Abstract
Objective:
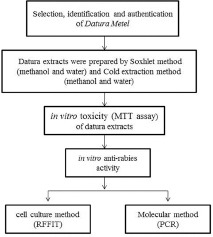
The soxhlet and cold extracts of Datura metel Linn. were evaluated for in vitro antirabies activity.
Materials and Methods:
Soxhlet and cold extraction method were used to extract Datura (fruit and seed) extracts. In vitro cytotoxicity assay was performed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay. Based on the CC50 range, the in vitro antirabies activity of the extracts was screened by rapid fluorescent focus inhibition test and molecular method.
Results:
The Datura (fruit and seed) extracts were not cytotoxic below 5 mg/ml (CC50). Titer of 10−4 rabies virus challenge virus standard (RV CVS) (1 50% tissue culture infective dose [1 TCID50]) was obtained by RFFT method and the challenge dose of 10 TCID50 was used for antirabies assay. Datura fruit and seed (soxhlet and cold) extracts showed 50% inhibition of RV CVS at 2.5 mg/ml and 1.25 mg/ml (inhibitory concentration 50% [IC50]), respectively. The tested extracts showed selectivity index (CC50/IC50) ranging from 2 to 4. The viral RNA was extracted and real-time reverse transcription-polymerase chain reaction was performed which also revealed a 2-fold reduction of viral load at 1.25 mg/ml of the Datura seed (soxhlet methanolic and cold aqueous) extracts.
Conclusion:
To the best of our knowledge, this is the first study of in vitro antiviral activity of D. metel Linn. against rabies virus. Datura seed extracts have a potential in vitro antirabies activity and, in future, can be further screened for in vivo activity against rabies virus in murine model.
SUMMARY
In the present study, Datura metel. Linn showed and in-vitro anti rabies activity in Vero cell line which was determined by RFFIT method and PCR method
Key words: Antirabies, antiviral assay, cytotoxicity, Datura, rapid fluorescent focus inhibition test
INTRODUCTION
Rabies or “hydrophobia” as it was previously known is caused by the highly neurotropic rabies virus and produces almost uniformly fatal encephalitis in humans and most other mammalian species. Rabies causes about 55,000 human deaths, annually, worldwide and 95% of human deaths due to rabies occur in Asia and Africa.[1] Roughly 97% of human rabies cases result from dog bites. India has the highest rate of human rabies in the world, primarily because of stray dogs, whose number has greatly increased since a 2001 law forbade the killing of dogs. Around 20,000 people are estimated to die every year from rabies in India which is more than a third of the global toll.[1,2] Vaccines are available, but it is ineffective once the virus enters the central nervous system (CNS) and there are no effective antiviral drugs against rabies virus. Rabies infection is fatal once the virus enters the CNS. There was only one reported case of a female survival from rabies virus by inducing coma and treating her with combination of known antiviral drugs.[3] Therefore, the search for more effective antiviral agents against viral infections of the CNS is a necessary and highly desirable task.
According to the literature, many traditional medicinal plants have been reported to have strong antiviral activity. Datura metel, although known for its toxicity, also contains medicinal properties. Datura intoxication typically produces delirium, hyperthermia, tachycardia, and bizarre behavior but it also has medicinal properties. Datura has long been used as an extremely effective treatment for asthma symptoms. The active antiasthmatic agent is atropine, which causes paralysis of the pulmonary branches of the lungs, eliminating the spasms that cause the asthma attacks. The leaves are generally smoked either in a cigarette or a pipe. This practice of smoking Datura to relieve asthma has its origins in traditional ayurvedic medicine in India.[4,5] Datura also has a wide range of traditional application including epilepsy, hysteria, insanity, and heart and skin diseases. In Ayurveda, Datura is used to cure mental diseases and relieve pain. In Indian ayuvedic medicine, Datura is used as a medication for the treatment of hydrophobia (classical symptom for rabies), which is rabies infection.[6] However, its mechanism of inhibition and interaction with the virus are yet to be elucidated, hence the present study intends to assess the in vitro antiviral activity of D. metel extracts against rabies virus.
MATERIALS AND METHODS
Collection and identification of sample
Datura fruits were collected from Dadar flower market, Mumbai. These were then identified and authenticated at the Blatter herbarium, St. Xavier's College, Mumbai, Maharashtra, India. The plant parts (Datura seed and Datura fruit pulp) were separately washed, shade dried, and powdered in grinder mixer. About 30 g of powdered material was extracted in soxhlet extraction apparatus (continuous hot percolation) with 600 ml of each of the following solvents: Methanol and water. Methanol used for the extraction was of analytical grade. The separated extracts were then filtered through Whatman's No. 1 filter paper and the aqueous and methanol filtrate were separately concentrated for dryness using a rotary evaporator to remove the water and methanol. The crude plant extracts obtained were weighed and stored in refrigerator for further use.[7]
Cell line and virus
Vero (African green monkey canine kidney) cell line was procured from NCCS, Pune. Vero cell lines were grown in minimum essential medium (MEM) with L-glutamine (2 mM), penicillin (100 IU/ml), streptomycin (100 μg/ml), and gentamicin (10 μg/ml) and supplemented with 10% fetal bovine serum (FBS). The flasks were incubated at 37°C, in 5% CO2 incubator for 2–3 days until a confluent monolayer is obtained. Rabies virus challenge virus standard (RV CVS) used in the present study was provided by the Department of Virology, Haffkine Institute. The virus stocks were stored at −80°C temperature for further use. The in vitro cytotoxicity and in vitro antiviral assay was also performed in Vero cell line.[8]
Virus titration
RV CVS stocks were grown in Vero cell lines, and titer of infectious virus was measured by 50% tissue culture infective dose (TCID50) titration by staining it with Immunofluorescent assay (IFA). Briefly, serial 10-fold dilutions (10−1 to 10−7) of virus in serum-free MEM were added onto the confluent monolayer in 96-well tissue culture plate and incubated for 72 h at 37°C with 5% CO2. After incubation, the medium was decanted and the cells were fixed by adding cold acetone (50 µl per well) and kept at 4°C for 30 min. After discarding the acetone, cells were stained by direct polyclonal florescent-labeled antibody (1:20) for 30 min and then washed (3 times) with 0.01 M phosphate buffer saline (PBS), air dried, and visualized under an inverted fluorescence microscope. The titer wby usas calculated using Reed and Muench method and expressed as TCID50.[9]
In vitro cytotoxicity assay
The in vitro cytotoxicity (CC50) assay of the plant extracts was performed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay in Vero cell line. Briefly, Vero cells were trypsinized and cultured onto 96-well plate at the density of 0.2 × 106 cells/ml. Once the monolayer is formed after 24 h, different concentrations (15–0.15 mg/ml) of Datura seed and Datura fruit (soxhlet and cold) extracts were serially diluted in DMEM (without phenol red) and added to each culture wells in triplicate and incubated at 37°C with 5% CO2 for 24 h. After incubation, the medium with plant extracts were removed and 10% of 5 mg/ml MTT (100 μl) was added to each well onto the Vero cells in 96-well plate and incubated for 3 h at 37°C. After incubation, MTT (100 μl) was removed and the crystal formed (formazan) was solubilized by adding dimethyl sulfoxide to each well. The absorbance was read at 550 nm by an ELISA reader.[10]
In vitro antirabies assay
The minimal cytotoxic concentrations (CC50) of Cold and Soxhlet extracts of Datura seed and fruit samples (50 µL) was mixed with a challenged dose of 10 TCID50 RV CVS (50 µL) and incubated for 1 hr in 96 well tissue culture plate. The reference serum (5 IU/ml) was serially diluted 2-fold from 1:00 to 1:2800 using MEM with 2% FBS in sterile tubes which serve as a positive control. Vero cell line was trypsinized and 0.2 × 106 cells/ml added to 96-well tissue culture plate. This was incubated at 37°C in a CO2 incubator for 72 h. The medium was decanted and the cells were fixed by adding cold acetone (50 µl per well) and kept at 4°C for 30 min. After discarding the acetone, cells were stained by direct polyclonal florescent-labeled antibody (1:20) for 30 min and then washed (3 times) with 0.01 M PBS, air dried, and visualized under an inverted fluorescence microscope.[9]
Viral load
The viral RNA was extracted from Vero cells from the 96-well plate after in vitro antiviral assay using the spin column based QIAamp-Viral RNA Mini Kit (QIAGEN GmbH, Hilden, Germany) as per the manufacturer's instructions. Reverse transcriptase real time-polymerase chain reaction (rRT-PCR) was carried out using a StepOne Real-time PCR instrument (M/s ABI) using 2X PCR mix and Super-ScriptTMIIRT/Platinum Taq Enzyme mix (M/s Takara) and rabies N gene primers[11] [Table 1]. The cycling condition for the rRT-PCR was 42°C for 5 min of reverse transcription followed by denaturation at 95°C for 10 s and PCR amplification (38 cycles) of 95°C for 1 min, 55°C for 1 min, and extension at 72°C for 1 min 30 s. The antirabies activity was quantified by Delta Delta Ct method.[12]
Table 1.
The rabies virus N gene primer sequence

RESULTS
Virus titration
RV CVS was titrated ((10−1 to 10−6) in Vero cell line in 96-well plate. On the 3rd day post infection (p.i.), the medium was decanted and the cells were fixed with acetone (chilled) and stained with fluorescently labeled antibody (IFA) and observed under fluorescent microscope and quantified by rapid fluorescent focus inhibition test (RFFIT) method. TCID50 was calculated by Reed–Muench method and the titer of 10−4 TCID50/0.1 ml of RV CVS (1 TCID50) was obtained [Figure 1]. Based on 1 TCID50, the challenge dose of 10 TCID50 was prepared for in vitro antiviral assay.
Figure 1.
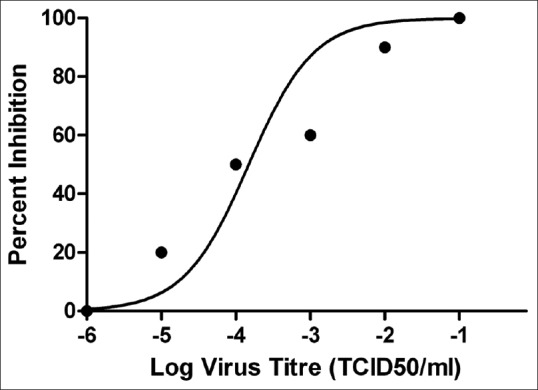
Titration of rabies virus challenge virus standard was performed in Vero cell line and virus 50% tissue culture infective dose was calculated by Reed–Muench method. The figure log virus titer with R2 value – 0.8555 using GraphPad Prism version 5.0 software
In vitro cytotoxicity assay
Different concentration (10 –0.1 mg/ml) of soxhlet and cold extracts of Datura seed and Datura fruit were added to Vero cell line (0.3 × 106 cells/ml) in 96-well plate and was analyzed by MTT based cytotoxicity assay. The CC50 of soxhlet extract of Datura fruit (water and methanol) was found to be 5 mg/ml and 4 mg/ml respectively whereas of cold extracts of Datura fruit (water and methanol) was found to be 7 mg/ml and 3 mg/ml respectively [Figure 2]. Whereas the CC50 of soxhlet extracts of Datura seed (water and methanol) was found to be 7.5 mg/ml and 5 mg/ml respectively. Whereas CC50 of cold extracts of Datura seed (water and methanol) was found to be 3.5 mg/ml and 5.5 mg/ml respectively [Figure 3]. The CC50 was calculated and the percent cytotoxicity was represented using GraphPad Prism version 5.0 software (California corporation).
Figure 2.
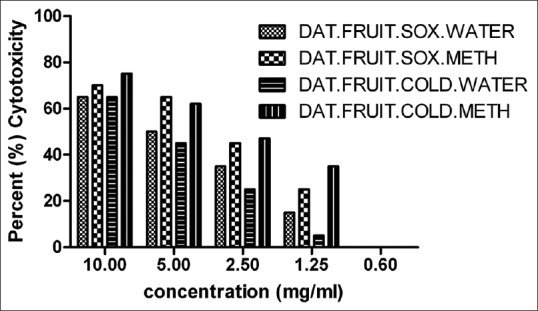
In vitro cytotoxicity assay of Datura fruit (soxhlet and cold) extracts of different concentration (10–0.6 mg/ml) was performed in Vero cell line by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay. CC50 value was calculated using GraphPad Prism version 5.0 software
Figure 3.
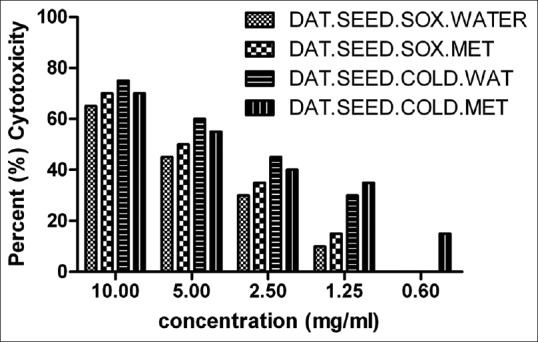
In vitro cytotoxicity assay of Datura seed (soxhlet and cold) extracts of different concentration (10–0.6 mg/ml) was performed in Vero cell line by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide assay. CC50 value was calculated using GraphPad Prism version 5.0 software
In vitro antiviral activity
Minimal cytotoxic concentration of Datura fruit and Datura seed (soxhlet and cold) extract was selected below the CC50 range (5–0.3 mg/ml) and exposed with the challenge dose of 10 TCID50 RV CVS for antiviral assay. After 72 h postinoculation (p.i.), the antiviral assay was quantified by RFFIT method and also by molecular method by PCR.
We observed 100% inhibition at 5 mg/ml and IC50 (50% inhibition) was found to be 2.5 mg/ml of Datura fruit (soxhlet and cold) extract [Figure 4]. For Datura seed (soxhlet and cold) extracts, we observed 100% inhibition of 10 TCID50 RV CVS at 2.5 mg/ml of Datura seed (methanol soxhlet and cold extracts) whereas 50% inhibition (IC50) of Datura seed (water soxhlet) was observed at 2.5 mg/ml. The IC50 of Datura seed (methanol soxhlet and cold extract) was found at 1.25 mg/ml by RFFIT method [Figure 5].
Figure 4.
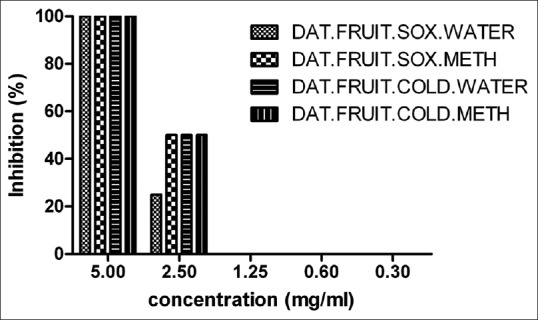
In vitro antiviral activity of Datura fruit (soxhlet and cold) extract against rabies virus challenge virus standard. The IC50 was calculated by GraphPad Prism version 5.0 software
Figure 5.
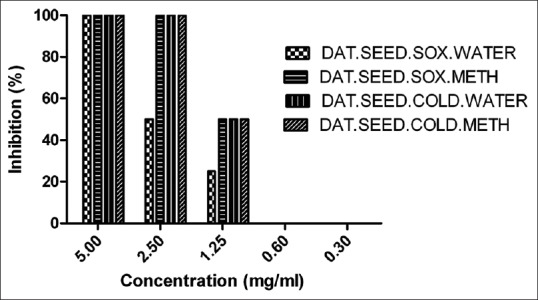
In vitro antiviral activity of Datura seed (soxhlet and cold) extract against rabies virus challenge virus standard. The IC50 was calculated by GraphPad Prism version 5.0 software
After 72 h post inoculation (p.i.), the RNA was extracted from the Vero cell line and rRT-PCR was performed to estimate the relative viral load in presence of the experimental extracts. The Datura seed cold methanol extracts exhibited >9000-fold reduction in the viral load with respect to control both at 5 mg/ml and 2.5 mg/ml concentration. Over 7000-fold reduction in the viral load was shown by the dautra ayurvedic extract and Datura seed cold (water) extract at 5 mg/ml and 2.5 mg/ml and Datura seed soxhlet (water and methanol) exhibited up to 5000-fold reduction in the viral load with respect to the virus control both at 5 mg/ml and 2.5 mg/ml. Whereas Datura fruit (soxhlet methanol and cold water) extracts exhibited up to 8000-fold reduction in the viral load with respect to control at 5 mg/ml concentration. The Datura fruit (soxhlet water) extract exhibited up to 4000-fold reduction and Datura fruit (cold methanol) extracts exhibited >4000-fold reduction in the viral load as compared to the virus control at 5 mg/ml. No fold reduction of the viral load was observed in the Datura seed and fruit (soxhlet and cold) extracts at 1.25 mg/ml.
DISCUSSION
Rabies, a disease caused by highly neurotropic rabies virus, is of public health importance especially since it invariably proves to be fatal after the onset of symptoms. Despite being fully preventable provided wound toileting and post-exposure prophylaxis is opted for, it is still responsible for nearly 20,000 deaths annually from India alone. Rabies infection is fatal once the virus enters the CNS. Antiviral chemotherapy is an important component in the management of viral diseases. However, at present, potent antivirals for clinical usage are available only against limited number of family of viruses (Orthomyxoviridae, Herpesviridae, and Retroviridae). There are no effective antiviral drugs available for rabies virus only palliative support and some experimental protocols to avoid death have been reported in the past.[13] Plant secondary metabolites have tremendous potential to be antiviral agents. D. metel has long been known for its sedative action and known to cure hydrophobia in Ayurveda. Thus, this medicinal plant may, therefore, be explored for potential antiviral activity against Rabies virus causing encephalitis.
In the present study, we evaluated the antiviral activity of D. metel against rabies virus. In vitro cytotoxic activity of soxhlet and cold extracts of Datura (fruit and seed) was performed in Vero cell line using MTT assay. The CC50 of Datura fruit (water and methanol) soxhlet extracts was found to be 5 mg/ml and 4 mg/ml, respectively, and of cold extracts (water and methanol) was found to be 7 mg/ml and 3 mg/ml, respectively [Figure 2]. Whereas the CC50 Datura seed (water and methanol) soxhlet extracts were found to be 7.5 mg/ml and 5 mg/ml, respectively, and of cold extracts (water and methanol) was found to be 3.5 mg/ml and 5.5 mg/ml, respectively [Figure 3]. The CC50 was calculated and the percent cytotoxicity was represented using GraphPad Prism version 5.0 software.
RV CVS was titrated (10−1 to 10−6) in Vero cell line in 96-well plate and the TCID50 was calculated by Reed–Muench method and the titer of 104 TCID50/ml of RV CVS was obtained [Figure 1]. The challenge dose of 10 TCID50 (10−3 TCID50/ml) was prepared for in vitro antiviral assay. Based on the CC50 of Datura extracts, minimum cytotoxic concentration (5–0.3 mg/ml) was used against the challenge dose of 10 TCID50 RV CVS. The IC50 (50% inhibition) of Datura fruit (soxhlet and cold) extract was found to be 2.5 mg/ml [Figure 4] and of Datura seed (water Soxhlet) was found to be 2.5 mg/ml whereas the IC50 of Datura seed (methanol soxhlet and cold extract) was found at 1.25 mg/ml [Figure 5]. The overall selectivity index (SI = CC50/IC50) of Datura seed extracts (SI = 4) was found to be higher than the Datura fruit extracts (SI = 2) which indicated that the Datura seed extracts had a higher antiviral activity against rabies virus.
Similar studies were conducted by Müller et al. where antirabies virus activity was detected for Allamanda schottii (MeOH extract-leaves) up to 230 µg/ml with an SI = 5.6.[14] Ribavirin has shown an in vitro antiviral activity against rabies virus infected with neuronal cells but fails to provide benefit in experimental rabies in mice.[15]
Molecular analysis by rRT-PCR also demonstrated reduction in the viral load by the extracts. High level of viral load reduction was found in all the extracts at 2.5 mg/ml. Also, the seed soxhlet methanol, seed cold aqueous, and the fruit cold aqueous extracts exhibited a 2-fold reduction of viral load at 1.25 mg/ml. Previous studies also used RT-PCR-based viral load detection as a measure of antiviral activity [Figure 6].[16,17]
Figure 6.
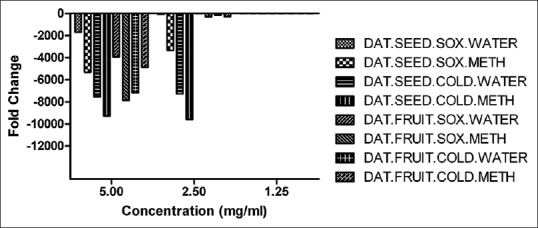
In vitro antiviral activity of Datura (soxhlet and cold) extract against rabies virus challenge virus standard by real-time reverse transcription-polymerase chain reaction method and fold change of the rabies virus represented by GraphPad Prism version 5.0 software
Earlier studies suggest that D. metel extracts have a wide range of antibacterial and antifungal activity.[18] One of the earliest attempts reports back to 1978 where Tsiang et al. showed that a condensed mineral ion, ammonium-5-tungsto-2-antimoniate inhibited in vitro multiplication of rabies virus without affecting the viability of cells.[19] More recently, Chávez et al. evaluated 24 synthetic phenolic compounds for their activity against PV strain of rabies virus.[20] Based on the preliminary results, they suggested the presence of some structure-activity relationship existing between the six compounds that inhibited viral cytopathic effect.
Datura is a wild plant having various medicinal and pharmacological properties. Phytochemical of the plant are alkaloids, atropine, scopolamine, tannin, saponine, glycosides, phenol, sterols, lignins, fats, carbohydrates, and proteins.[21]
Datura plant contains high levels of tropane alkaloids such as atropine, hyoscyamine, and scopolamine. Atropine and scopolamine are muscarinic antagonists, which can be used to treat Parkinson's disease and parasympathetic stimulation of the eye, heart, urinary, respiratory, and gastrointestinal tract. Atropine and scopolamine are a class of compounds that inhibit parasympathetic nerve impulses by selectively blocking the binding of the neurotransmitter acetylcholine to its receptor in nerve cells.[6] Atropine also reported to have an antiviral activity. It inhibits the growth of enveloped viruses such as herpes simplex virus, influenza virus, new castle disease virus, sindbis, vaccinia, adenovirus, and Japanese encephalitis virus.[22]
Atropine also blocks the glycosylation of viral proteins of herpes virus, and hence the production of new infectious virus particles (virions). Virions formed in the presence of atropine are noninfectious.[23]
Our laboratory is actively involved in research of novel experimental moieties and exploring the antiviral activity of herbal and ayurvedic formulations against rabies virus and other viral encephalitis for potential antiviral drug. Thus, in our study, we observed that Datura had a potential antiviral activity of which Datura seed extracts had a greater (SI = 4)in vitro antiviral activity against rabies virus.
CONCLUSION
The study has explored the potential in vitro antirabies activity of D. metel (seed) extracts by means of RFFIT and PCR methods. Further research will be to scale up for in vivo toxicity and in vivo antirabies trial in murine model to evaluate the efficacy of D. metel (seed) extracts in future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR
Dr. Soumen Roy, Department of Virology, Haffkine Institute for Training, Research and Testing, Mumbai, Maharashtra, India. E-mail: soumenroy118@gmail.com
Acknowledgments
We would like to thank NCCS, Pune, for providing cell line and NIMHANS, Bengaluru, for providing RV CVS for the research work.
REFERENCES
- 1.Lafon M. Evasive strategies in rabies virus infection. Adv Virus Res. 2011;79:33–53. doi: 10.1016/B978-0-12-387040-7.00003-2. [DOI] [PubMed] [Google Scholar]
- 2.Rabies vaccines. WHO position paper. Wkly Epidemiol Rec. 2007;82:425–35. [PubMed] [Google Scholar]
- 3.Willoughby RE, Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352:2508–14. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- 4.Barceloux DG. Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals. Los Angeles, California: General and Introductory Medical Science: John Wiley and Sons; 2008. pp. 1877–8. [Google Scholar]
- 5.Pennachio M, Jefferson L, Havens K. Uses and Abuses of Plant Derived Smoke: Ethnobotany as Hallucinogen, Perfume, Incense, and Medicine. 198 Madison Avenue, New York, New York 10016: Oxford University Press; 2010. pp. 6–7. [Google Scholar]
- 6.Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JF. Primer on the Autonomic Nervous System. Elservier: Academic Press; 2011. pp. 77–8. [Google Scholar]
- 7.Patil D, Roy S, Dahake R, Rajopadhye S, Kothari S, Deshmukh R, et al. Evaluation of Jatropha curcas Linn. leaf extracts for its cytotoxicity and potential to inhibit hemagglutinin protein of influenza virus. Indian J Virol. 2013;24:220–6. doi: 10.1007/s13337-013-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meslin FX, Kaplan M, Koprowski H. Laboratory Techniques in Rabies. 4th ed. Geneva: WHO; 1996. pp. 181–91. [Google Scholar]
- 9.Mahy BW, Kangaro HO, editors. Virology Methods Manual. London; Sydney: Academic Press; 1996. [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Nagaraj T, Vasanth JP, Desai A, Kamat A, Madhusudana SN, Ravi V. Ante mortem diagnosis of human rabies using saliva samples: Comparison of real time and conventional RT-PCR techniques. J Clin Virol. 2006;36:17–23. doi: 10.1016/j.jcv.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Albertini AA, Ruigrok RW, Blondel D. Rabies virus transcription and replication. Adv Virus Res. 2011;79:1–22. doi: 10.1016/B978-0-12-387040-7.00001-9. [DOI] [PubMed] [Google Scholar]
- 14.Müller V, Chávez JH, Reginatto FH, Zucolotto SM, Niero R, Navarro D, et al. Evaluation of antiviral activity of South American plant extracts against herpes simplex virus type 1 and rabies virus. Phytother Res. 2007;21:970–4. doi: 10.1002/ptr.2198. [DOI] [PubMed] [Google Scholar]
- 15.Appolinário CM. Ribavirin has an in vitro antiviral effect in rabies virus infected neuronal cells but fails to provide benefit in experimental rabies in mice. J Virol Antivir Res. 2013;2:1–5. [Google Scholar]
- 16.Rothan HA, Zulqarnain M, Ammar YA, Tan EC, Rahman NA, Yusof R. Screening of antiviral activities in medicinal plants extracts against dengue virus using dengue NS2B-NS3 protease assay. Trop Biomed. 2014;31:286–96. [PubMed] [Google Scholar]
- 17.Li J, Huang H, Feng M, Zhou W, Shi X, Zhou P. In vitro and in vivo anti-hepatitis B virus activities of a plant extract from Geranium carolinianum L. Antiviral Res. 2008;79:114–20. doi: 10.1016/j.antiviral.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Kaushik P, Goyal P. In vitro evaluation of Datura innoxia (thorn-apple) for potential antibacterial activity. Indian J Microbiol. 2008;48:353–7. doi: 10.1007/s12088-008-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsiang H, Atanasiu P, Chermann JC, Jasmin C. Inhibition of rabies virus in vitro by the ammonium-5-tungsto-2-antimoniate. J Gen Virol. 1978;40:665–8. doi: 10.1099/0022-1317-40-3-665. [DOI] [PubMed] [Google Scholar]
- 20.Chávez JH, Leal PC, Yunes RA, Nunes RJ, Barardi CR, Pinto AR, et al. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet Microbiol. 2006;116:53–9. doi: 10.1016/j.vetmic.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Sayyed A, Shah M. Phytochemistry, pharmacological and traditional uses of Datura stramonium L. Rev J Pharmacogn Phytochem. 2014;2:123–5. [Google Scholar]
- 22.Yamazaki Z, Tagaya I. Antiviral effects of atropine and caffeine. J Gen Virol. 1980;50:429–31. doi: 10.1099/0022-1317-50-2-429. [DOI] [PubMed] [Google Scholar]
- 23.Alarcón B, González ME, Carrasco L. Antiherpesvirus action of atropine. Antimicrob Agents Chemother. 1984;26:702–6. doi: 10.1128/aac.26.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]


