalences of inadequate and excessive iodine intakes. Am J Clin Nutr 2016;104(Suppl):888S–97S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carriquiry AL, Spungen JH, Murphy SP, Pehrsson PR, Dwyer JT, Juan WY, Wirtz MS. Variation in the iodine concentrations of foods: considerations for dietary assessment. Am J Clin Nutr 2016;104(Suppl):877S–87S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gahche JJ, Bailey RL, Mirel LB, Dwyer JT. The prevalence of using iodine-containing supplements is low among reproductive-age women, NHANES 1999–2006. J Nutr 2013;143:872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips KM, Patterson KY, Rasor AS, Exler J, Haytowitz DB, Holden JM, Pehrsson PR. Quality-control materials in the USDA National Food and Nutrient Analysis Program (NFNAP). Anal Bioanal Chem 2006;384:1341–55. [DOI] [PubMed] [Google Scholar]
- 39.Luchtefeld RG. Semi-automated method for the determination of iodine in Total Diet market baskets. Rockville (MD): US Food and Drug Administration; 1974. (Lab Inform Bulletin 1678.) [Google Scholar]