Abstract
Background:
Mammea siamensis (Miq.) T. Anders. is used as a medicinal plant in Thailand and has several traditional therapeutic properties. In a previous study, we isolated eight compounds from the flower of M. siamensis and demonstrated that kayeassamin A (KA) exhibited potent antiproliferative activity against human leukemia and stomach cancer cell lines.
Objective:
In this study, we investigated the effect of KA on cell viability and apoptotic mechanisms in HL-60 human leukemia cells.
Materials and Methods:
Cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Nuclear morphology and DNA fragmentation were observed using Hoechst 33258 staining and agarose gel electrophoresis, respectively. The sub-G1 phase of cells was analyzed by flow cytometry after the cellular DNA had been stained with propidium iodide. The protein levels of poly (ADP-ribose) polymerase (PARP) and caspases were determined by Western blotting.
Results:
KA exhibited a significant cytotoxic effect in a dose- and time-dependent manner, and induced chromatin condensation, DNA fragmentation, and sub-G1 phase DNA content, known as molecular events associated with the induction of apoptosis. In addition, KA strongly induced the activation of PARP and caspase-3 and -8, with weak caspase-9 activation. Furthermore, KA-induced DNA fragmentation was abolished by pretreatment with z-VAD-FMK (a broad caspase inhibitor), z-DEVD-FMK (a caspase-3 inhibitor), and z-IETD-FMK (a caspase-8 inhibitor), but not by z-LEHD-FMK (a caspase-9 inhibitor) pretreatment.
Conclusion:
These results indicate that KA triggers apoptotic cell death by activation of caspase-3 and -8 in HL-60 cells.
SUMMARY
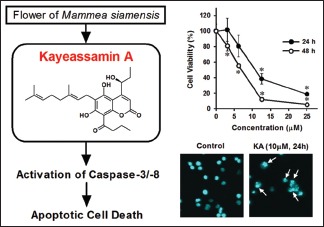
Kayeassamin A (KA) isolated from the flower of Mammea siamensis exhibited a significant cytotoxic effect in HL-60 human leukemia cells. KA triggers apoptotic cell death by activating caspase-3/-8.
Abbreviations Used: KA: Kayeassamin A; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PARP: Poly (ADP-ribose) polymerase; PI: Propidium iodide; CA: Corosolic acid
Key words: Apoptosis, caspase, kayeassamin A, leukemia, Mammea siamensis
INTRODUCTION
Mammea siamensis (Miq.) T. Anders., distributed throughout Thailand where it is known as “Sarapee,” is a small evergreen tree with fragrant yellow or white flowers that belongs to the family Calophyllaceae.[1,2] In traditional medicine, its flowers have been used as an analgesic and a heart tonic, and for boosting appetite.[3] Previous phytochemical studies demonstrated that the flower of M. siamensis is a rich source of various coumarins.[4,5] Mahidol et al. isolated the series of typical coumarins from M. siamensis.[5] Laphookhieo et al.[6] and Morikawa et al.[7] also isolated several coumarins from M. siamensis and confirmed the cytotoxic activity and the inhibition activity against nitric oxide production in lipopolysaccharide-activated RAW264.7 cells, respectively. Win et al. isolated the structurally-related coumarins including typical derivatives such as kayeassammins from Kayea assamica.[8,9] In our previous study, we also isolated eight coumarin constituents from M. siamensis flowers including mammeanoyl, a novel geranylated coumarin, and confirmed antiproliferative effects using several cell lines.[10]
Apoptosis is the process of programmed cell death that occurs in multicellular organisms and is characterized by unique cellular mitochondrial fragmentation and dysfunction, nuclear condensation, cytoplasmic shrinkage, blebbing of the plasma membrane, and phagocytosis by neighboring cells.[11] Apoptosis is regulated by two major pathways, the mitochondrial (or intrinsic) pathway and membrane death receptor (or extrinsic) pathway.[12] The mitochondrial mediated pathway, which activates caspase-9, and the death receptor-activated pathway, which activates caspase-8, converge at caspase-3, and finally induce cell death.[13] Thus, the induction of apoptosis is a key target in chemotherapy and the development of novel anticancer agents.[14,15]
Our previous study demonstrated that three structurally-related coumarins, isolated from the flower of M. siamensis, showed statistically significant antiproliferative activity in human leukemia and stomach cancer cell lines. Of these, kayeassamin A (KA) [Figure 1] was the most potent antiproliferative compound.[10] Structure and activity relations in a naturally occurring coumarin antibiotics, novobiocin have been reviewed well resulted that a free hydroxyl group in coumarin nucleus was found to be important for its antifungal and antibacterial activity and substituent at C-4 or C-7 on coumarin nucleus contributed to high activity.[16] From the above evidences we selected KA as a candidate of anticancer agent through apoptosis phenomena. In this study, we aimed to investigate the antiproliferative and apoptosis-inducing mechanisms of KA in the human promyelocytic leukemia HL-60 cell line.
Figure 1.
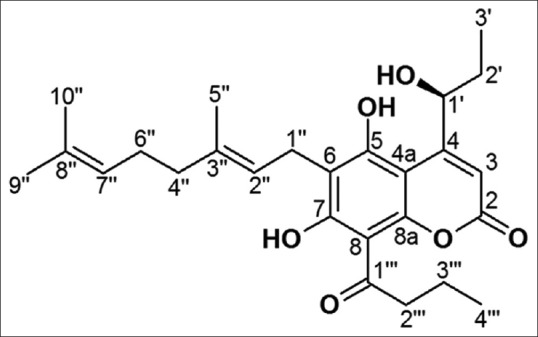
Chemical structure of kayeassamin A
MATERIALS AND METHODS
Reagents
Antibodies against caspase-3, -8, -9, and poly (ADP-ribose) polymerase (PARP) were obtained from Cell Signaling Technology (Beverly, MA, USA). Antibody against β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Fetal bovine serum (FBS) was from GIBCO (Gaithersburg, MD, USA). Caspase inhibitors were from Calciochem (San Diego, CA, USA). All other chemicals were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of kayeassamin A
KA used for this study was isolated form the flower of M. siamensis as previously described.[10] Briefly, the air-dried sample of 100 g was extracted with MeOH. After the removal of solvent, the obtained residue (24.5 g) was suspended in water and successively partitioned with hexane, CH2 Cl2, EtOAC, and n-BuOH to obtain soluble fractions of hexane (6.14 g), CH2 Cl2 (1.31 g), EtOAC (1.31 g), and n-BuOH (6.42 g). The hexane and CH2 Cl2 fractions were combined and then subjected to a silica gel column chromatography and a reverse-phase column chromatography for the purification of KA (150 mg).
Cell culture and treatment
HL-60 cell line was obtained from the RIKEN BioResource Center Cell Bank and cultured at 37°C in a 5% CO2 atmosphere in RPMI1640 medium containing 10% FBS. For cell treatment, KA and caspase inhibitors were dissolved in dimethyl sulfoxide (DMSO) and were stored at −20°C before use. DMSO concentrations in the cell culture medium did not exceed 0.2% (v/v), and the controls were always treated with the same amount of DMSO as used in the corresponding experiments.
Cell viability assay
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, the cells (1 × 104 cells/well) were treated with KA at various concentrations for 24 h or 48 h. At the end of treatment, MTT solution was added to each well, and the cells were incubated for another 4 h. The precipitated MTT-formazan was dissolved with 0.04 N HCl-isopropanol, and the amount of formazan was measured at 595 nm using a microplate reader (iMark, BioRad, Tokyo, Japan). Cell viability was expressed as a percentage of the control culture.
Nuclear staining by Hoechst 33258
Cells (1 × 106 cells) were treated with KA for 24 h, and then the cells were harvested, washed with PBS, and fixed with 1% glutaraldehyde for 30 min. After washing with PBS, the cells were stained with Hoechst 33258 for 10 min. The cells were washed with PBS, and nuclear morphology was observed by a fluorescent microscopy (Eclipse E600, Nikon, Tokyo, Japan).
DNA fragmentation analysis
Cells (1 × 106 cells) were treated with KA, and then cells were washed with ice-cold PBS and resuspended in lysis buffer (50 mM Tris-HCL, pH 8.0, 10 mM ethylenediaminetetraacetic acid, and 0.5% sodium dodecyl sulfate) with 0.2 mg/ml RNase A for 30 min at 50°C. Proteinase K was then added and incubated for overnight. The DNA was separated on 2% agarose gel and visualized under ultraviolet illumination after staining with ethidium bromide.
Flow cytometric detection of apoptosis cell by propidium iodide staining
Cell cycle analysis to detect the sub-G1 phase was performed using a cell cycle phase determination kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions. Briefly, cells (1 × 106 cells) were treated with KA for 24 h, and then the cells were washed and fixed with fixative and suspended with staining solution containing propidium iodide (PI) and RNase A. The sub-G1 peak was measured and analyzed in the FL2 channel of a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) with a 488 nm excitation laser. A total of 10,000 cells were analyzed per sample.
Western blot analysis
Cells (1 × 106 cells) were plated in 6 cm dish. After treatment with KA for various periods, the harvested cells were lysed, and the supernatants were boiled for 5 min. Protein concentration was determined by using a dye-binding protein assay kit according to the manufacturer's manual (Bio-Rad, Richmond, CA, USA). Equal amounts of lysate protein were subjected to SDS-polyacrylamide gel electrophoresis. Proteins were electrotransferred to polyvinylidene fluoride membranes and were detected as described previously.[17,18]
Statistical analyses
The experimental results are presented as mean ± standard error of the mean. Each experiment was repeated at least three times. Data were analyzed by ANOVA followed by Dunnett's test using GraphPad Prism 6 (GraphPad software, San Diego, CA, USA). P < 0.05 was considered statistically significant.
RESULTS
Effect of kayeassamin A on cell proliferation
To determine the cytotoxicity of KA in HL-60 cells, we first examined cell viability after treatment with various concentrations of KA for 24 h and 48 h by using the MTT assay. A dose- and time-dependent decrease in viability was observed in the presence of KA [Figure 2]. The IC50 values at 24 h and 48 h were 11.9 µM and 6.2 µM, respectively.
Figure 2.
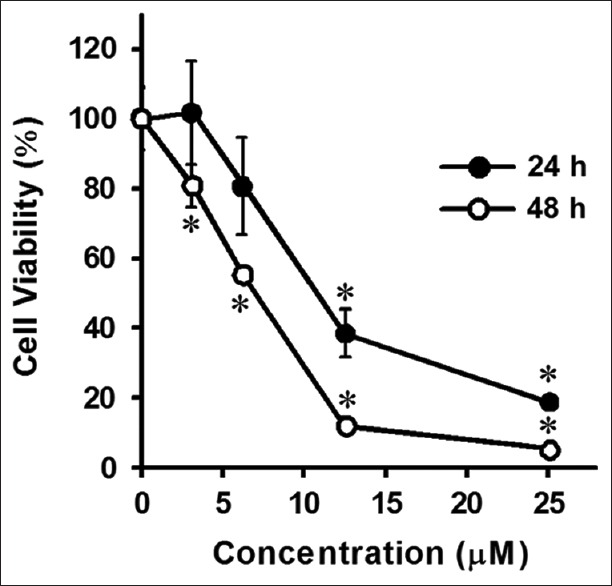
Effects of kayeassamin A on cell proliferation in HL-60 cells. Cells were treated with kayeassamin A at various concentrations for 24 h or 48 h, and the cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The data represent the mean ± standard error of the mean for three individual experiments. *P < 0.05 compared with the control group
Effect of kayeassamin A on the induction of apoptosis
To explore whether the antiproliferative activity of KA is related to apoptosis, we evaluated the presence of markers of apoptosis, including nuclear morphological changes and DNA fragmentation, in HL-60 cells. Following treatment with 10 µM KA for 24 h, nuclear morphology was determined using Hoechst 33258 staining. As shown in Figure 3a, control cells exhibited normal nuclear morphology whereas cells treated with KA showed chromatin condensation. Furthermore, DNA fragmentation was examined based on the classical DNA laddering using agarose gel electrophoresis. Figure 3b shows that KA treatment (10 µM) led to the appearance of a DNA ladder in a time-dependent manner. Furthermore, we analyzed the sub-G1 phase of cells by flow cytometry after the cellular DNA had been stained with PI. As shown in Figure 3c, KA increased the accumulation of cells in the sub-G1 phase from 9.8% (control) to 68.1% (KA, 10 µM). In summary, these results clearly indicate that KA exerted its antiproliferative effect via the induction of apoptotic cell death.
Figure 3.
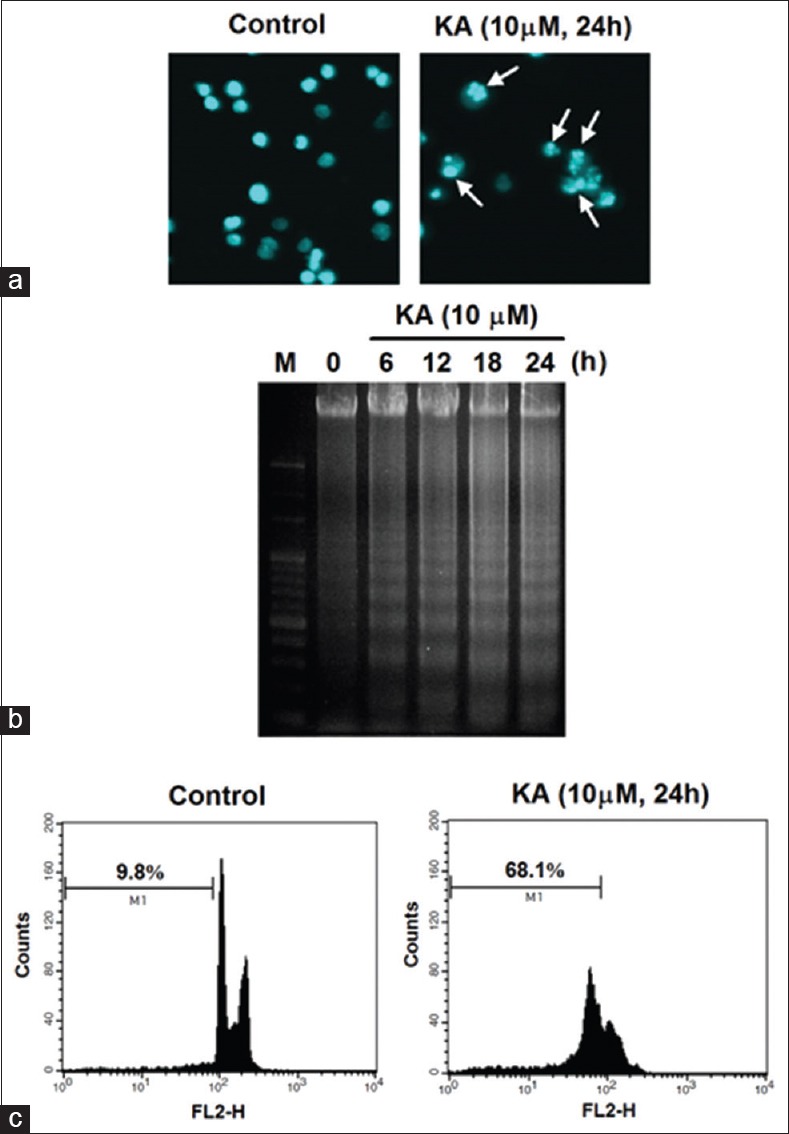
Effect of kayeassamin A on apoptosis induction. (a) Inductionof chromatin condensation by kayeassamin A. HL-60 cells were treated with kayeassamin A (10 μM) for 24 h and were stained with Hoechst 33258. (b) Induction of DNA fragmentation by kayeassamin A. HL-60 cells were treated with kayeassamin A (10 μM) for indicated times and the DNA fragmentation was analyzed by agarose gel electrophoresis. M is the 100-bp DNA marker. (c) Increase in the sub-G1 phase cells by kayeassamin A. HL-60 cells were treated with the indicated concentration of kayeassamin A (10 μM) for 24 h and were then analyzed by flow cytometry after staining with propidium iodide. The data shown are representative of 3 independent experiments that had similar results
Effect of kayeassamin A on the caspase cascade
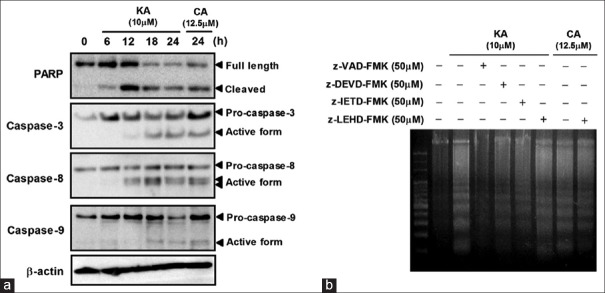
In order to confirm the role of caspases in the induction of apoptosis by KA, we determined the protein levels of PARP and caspase-3, -8, and -9. Caspase-3 is the main downstream effector in the induction of apoptotic cell death, and PARP is the endogenous substrate protein of caspase-3.[19] As a positive control, we used corosolic acid (CA), which induces apoptosis through the activation of caspase-3, -8, and -9.[20] KA caused the cleavage of PARP and activated caspase-3 in a time-dependent manner [Figure 4a]. The activation of caspase-3 requires the activation of initiator caspases such as caspase-8 and -9. Our data demonstrated that KA strongly activated caspase-8, although it weakly induced the activation of caspase-9. To further confirm the involvement of specific caspases in KA-induced apoptosis, cells were treated with KA in the absence or presence of caspase inhibitors. As shown in Figure 4b, KA-induced DNA fragmentation was abolished by pretreatment with z-VAD-FMK (a broad caspase inhibitor), z-DEVD-FMK (a caspase-3 inhibitor), and z-IETD-FMK (a caspase-8 inhibitor). However, z-LEHD-FMK (a caspase-9 inhibitor) did not suppress KA-induced DNA fragmentation although it did inhibit DNA fragmentation induced by CA as a positive control. These results indicate that KA-induced apoptosis involves a caspase-3/8-dependent pathway in HL-60 cells.
Figure 4.
Involvement of the caspase cascade in kayeassamin A-induced apoptosis. (a) The effect of kayeassamin A on poly (ADP-ribose) polymerase cleavage and the activation of caspase-3, -8, -9. HL-60 cells were treated with kayeassamin A (10 μM) for the times indicated. The cells were lysed, and poly (ADP-ribose)polymerase, caspase-3, -8, -9, and β-actin protein levels were determined by Western blotting. (b) The effect of caspase inhibitors on kayeassamin A-induced DNA fragmentation. After pretreatment with 50 μM caspase inhibitors for 1 h, HL-60 cells were treated with kayeassamin A (10 μM) for 18 h. DNA fragmentation was analyzed by agarose gel electrophoresis. The data shown are representative of three independent experiments with similar results. Corosolic acid is a positive control
DISCUSSION
Apoptosis is a highly-regulated programmed cell death process in which cells undergo inducible nonnecrotic cellular suicide, and therefore plays an important role in anticarcinogenesis. The induction of apoptosis involves the activation of caspases.[12] There are two major pathways in the caspase cascade; the intrinsic pathway or mitochondrial pathway involves spase-9 activation, while the extrinsic pathway involves death receptor-mediated activation via caspase-8.[12,13] Initiator caspases, such as caspase-8 and -9, self-activate in response to apoptotic stimuli, whereas the activation of effector caspases, such as caspase-3, requires the activation of initiator caspases.[12,13] The induction of apoptosis is the most frequently observed mechanism of anticancer agents. The strategy to selectively induce apoptosis in cancer cells is therefore important for cancer chemoprevention and chemotherapy.[14,15]
In our previous anticancer phytotherapeutic research, we reported the potent anticancer activity of alkannin derivatives from Alkanna tinctoria[21] and of crocin from saffron (Crocus sativus).[22,23] As part of an ongoing study, our preliminary screening of Thai medicinal plants showed promising antiproliferative effects of a methanol extract from M. siamensis flowers on several human cancer cell lines. In our previous study, bioassay-guided fractionation and a chemical investigation of the methanol extract of M. siamensis flower resulted in the isolation and identification of eight compounds, including a novel geranylated coumarin (mammeanoyl) and seven known compounds. Among the isolated compounds, three structurally-related coumarins showed statistically significant antiproliferative activities against human leukemia and stomach cancer cell lines. Of these, KA exerted the strongest activity.[10] Moreover, these compounds did not affect cell viability in colon cancer, hepatoma, and normal skin fibroblast cell lines.[10]
In this study, we investigated the mechanism of action of KA in HL-60 human leukemia cells. Our results indicated that KA strongly suppressed cell growth, with 24 h and 48 h IC50 values of 11.9 µM and 6.2 µM, respectively. Moreover, KA-induced apoptosis was shown to implicate a caspase-dependent pathway involving caspase-3 and its initiator caspases-8, but not caspase-9. The extrinsic pathway via caspase-8 involved binding of a ligand to one of the tumor necrosis factor families of death receptors, followed by activation of caspase-8 and -3.[24] To examine the effect of KA on the Fas/FasL system, we investigated the expression of Fas and FasL. However, KA had no effect on either Fas or FasL protein expression (data not shown). To elucidate the mechanism by which KA triggers the activation of caspase-8, further investigation of the molecular mechanism of KA-induced apoptosis is required. Our findings suggest that KA is a potential anticancer candidate for use in the treatment of leukemia as we previously selected KA as a candidate of anticancer drug depending on the structure and activity relations.[16] Moreover, KA has three hydroxyl groups in a molecule. A phenolic hydroxyl group at C-5 can function chelating with some donor between a β-hydroxyl group at C-1´. Other phenolic hydroxyl group at C-7 makes chelation junction with carboxyl group at C-1´. These structural functions may further promote activity. It would be interesting to determine whether KA-induced apoptosis contributes to anticancer activity in vivo.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Yukihiro Shoyama
Yukihiro Shoyama, Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Nagasaki International University, Japan.
REFERENCES
- 1.Maneesri J, Masniyom P, Pongpiriyadacha Y. Bacterial cellulose film containing flavonoids from “Sarapee” (Mammea siamensis) flower extract against Salmonella Typhimurium TISTR 292. J Agric Sci Technol A. 2012;2:86–9. [Google Scholar]
- 2.Poobrasert O, Constant HL, Beecher CW, Farnsworth NR, Kinghorn AD, Pezzuto JM, et al. Xanthones from the twigs of Mammea siamensis. Phytochemistry. 1998;47:1661–3. doi: 10.1016/s0031-9422(97)00820-0. [DOI] [PubMed] [Google Scholar]
- 3.Subhadhirasakul S, Pechpongs P. A terpenoid and two steroids from the flowers of Mammea siamensis. Songklanakarin J Sci Technol. 2005;27:555–61. [Google Scholar]
- 4.Prachyawarakorn V, Mahidol C, Ruchirawat S. Pyranocoumarins from the twigs of Mammea siamensis. Phytochemistry. 2006;67:924–8. doi: 10.1016/j.phytochem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Mahidol C, Kaweetripob W, Prawat H, Ruchirawat S. Mammea coumarins from the flowers of Mammea siamensis. J Nat Prod. 2002;65:757–60. doi: 10.1021/np010579u. [DOI] [PubMed] [Google Scholar]
- 6.Laphookhieo S, Promnart P, Syers JK, Kanjana-Opas A, Ponglimanont C, Karalai C. Coumarins and xanthones from the seeds of Mammea siamensis. J Braz Chem. 2007;18:107–80. [Google Scholar]
- 7.Morikawa T, Sueyoshi M, Chaipech S, Matsuda H, Nomura Y, Yabe M, et al. Suppressive effects of coumarins from Mammea siamensis on inducible nitric oxide synthase expression in RAW264.7 cells. Bioorg Med Chem. 2012;20:4968–77. doi: 10.1016/j.bmc.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Win NN, Awale S, Esumi H, Tezuka Y, Kadota S. Novel anticancer agents, kayeassamins A and B from the flower of Kayea assamica of Myanmar. Bioorg Med Chem Lett. 2008;18:4688–91. doi: 10.1016/j.bmcl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Win NN, Awale S, Esumi H, Tezuka Y, Kadota S. Novel anticancer agents, kayeassamins C-I from the flower of Kayea assamica of Myanmar. Bioorg Med Chem. 2008;16:8653–60. doi: 10.1016/j.bmc.2008.07.091. [DOI] [PubMed] [Google Scholar]
- 10.Tung NH, Uto T, Sakamoto A, Hayashida Y, Hidaka Y, Morinaga O, et al. Antiproliferative and apoptotic effects of compounds from the flower of Mammea siamensis (Miq.) T. Anders. on human cancer cell lines. Bioorg Med Chem Lett. 2013;23:158–62. doi: 10.1016/j.bmcl.2012.10.127. [DOI] [PubMed] [Google Scholar]
- 11.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–67. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 13.Ulukaya E, Acilan C, Yilmaz Y. Apoptosis: Why and how does it occur in biology? Cell Biochem Funct. 2011;29:468–80. doi: 10.1002/cbf.1774. [DOI] [PubMed] [Google Scholar]
- 14.Okun I, Balakin KV, Tkachenko SE, Ivachtchenko AV. Caspase activity modulators as anticancer agents. Anticancer Agents Med Chem. 2008;8:322–41. doi: 10.2174/187152008783961914. [DOI] [PubMed] [Google Scholar]
- 15.Hensley P, Mishra M, Kyprianou N. Targeting caspases in cancer therapeutics. Biol Chem. 2013;394:831–43. doi: 10.1515/hsz-2013-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr Med Chem. 2005;12:887–916. doi: 10.2174/0929867053507315. [DOI] [PubMed] [Google Scholar]
- 17.Uto T, Tung NH, Taniyama R, Miyanowaki T, Morinaga O, Shoyama Y. Anti-inflammatory activity of constituents isolated from aerial part of Angelica acutiloba Kitagawa. Phytother Res. 2015;29:1956–63. doi: 10.1002/ptr.5490. [DOI] [PubMed] [Google Scholar]
- 18.Uto T, Tung NH, Appiah-Opong R, Aning A, Morinaga O, Edoh D, et al. Antiproliferative and pro-apoptotic activity of diarylheptanoids isolated from the bark of Alnus japonica in human leukemia cell lines. Am J Chin Med. 2015;43:757–67. doi: 10.1142/S0192415X15500470. [DOI] [PubMed] [Google Scholar]
- 19.D’Amours D, Germain M, Orth K, Dixit VM, Poirier GG. Proteolysis of poly (ADP-ribose) polymerase by caspase 3: Kinetics of cleavage of mono (ADP-ribosyl) ated and DNA-bound substrates. Radiat Res. 1998;150:3–10. [PubMed] [Google Scholar]
- 20.Uto T, Sakamoto A, Tung NH, Fujiki T, Kishihara K, Oiso S, et al. Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in human leukemia cell lines. Int J Mol Sci. 2013;14:4106–20. doi: 10.3390/ijms14024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tung NH, Du GJ, Yuan CS, Shoyama Y, Wang CZ. Isolation and chemopreventive evaluation of novel naphthoquinone compounds from Alkanna tinctoria. Anticancer Drugs. 2013;24:1058–68. doi: 10.1097/CAD.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29:175–80. [PMC free article] [PubMed] [Google Scholar]
- 23.Konoshima T, Takasaki M, Tokuda H, Morimoto S, Tanaka H, Kawata E, et al. Crocin and crocetin derivatives inhibit skin tumour promotion in mice. Phytother Res. 1998;12:400–4. [Google Scholar]
- 24.Peter ME, Scaffidi C, Medema JP, Kischkel F, Krammer PH. The death receptors. Results Probl Cell Differ. 1999;23:25–63. doi: 10.1007/978-3-540-69184-6_3. [DOI] [PubMed] [Google Scholar]