Abstract
Background:
Fruits are considered one of the richest sources of natural antioxidants. Their consumption has been linked to the prevention of oxidative stress-induced diseases.
Objective:
In this study, in vitro antioxidant activities of blueberry, jackfruit, blackberry, black raspberry, red raspberry, strawberry, and California table grape extracts were evaluated.
Materials and Methods:
Antioxidant activities were determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant potential (FRAP), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), nitric oxide (NO), superoxide anion (O2−) scavenging assays, and ferric reducing power.
Results:
Black raspberry extract had the highest phenolic (965.6 ± 2.9 mg gallic acid equivalents [GAE]/g), flavonoid (186.4 ± 1.7 mg quercetin equivalents/g), and proanthocyanidin (2677 ± 71.1 mg GAE/g) contents. All fruit extracts exhibited increasing radical scavenging activities with increased concentrations. At 100 μg/ml, red raspberry extract showed the highest ferric reducing power (A700 =0.3 ± 0.0052) and FRAP activity (A593 =11.43 mM Fe2+/g). Black raspberry extract (100 μg/ml) exhibited the highest DPPH activity (A517 =89.03 ± 0.0471). Jackfruit extract (100 μg/ml) had the highest ABTS (A734 =35.6 ± 0.613), NO (A540 =81.7 ± 0.2), and O2− radical scavenging (A230 =55.5 ± 0.2) activities. Positive correlations were observed between IC50 values for different radical scavenging activities and different polyphenolics. Red raspberry extract had the highest Pearson's coefficient values (0.952–1) between total phenolics, flavonoids, and proanthocyanidins and DPPH and superoxide radical scavenging activities.
Conclusions:
The antioxidant rich fruits in this study are good source of functional food and nutraceuticals that have the potential to improve human health.
SUMMARY
All fruit extracts exhibited increasing radical scavenging activities with increased concentrations
Black raspberry extract is enriched in total phenols, flavonoids, and proanthocyanidins and showed the highest 2,2-diphenyl-1-picrylhydrazyl scavenging activity and red raspberry extract showed the highest ferric reducing power and ferric reducing antioxidant potential activity
Jackfruit extract exhibited the highest 2,2′azino-bis (3-ethylbenzothiazoline-6-sulfonicacid) diammonium salt, nitric oxide, O2- scavenging activities
Positive correlations were observed between IC50 values for different radical scavenging activities and different polyphenolics.
Abbreviations Used: Abs: Absorbance, ABTS: 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, BHT: Butylated hydroxytoluene, DPPH: 2,2-diphenyl-1-picrylhydrazyl, DW: Dry weight, FRAP: Ferric reducing antioxidant potential, FW: Fresh weight, GAE: Gallic acid equivalents, NADH: β-nicotinamide adenine dinucleotide hydrate, NFL: The National Food Laboratories, NO: Nitric oxide, ONPG: ortho-nitrophenyl-β-galactoside, PBS: Phosphate buffered saline, PMS: Phenazine methosulfate, QE: Quercetin equivalents, ROS: Reactive oxygen species, SD: Standard deviation, SOD: Superoxide dismutase, TCA: Trichloroacetic acid, TPTZ: 2,4,6-tris(2-pyridyl)-s-triazine, Trolox: (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid.
Key words: Antioxidant activity, flavonoids, IC50, phenols, proanthocyanidins, radical scavenging
INTRODUCTION
Free radicals are generated in all living cells as part of normal cellular function. However, generation of excess free radical either from exogenous or endogenous sources is responsible for many diseases. Ageing, immunosuppression, and many chronic and degenerative diseases, including cancer, atherosclerosis, diabetes mellitus, and neurodegenerative diseases are examples of free radical mediated oxidative stress.[1] To protect themselves from free radical-mediated oxidative stress, cells employ different cellular antioxidant systems, such as low molecular mass antioxidants (glutathione, tocopherols, ascorbic acid); enzymes interacting with reactive oxygen species (ROS) such as superoxide dismutase, peroxidases, catalases; and other enzymes generating reduced forms of antioxidants.[2] Epidemiological studies have established a positive correlation between the consumption of fruits and vegetables and prevention of diseases associated with oxidative stress.[1,3]
Plant tissues contain different antioxidants such as flavonoids, tannins, and lignin precursors, which act as ROS-scavenging compounds.[2] Berries, small fleshy fruits consumed fresh or processed, are rich in phenolic compounds such as phenolic acids, stillbenoids, proanthocyanidins (condensed tannins), ellagitannins and gallotannins (hydrolysable tannins), and flavonoids (flavonols, flavanols, anthocyanins).[3] In North America, the commonly consumed berries are blackberry, black raspberry, blueberry, cranberry (Vaccinium macrocarpon, Ericaceae), red raspberry, strawberry, and grape.[3] Jackfruit, mostly consumed in Asia, is another rich source of phenolic compounds providing health benefits.[4]
In this study,in vitro antioxidant activities of seven fruit extracts of blueberry (Vaccinium corymbosum, Ericaceae), jackfruit (Artocarpus heterophyllus, Moraceae), blackberry (Rubus fruticosus, Rosaceae), black raspberry (Rubus occidentalis, Rosaceae), red raspberry (Rubus idaeus, Rosaceae), strawberry (Fragaria x ananassa, Rosaceae), and California table grape (Vitis vinifera, Vitaceae) were determined. The aim of this study was to evaluate and compare the polyphenolic contents and antioxidant activities of commercially available fruits and selected freeze-dried fruit powders used in shakes and smoothies. All fruit extracts under study exhibited strong radical scavenging activities.
MATERIALS AND METHODS
Chemicals
Ethyl alcohol (95%), ortho-nitrophenyl-β-galactoside, Folin–Ciocalteu's phenol reagent, Griess' reagent for nitrite, aluminum chloride, β-nicotinamide adenine dinucleotide hydrate (NADH), phenazine methosulfate (PMS), 2,4,6-tris (2-pyridyl)-s-triazine (TPTZ), sodium acetate, L-ascorbic acid, quercetin, gallic acid, ferrous chloride, ferrous sulfate heptahydrate, trichloroacetic acid (TCA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), (±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), potassium persulfate, sodium nitroprusside, butylated hydroxytoluene (BHT) were purchased from Sigma Aldrich (St. Louis, MO, USA).
Preparation of fruit extracts
Fresh blueberry, blackberry, and strawberry fruits (all packed by Driscoll's) were purchased at a local market in Denton, Texas. Jackfruit was purchased at a Vietnamese market in Carrollton, Texas. Freeze-dried powders of red raspberry, black raspberry, and California table grape were obtained from The National Food Laboratories and California table grape from California Table Grape Commission. Fruit extracts were arranged alphabetically according to their respective plant families in tables and figures. Fresh fruits were frozen and milled in an SPEX Sample Prep 6870 Freezer/Mill (Metuchen, NJ, USA) before extraction. Fruit powders were extracted in 95% ethanol (1:4 w/v) at room temperature for 2 days and then centrifuged at 3500 rpm for 20 min. Supernatants were filtered through Whatman #54 filter paper and stored at −20°C until further analysis.
Determination of total phenolics, flavonoids, and proanthocyanidins
Total phenolic content of fruit extracts was determined by Folin–Ciocalteu method. Four hundred microliter of fruit extracts (20 µg/ml–100 µg/ml), 1.6 ml of sodium carbonate (7.5% dissolved in deionized water), and 2 ml of Folin–Ciocalteu reagent (diluted 10 times in deionized water) were added. The reaction mixtures were incubated at room temperature for 1 h, and absorbances were measured at 765 nm.[5] Gallic acid was used as a standard and the total phenolic content in fruit extracts was expressed as mg gallic acid equivalents (GAE)/g of fruit fresh weight (FW) or dry weight (DW) (powder). Total flavonoids were determined by the method of Ordonez et al.[6] Five hundred microliters of 2% aluminum chloride prepared in ethanol were added to 500 µl of each fruit extract (20 µg/ml–100 µg/ml). The reaction mixtures were incubated at room temperature for 1 h, and the absorbances were measured at 430 nm. Quercetin was used as a standard, and total flavonoid content of fruit extracts was expressed as mg quercetin equivalents (QE)/g of fruit FW or DW. Total proanthocyanidins were determined by the method of Sun et al.[7] Three milliliters of vanillin-methanol (4% v/v) and 1.5 ml of hydrochloric acid were added to 0.5 ml of each fruit extract (20 µg/ml–100 µg/ml) and vortexed thoroughly. The resulting mixtures were allowed to stand at the room temperature for 15 min, and absorbance was measured at 500 nm. Total proanthocyanidin content in fruit extracts was expressed as mg GAE/g of fruit FW or DW.
Ferric reducing power
Ferric reducing power was determined by the method of Oyaizu.[8] Each reaction mixture contained 2.5 ml of 0.2 M phosphate buffer (pH 6.6), 2.5 ml of K3 Fe (CN)6(1% w/v), and 1 ml of each fruit extract (20 µg/ml–100 µg/ml). The resulting mixtures were incubated at 50°C for 20 min. After addition of 2.5 ml TCA (10% w/v), the mixtures were centrifuged at 3000 rpm for 10 min. Supernatants (2.5 ml) were mixed with 2.5 ml of distilled water and 0.5 ml of FeCl3 (0.1%, w/v), and absorbance was measured at 700 nm.
Ferric reducing antioxidant potential assays
Ferric reducing antioxidant potential (FRAP) reagent was freshly prepared by mixing 300 mM acetate buffer (pH 3.6), 10 mM TPTZ in 40 mM hydrochloric acid, and 20 mM ferric chloride in a ratio of 10:1:1. The assays were performed by the method of Othman et al.[9] Reaction mixtures consisted of 16 µl of each fruit extract (20 µg/ml–100 µg/ml), 500 µl FRAP reagent, and 50 µl distilled water. Mixtures were incubated at room temperature for 4 min and absorbances were measured at 593 nm. A FeSO4 ×7H2O standard curve was used to estimate FRAP activity of fruit extracts, which was expressed as µmol Fe2+/g.
In vitro free radical scavenging activities of fruit extracts
2,2-Diphenyl-1-picrylhydrazyl radical scavenging activity
The DPPH radical scavenging activity of fruit extracts was determined by the modified method of Gülçin et al.[10] Reaction mixtures contained 50 µl of fruit extract (20 µg/ml–100 µg/ml) and 2.95 ml of 0.1 mM DPPH in methanol. Mixtures were vortexed thoroughly and kept in the dark at room temperature for 30 min. After 30 min of incubation, the absorbances were measured at 517 nm. Ascorbic acid was used as a standard. The percentage of DPPH* radical scavenging was calculated according to equation 1, in which Abs = absorbance at 517 nm.
% of free radical scavenging activity = ([Abscontrol − Abssample]/Abscontrol) × 100(1)
2,2'-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activity
The ABTS radical scavenging activity was determined by the method of Re et al.[11] The ABTS working solution was prepared by mixing 7 mM ABTS and 2.4 mM potassium persulfate in equal amounts and allowed to react in the dark at room temperature for 12 h. One ml of ABTS+ solution was mixed with 60 ml of methanol to obtain an absorbance of 0.76 ± 0.001 at 734 nm. About 1 ml of each fruit extract (20 µg/ml–100 µg/ml) was allowed to react with 1 ml ABTS+ solution, and absorbances were measured at 734 nm after 7 min. Ascorbic acid was used as a standard. The percentage of ABTS* radical scavenging was calculated according to Eq. 1.
Nitric oxide scavenging activity
Nitric oxide (NO) scavenging activity was determined by the modified method of Balakrishnan et al.[12] About 2 ml of sodium nitroprusside in phosphate buffered saline was mixed with 1 ml of each fruit extract (20 µg/ml–100 µg/ml). Mixtures were incubated at 25°C for 150 min, after which 0.5 ml of incubation solution was mixed with Griess reagent. Mixtures were allowed to stand at the room temperature for 30 min, and absorbances were measured at 540 nm. Quercetin was used as a standard. The percentage of NO* radical scavenging was calculated according to Eq. 1 at Abs540.
Superoxide anion scavenging activity
The superoxide anion scavenging activity was determined by the method of Yen and Chen.[13] The reaction mixtures contained 1 ml of each fruit extract (20 µg/ml–100 µg/ml), 1 ml PMS (60 µM) prepared in 0.1 M (pH 7.4) phosphate buffer, and 1 ml NADH prepared in phosphate buffer. Mixtures were incubated at 25°C for 5 min, and absorbances were measured at 230 nm against phosphate buffer blank. Quercetin was used as a standard. The percentage of superoxide anion radical scavenging was calculated according to Eq. 1 at Abs230.
IC50
IC50 values (concentrations of samples required to scavenge 50% of free radicals) were calculated by linear regression analysis.
Statistical analysis
Means and standard deviations of three experiments were calculated. One-way ANOVA was performed and significance of differences among means was determined by Tukey's test (P ≤ 0.01 or P ≤ 0.05). Pearson's correlation was performed to indicate the relationship between total phenolics, flavonoids, proanthocyanidins, and radical scavenging activities of fruit extracts.
RESULTS AND DISCUSSION
Total phenolics, flavonoids, and proanthocyanidins of fruit extracts
Total phenolics, flavonoids, and proanthocyanidins were estimated Table 1 to identify what phytochemicals correlated with specific in vitro free radical scavenging activities of fruit extracts. Berries are known to contain high levels of diverse phytochemicals, most of which are the products of phenylpropanoid pathway, such as anthocyanins, flavonols, flavanols, proanthocyanidins, ellagitannins, and phenolic acids.[3] Black raspberry extract contained the highest phenolics (965.6 ± 2.9 mg GAE/g), flavonoids (186.4 ± 1.7 mg QE/g), and proanthocyanidins (2677 ± 71.1 mg GAE/g) Table 1. Study by Wu et al.[14] reported protocatechuic acid as the major phenolic acid in black raspberry followed by p-coumaric, caffeic, ferulic, and 3-hydroxybenzoic acids. In another study, Tulio et al.[15] reported two anthocyanins, cyanidin 3-rutinoside, and cyanidin 3-xylosylrutinoside to significantly contribute to the antioxidant activity of black raspberry.
Table 1.
Total phenolics, flavonoids, and proanthocyanidins contents of fruit extracts
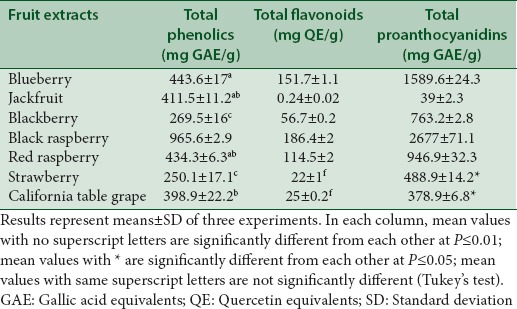
Total phenolic contents of fruit extracts ranged from 250.1 ± 17.12 to 965.6 ± 2.9 mg GAE/g of fruit FW or DW Table 1. The total phenolic content of fruit extracts in our study ranked as follows: Black raspberry >blueberry, jackfruit, and red raspberry >California table grape >blackberry >strawberry Table 1. Pantelidis et al.[16] reported higher phenolic content in two varieties (Autumn Bliss and Heritage) of red raspberry ranging from 1052 ± 75 to 2494 ± 77 mg GAE 100/g DW, whereas the present study showed the phenolic content of red raspberry as 434.3 ± 6.31 mg GAE/g DW. Pantelidis et al.[16] also reported higher phenolic content in four varieties (Choctaw, Thornless Evergreen, Chester Thornless, Hull Thornless) of blackberry extracts ranging from 1703 ± 71 to 2349 ± 1531 mg GAE/g DW compared to the present study where the total phenolic content of blackberry extract was 269.51 ± 16 mg GAE/g FW. Total phenolic contents of strawberry and white grape extracts reported by Park et al.[17] were 7.8 ± 0.7 g/kg FW and 5.5 ± 0.5 g/kg FW, respectively. Study by Shrikanta et al.[18] showed low phenolic (1.04 mg GAE/g FW) content of grape pulp. In the present study, total phenolic contents of strawberry and California table grape extracts were 250.1 ± 17.12 mg GAE/g FW and 398.93 ± 22.2 mg GAE/g DW, respectively. Studies by Jagtap et al.[4] and Shrikanta et al.[18] reported low phenol content (0.21 ± 0.012 mg GAE/g FW and 1.27 ± 0.33 mg GAE/g, respectively), in jackfruit pulp extracts. The present study however reported statistically significant higher amount of total phenol (411.5 ± 11.23 mg GAE/g FW) as compared to the aforementioned studies. These differences in phenolic contents of fruit extracts among different studies may be due to different quantification methods (including use of different standards) as well as other factors such as the genus, species, cultivar of the plants used, and environmental differences at the plant growing location such as climate, soil composition, light, temperature, pest exposure, ripening stage, and handling and storage of fruit.[19,20]
Total flavonoid contents of fruit extracts ranged from 0.24 ± 0.02 to 186.4 ± 1.7 mg GAE/g of fruit FW or DW ranking as follows: Black raspberry >blueberry >red raspberry >blackberry >strawberry and California grape >jackfruit Table 1. In our study, jackfruit has the lowest total flavonoid content, 0.24 ± 0.02 mg QE/g FW Table 1. This result is consistent with results of previous studies by Jagtap et al.[4] and Shrikanta et al.,[18] which reported that jackfruit pulp extracts contained low level of flavonoids (1.20 mg of rutin equivalents/g FW and 0.11 ± 0.03 mg catechin equivalents [CE]/g FW, respectively). Sharma et al.[21] reported higher amount of flavonoid content (10.5 ± 0.21 mg CE/g FW) in jackfruit shell powder. Study by Shrikanta et al.[18] showed low flavonoid (0.23 ± 0.02 mg CE/g FW) contents of grape pulp, whereas the present study reported a higher flavonoid content of 25 ± 0.2 mg QE/g DW in California table grape extract. The differences in flavonoid content among studies may be the result of different grapevine varieties used, as well as the use of different standards (catechin vs. quercetin) to estimate total flavonoids in fruit extracts and way of reporting flavonoid content, FW versus DW.
Total proanthocyanidins contents of fruit extracts in this study ranged from 39 ± 0.23 to 2677 ± 71.1 mg GAE/g of fruit FW or DW and ranked as follows: Black raspberry >blueberry >red raspberry >blackberry >strawberry >California table grape >jackfruit Table 1. Huang et al.[22] using high-performance liquid chromatography showed high levels of proanthocyanidins in blueberry extracts obtained from fruit cultivated in Nanjing, which contributed to high DPPH and ABTS radical scavenging activities. Hwang et al.[23] reported lower (11.8 ± 5.0 mg CE/g FW) total proanthocyanidin content of blueberry extract cultivated in Korea as compared to the present study (1589.6 ± 24.3 mg GAE/g FW). The difference may attribute to the different cultivars used for plant extracts as well as the use of different standards, catechin versus gallic acid, in estimating the proanthocyanidin content.
Determination of ferric reducing power
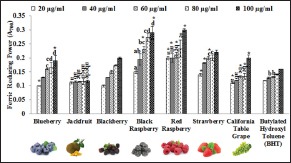
Ferric reducing power assay is based on the reduction of Fe3+ to Fe2+ by antioxidant compounds visible in changing the yellow color of the test solution to various shades of green and blue, depending on the reducing power of antioxidants. Increased absorbance at 700 nm indicates high ferric reductive ability. In general, except for jackfruit extract, all fruit extracts showed increased ferric reducing power with increasing concentrations [Figure 1]. All fruit extracts at 100 µg/ml, except for jackfruit extract, showed higher ferric reducing power than BHT [Figure 1]. At 100 µg/ml, black and red raspberry extracts exhibited the highest reducing power of 0.27 and 0.29, respectively. The range of reducing power for other fruit extracts at 100 µg/ml was 0.11–0.29. Hyun et al.[24] reported that Korean black raspberry extracts (100–200 µg/ml) obtained from fruit at different ripening stages exhibited a range of 0.13–0.28 reducing power. Weidner et al.[25] reported a range of 0.553–0.931 ferric reducing power of extracts from California grape germinating seed, after seeds were exposed to three temperature conditions: Chill stress, optimal condition, and postchill recovery. The above results show that ripening stages and stressful condition for germination affect ferric reducing power of fruit extracts. Fruits have different polyphenolic content at different developmental stages.[26] It is known that secondary metabolites in plants including antioxidants are intensively synthesized under stress.[27]
Figure 1.
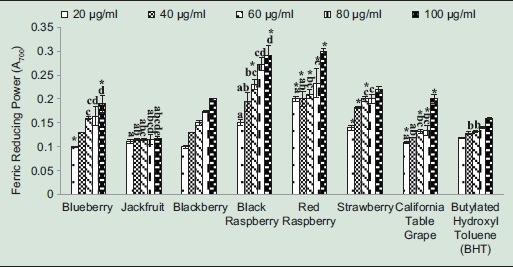
Ferric reducing power of fruit extracts. Butylated hydroxyl toluene was used as a standard. Results represent means ± standard deviation of three experiments. Bars for each plant extract with no superscript letters are significantly different from each other at P ≤ 0.01. Bars for each plant extract with * are significantly different from each other at P ≤ 0.05 (Tukey's test)
Ferric inducing antioxidant potential assays
FRAP assay is based on the reduction at low pH of a colorless ferric complex (Fe3+-tripyridyltriazine) to a blue-colored ferrous complex (Fe2+-tripyridyltriazine) by the action of electron-donors. Red raspberry exhibited the highest FRAP activity (103.9 ± 0.9 µM Fe2+/g). The activities of other extracts ranked as follows: Strawberry >blackberry >black raspberry and blueberry >jackfruit >California table grape [Figure 2]. Borges et al.[28] reported the FRAP activity of blueberry extract as 30 ± 1.9 μM of Fe2+/g, whereas present study reported it as 22.5 ± 0.6 µM Fe2+/g. Jagtap et al.[4] studied the FRAP activity of jackfruit pulp extracts in different solvents (acetone, ethanol, methanol, water) and found it to increase with increased concentrations of fruit per volume of solvent (1–5 mg/ml). Different studies reported FRAP activity in different ways. At 5 mg/ml, jackfruit pulp extract in one study showed a FRAP activity of 1.38 mM TEAC/g,[4] whereas our study reports the FRAP activity of jackfruit extract as 12.63 ± 0.1 µM Fe2+/g. Pantelidis et al.[16] reported FRAP activities of two red raspberry and two blackberry cultivars ranging from 77.7 to 145.4 µM ascorbic acid per g DW and 113.6–169 µM ascorbic acid per g DW, respectively. Another study by Koca and Karadeniz[29] reported the FRAP activities of different varieties of blackberries and blueberries ranging from 35.05 to 70.41 µM/g and 7.41 to 57.92 µM/g, respectively. Therefore, no comparison can be made between our results and those reported in other studies due to use of different standards (FeSO4, ascorbic acid, Trolox) and reporting FRAP results.
Figure 2.
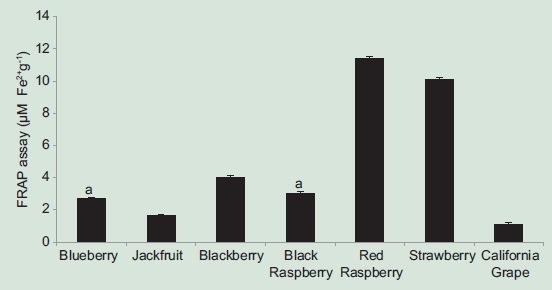
Ferric reducing antioxidant potential of fruit extracts. Results represent means ± standard deviation of three experiments. Bars with no superscript letters are significantly different from each other at P ≤ 0.01 (Tukey's test). Bars with same superscript letters are not significantly different
In vitro free radical scavenging activities of fruit extracts
In vitro antioxidant activities of fruit extracts were determined by DPPH, ABTS, NO, and superoxide anion scavenging activities [Table 2]. Blueberry, black and red raspberry extracts (20–100 µg/ml) show the highest percentage of DPPH radical inhibition ranging from 38.5% to 87.9%, 64.2% to 89%, and 37.6% to 87%, respectively [Table 2]. Hwang et al.[23] reported the DPPH radical scavenging activities of Korean blueberry extracts at different concentrations (10, 50 and 500 μg/ml) to be 29.4%, 29.6%, and 40.6%, respectively, which are lower than that obtained in the present study where 100 μg/ml of blueberry extract show 87.94% of DPPH scavenging activity. The DPPH radical scavenging activities of other fruit extracts at 100 µg/ml ranked as follows: Blackberry >strawberry >California table grape >jackfruit [Table 2]. In the present study, the DPPH scavenging activity of jackfruit extract (100 µg/ml) was 4.5% similar to that reported by Jagtap et al.[4] Except for jackfruit, all other fruit extracts exhibited higher DPPH radical scavenging activities than that of ascorbic acid standard.
Table 2.
Radical scavenging capacity of fruit extracts
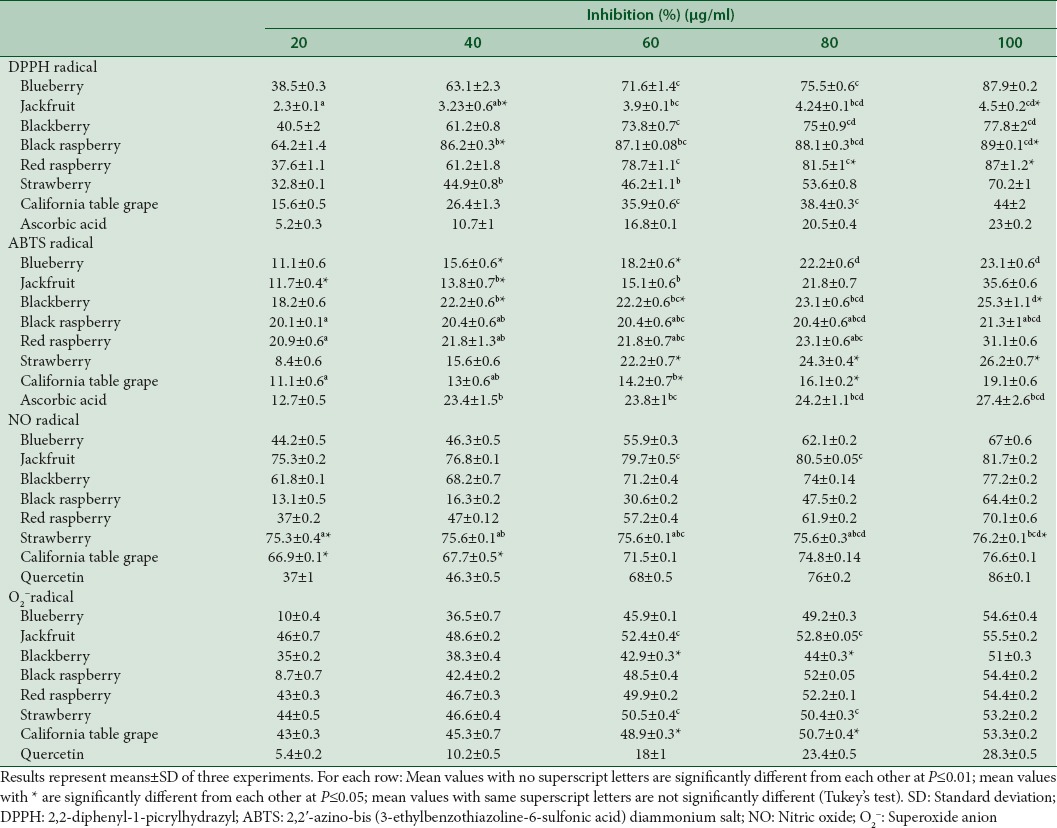
ABTS scavenging activity measures the reduction of the blue–green chromophore ABTS+ (2,2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) to colorless ABTS by an antioxidant. Jackfruit extract (20–100 µg/ml) showed the highest ABTS radical scavenging activity of 11.7–35.6% [Table 2]. The percentage of ABTS radical inhibition for other fruit extracts (100 µg/ml) are as follows: Red raspberry >blueberry, blackberry, black raspberry, and strawberry >California table grape. Blackberry, red and black raspberry extracts have the same or higher ABTS radical inhibition as ascorbic acid at all concentrations used. Jackfruit extract showed the highest ABTS radical scavenging activity of 35.6% at 100 µg/ml, similar to reports by Shanmugapriya et al.,[30] where jackfruit pulp extract (100 µg/ml) inhibited ABTS radical by 31.26%. Hwang et al.[23] showed that ABTS radical scavenging of Korean blueberry extract at concentrations of 10, 50 and 500 μg/ml were 2.3%, 4.2% and 8.6%, respectively, whereas the present study reports higher inhibition of ABTS radical by blueberry extracts (11.1–23.1%, 20–100 μg/ml).
NO is generated in biological tissues when specific NO synthases metabolize arginine to citrulline via a five electron oxidative reaction. Jackfruit extract (20–100 µg/ml) showed the highest inhibition of NO radical ranging from 75.3% to 81.7% [Table 2]. The percentage of inhibition for other fruit extracts (100 µg/ml) were as follows: Blackberry, strawberry, and California table grape >red raspberry >blueberry. Jagtap et al.[31] reported the NO inhibition by jackfruit wine (100–500 µl) ranging from 50% to 60%.
Superoxide anion radical (O2−) is one of the strongest ROS, which gets converted to other harmful ROS as well as free radicals such as hydrogen peroxide and hydroxyl radical in the cells. In this study, fruit extracts showed superoxide scavenging activity ranging from 51% to 55.5% at 100 μg/ml which were higher than those of quercetin standard [Table 2]. Wang and Jiao[32] reported inhibition of superoxide radicals by 100 μl of unknown concentration of blueberry, blackberry, black raspberry, red raspberry, and strawberry extracts as follows: Blueberry (52.5–69.3%), blackberry (43.3–72%), raspberries (40.8–66.9%), and strawberry (57.9–73.6%).
The differences between the radical scavenging activities in this study and previous studies could be attributed to different methods of extraction, usage of various concentrations, methods of reporting results, conditions of climate, soil composition, varieties, cultivar as well as different modes of processing. In some cases, no comparison can be made between our results and others mainly due to unknown extract concentration reported.[26]
IC50
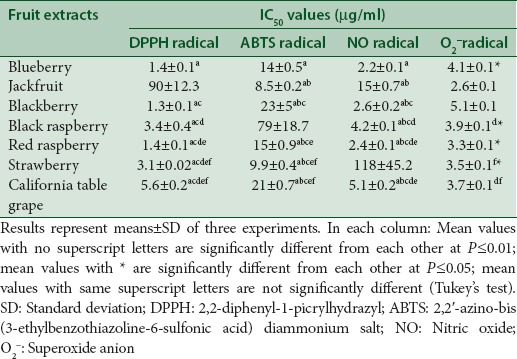
A lower IC50 value indicates greater antioxidant activity. In the present study, the lowest IC50 value (1.3 ± 0.1 µg/ml) required to quench at least 50% DPPH radicals was that of blackberry extract [Table 3]. Ivanovic et al.[33] reported that blackberry extract of “Čačanska Bestrna” cultivar showed lower IC50 values (96–118.1 µg/ml) for DPPH radical with increasing sonication time and/or temperature. In comparison to the Ivanovic et al.[33] study, the present study shows much lower IC50 value for DPPH radical (1.3 µg/ml). The differences in results can be attributable to the methods of extraction and different plant cultivars. Among the fruit extracts tested, jackfruit extract showed the lowest IC50 values for ABTS (8.5 ± 0.22 µg/ml) and O2− radicals (2.6 ± 0.1 µg/ml). The IC50 value for O2− radical (2.6 ± 0.1 µg/ml) in the present study is much lower than that reported by Ruikar et al.[34] for the jackfruit extract (24.3 ± 2.09 µg/ml). Blueberry extract showed the lowest IC50 value (2.23 ± 0.1 µg/ml) for NO radical. The IC50 value (100 µg/ml) of Korean blueberry extract for NO radical[35] was much lower than that reported in the present study (2.23 µg/ml) indicating differences in fruit varieties, methods of extraction, and other factors. These results indicate that aforementioned fruit extracts are the powerful radical scavengers at very low concentrations.
Table 3.
IC50 values of radical scavenging activities of fruit extracts
The antioxidant capacity of the fruit extracts was evaluated and correlated to chemical classes of polyphenols content. Table 4 shows the correlation between IC50 values of radical scavenging activities and total phenolics, flavonoids, and proanthocyanidins of fruit extracts. Jackfruit, blackberry, red raspberry, and California table grape fruit extracts show high correlation of total phenolics with DPPH radical scavenging activity, whereas jackfruit, black and red raspberry, strawberry show high correlations of total proanthocyanidins with DPPH radical scavenging activity. Except for black and red raspberry extracts, all fruit extracts show low correlations of total flavonoids with DPPH radical scavenging activity. These results indicate that total phenolics and proanthocyanidins resulted in a stronger DPPH scavenging activities of the fruit extracts than total flavonoids.
Table 4.
Correlation between IC50 of radical scavenging activities and phenolics, flavonoids, proanthocyanidins of fruit extracts
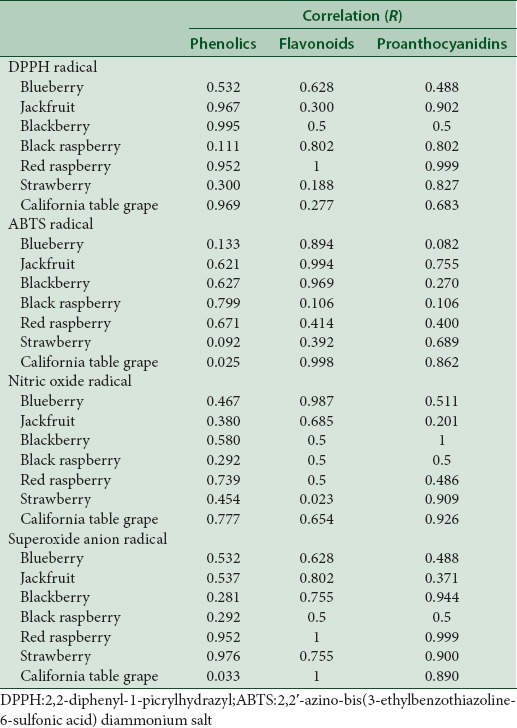
Blueberry, jackfruit, blackberry, and California table grape extracts show high correlations of ABTS scavenging activities with total flavonoids. In addition, California table grape extract has a high correlation between total proanthocyanidins content and ABTS scavenging activities. Black and red raspberry extracts show high correlation of ABTS scavenging activities with total phenolics. These results indicate that total flavonoids rather than total phenolics and proanthocyanidins in most fruit extracts resulted in stronger ABTS scavenging activities. Total flavonoids of blueberry, jackfruit, and California table grape extracts and total proanthocyanidins of blackberry, strawberry, and California table grape show high correlation with NO radical scavenging activity.
Red raspberry and strawberry extracts show high correlations of total phenolics, flavonoids, and proanthocyanidins with O2− radical scavenging activity. Furthermore, for O2− radical scavenging activity high correlations were observed with total proanthocyanidins in blackberry extract and total flavonoids and proanthocyanidins in California table grape extract. These results indicate that total phenolics, flavonoids, and proanthocyanidins could contribute to the NO radical scavenging activity [Table 4].
CONCLUSIONS
In the present study, we investigated the total phenolic, flavonoid, proanthocyanidin contents, and in vitro antioxidant activities of seven fruit extracts by employing different in vitro antioxidant assays with relevance to oxidative stress in human chronic diseases. Black raspberry among all fruit studied contains the highest amounts of phenols, flavonoids, and proanthocyanidins. The highest DPPH scavenging activity was exhibited by black raspberry extract and jackfruit extract showed the highest ABTS, NO, and O2− radical scavenging activities.
These antioxidant-rich aforementioned fruits are good candidates for functional food and nutraceuticals to help in the prevention of oxidative stress induced diseases. In spite of discrepancies in reported results among studies, attributed mostly to cultivar and methodology differences, our data add valuable information to current knowledge of nutritional properties of commercially available berries used fresh or processed. In conclusion red and black raspberry and California table grape powder in shakes, smoothies, and ice cream formulations will provide high levels of polyphenols and antioxidant properties.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Camelia Maier
Dr. Camelia Maier, has completed her PhD entitled ‘Phytoestrogens in two dioecious species: isolation, characterization and role in plant reproduction’ at the University of North Texas. She is an Associate Professor in the Department of Biology at Texas Woman's University in Denton, Texas, USA. Dr. Maier's research focuses on plant compounds with estrogenic and antioxidant activities. Texas Woman's University Biology Department Denton, Texas 56204-5799, USA
Acknowledgment
The authors express their special thanks to Drs. Shanil Juma, Parakat Vijayagopal, and Nancy DiMarco (TWU, Department of Nutrition and Food Sciences) for generously providing the following plant samples: Red raspberry, black raspberry, and California table grape and to Dinu Dixon and Sidney Cruz for technical support.
REFERENCES
- 1.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–86. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot. 2003;91:179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeram NP. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem. 2008;56:627–9. doi: 10.1021/jf071988k. [DOI] [PubMed] [Google Scholar]
- 4.Jagtap UB, Panaskar SN, Bapat VA. Evaluation of antioxidant capacity and phenol content in jackfruit (Artocarpus heterophyllus Lam.) fruit pulp. Plant Foods Hum Nutr. 2010;65:99–104. doi: 10.1007/s11130-010-0155-7. [DOI] [PubMed] [Google Scholar]
- 5.Singelton VR, Orthofer R, Lamuela-Raventos RM. Analysis of total phenol and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–78. [Google Scholar]
- 6.Ordonez AA, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq) Food Chem. 2006;97:452–8. [Google Scholar]
- 7.Sun JS, Tsuang YH, Chen IJ, Huang WC, Hang YS, Lu FJ. An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns. 1998;24:225–31. doi: 10.1016/s0305-4179(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 8.Oyaizu M. Studies on products of browning reactions: Antioxidant activities of products of browning reaction prepared from glucosamine. J Nutr. 1986;44:307–15. [Google Scholar]
- 9.Othman A, Ismail A, Ghani NA, Adenan I. Antioxidant capacity and phenolic content of cocoa beans. Food Chem. 2007;100:1523–30. [Google Scholar]
- 10.Gülçin I, Kireçci E, Akkemik E, Topal F, Hisar O. Antioxidant and antimicrobial activities of an aquatic plant: Duckweed (Lemna minor L.) Turk J Biol. 2010;34:175–88. [Google Scholar]
- 11.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 12.Balakrishnan N, Panda AB, Raj NR, Shrivastava A, Prathani R. The evaluation of nitric oxide scavenging activity of Acalypha indica Linn root. Asian J Res Chem. 2009;2:148–50. [Google Scholar]
- 13.Yen G, Chen H. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 14.Wu X, Pittman Iii HE, Hager T, Hager A, Howard L, Prior RL. Phenolic acids in black raspberry and in the gastrointestinal tract of pigs following ingestion of black raspberry. Mol Nutr Food Res. 2009;53(Suppl 1):S76–84. doi: 10.1002/mnfr.200800231. [DOI] [PubMed] [Google Scholar]
- 15.Tulio AZ, Jr, Reese RN, Wyzgoski FJ, Rinaldi PL, Fu R, Scheerens JC, et al. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J Agric Food Chem. 2008;56:1880–8. doi: 10.1021/jf072313k. [DOI] [PubMed] [Google Scholar]
- 16.Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis GR. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and cornelian cherries. Food Chem. 2007;102:777–83. [Google Scholar]
- 17.Park Y, Im MH, Ham K, Kang SG, Park YK, Namiesnik J, et al. Quantitative assessment of the main antioxidant compounds, antioxidant activities and FTIR spectra from commonly consumed fruits, compared to standard kiwi fruit. LWT Food Sci Technol. 2015;63:346–52. [Google Scholar]
- 18.Shrikanta A, Kumar A, Govindaswamy V. Resveratrol content and antioxidant properties of underutilized fruits. J Food Sci Technol. 2015;52:383–90. doi: 10.1007/s13197-013-0993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005;53:7749–59. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Garcia SN, Guevara-Gonzalez RG, Miranda-Lopez R, Feregrino-Perez AA, Torres-Pacheco I, Vasquez-Cruz MA. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res Int. 2012;54:1195–207. [Google Scholar]
- 21.Sharma A, Gupta P, Verma AK. Preliminary nutritional and biological potential of Artocarpus heterophyllus L. shell powder. J Food Sci Technol. 2015;52:1339–49. doi: 10.1007/s13197-013-1130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang WY, Zhang HC, Liu WX, Li CY. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci B. 2012;13:94–102. doi: 10.1631/jzus.B1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang SJ, Yoon WB, Lee OH, Cha SJ, Kim JD. Radical-scavenging-linked antioxidant activities of extracts from black chokeberry and blueberry cultivated in Korea. Food Chem. 2014;146:71–7. doi: 10.1016/j.foodchem.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Hyun TK, Rim K, Kim E, Kim JS. Variation in bioactive principles of Korean black raspberry (Rubus coreanus Miquel) during ripening. Plant Omics. 2014;7:510–6. [Google Scholar]
- 25.Weidner S, Chrzanowski S, Karamac M, Król A, Badowiec A, Mostek A, et al. Analysis of phenolic compounds and antioxidant abilities of extracts from germinating Vitis californica seeds submitted to cold stress conditions and recovery after the stress. Int J Mol Sci. 2014;15:16211–25. doi: 10.3390/ijms150916211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalt W. Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci. 2005;70:R11–19. [Google Scholar]
- 27.Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J Agric Food Chem. 2000;48:140–6. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- 28.Borges G, Degeneve A, Mullen W, Crozier A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J Agric Food Chem. 2010;58:3901–9. doi: 10.1021/jf902263n. [DOI] [PubMed] [Google Scholar]
- 29.Koca I, Karadeniz B. Antioxidant properties of blackberry and blueberry fruits grown in the Black Sea region of Turkey. Sci Hortic. 2009;121:447–50. [Google Scholar]
- 30.Shanmugapriya K, Saravana PS, Payal HS, Mohammed SP, Binnie W. Antioxidant activity, total phenolic and flavonoid contents of Artocarpus heterophyllus and Manilkara zapota seeds and its reduction potential. Int J Pharm Pharm Sci. 2011;3:256–60. [Google Scholar]
- 31.Jagtap UB, Waghmareb SR, Lokhande VH, Suprasannad P, Bapat VA. Preparation and evaluation of antioxidant capacity of jackfruit (Artocarpus heterophyllus Lam.) wine and its protective role against radiation induced DNA damage. Ind Crops Prod. 2011;34:1595–601. [Google Scholar]
- 32.Wang SY, Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem. 2000;48:5677–84. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 33.Ivanovic J, Tadic V, Dimitrijevic S, Stamenic M, Petrovic S, Zizovic I. Antioxidant properties of the anthocyanin-containing ultrasonic extract from blackberry cultivar “Čačanska Bestrna”. Ind Crops Prod. 2014;53:274–81. [Google Scholar]
- 34.Ruikar A, Torane R, Misar A, Mujumdar A, Puranik A, Deshpande N. Antioxidant screening of a medicinal Plant Artocarpus heterophyllus. Int J Pharm Sci Rev Res. 2015;33:35–3. [Google Scholar]
- 35.Samad NB, Debnath T, Ye M, Hasnat MD, Lim BO. In vitro antioxidant and anti-inflammatory activities of Korean blueberry (Vaccinium corymbosum L.) extracts. Asian Pac J Trop Biomed. 2014;4:807–15. [Google Scholar]


