Abstract
Background:
Carissa congesta (CC), Polyalthia longifolia (PL), and Benincasa hispida (BH) are economically important plants.
Objective:
Current research encompasses identification of quercetin and rutin and their analogues by liquid chromatography-mass spectroscopy (LC-MS) from the selected plant species.
Materials and Methods:
Fresh roots, leaves, and seeds of CC, PL, and BH plants respectively were shade-dried followed by extraction and elucidation of rutin and quercetin by LC-MS.
Results:
Structural elucidation of CC, PL, and BH extracts revealed the presence of flavonoids such as quercetin (m/z 301) and rutin (m/z 610) as the parent ions along with presence of close analogues such as quercetin-O-hexoside, Vicenin 2, quercetin-3-O-xyloside/arabinoside, and quercetin-3-O-glucoside were identified as fragments.
Conclusions:
Thus, CC, PL, and BH extracts revealed the presence of flavonoids belonging to the class of flavonols such as rutin and quercetin.
SUMMARY
Quercetin and rutin were identified from CC roots, PL leaves and BH seeds by liquid chromatography-mass spectroscopy.
Quercetin was characterized at (m/z 301) and rutin (m/z 610) as the parent ion peaks.
Analogues such as quercetin-O-hexoside, Vicenin 2 and quercetin-3-O-glucoside were identified as fragments.
Key words: Benincasa hispida, Carissa congesta, Liquid chromatography, Mass spectroscopy, Polyalthia longifolia, Quercetin, Rutin
INTRODUCTION
About 2% of all carbon photosynthesized by plants are converted into flavonoids or its closely related analogues.[1] They occur abundantly in flowers, fruits and leaves of plants.[2] Flavonoids may occur in various forms which can be obtained by hydroxylation, methylation and glycosylation. Aromatic and aliphatic acids, sulfate, prenyl, methylenedioxyl, or isoprenyl groups gets attached to the flavonoid nucleus while aglycones exist in wax coating on leaves and barks.[3] Flavonoids are commonly found as mixtures in various plants extracts. However, similarities in their structures and polarities have made identification of each compound difficult. However, emerging powerful advanced techniques of liquid chromatography coupled with mass spectroscopy (LC-MS) has paved the way for identification of flavonoids quiet easily.[4,5]
Flavonoids are produced by a series of condensation reactions between hydroxycinnamic acid and malonyl residues (B-ring and carbon atoms 2, 3 and 4 of the C-ring with A-ring) resulting in formation of C6-C3-C6 basic structure. In addition, the three-carbon bridges between the phenyl rings are commonly cyclized to form a third ring (C-ring). Cyclization with the degree of unsaturation and oxidation of the three-carbon segment allows them to be put into different classes.[3] Based on the C-ring, flavonols can be subdivided as flavonols (with 2, 3-double bond, 3-OH and 4-keto groups), flavones (with 2, 3-double bond and 4-keto group), dihydroflavonols or flavanonols (with 3-OH and 4-keto groups), flavanones (with 4-keto groups) and flavonols (with 3-OH groups).
In flavonoids, precise characteristic fragments of A- and B-rings have been interpreted while additional identification of unknown flavonoids can be studied based on their comparison with standard bio-markers and ultraviolet-visible (UV-Vis) spectra.[6] Two common absorbance bands are A and B (rutin and quercetin) that lies in the range of 310–350 nm (flavones) and 350–385 nm (flavonols). Band B with range of 250–290 nm are almost identical in all the above mentioned flavonoid subgroups. In case of flavanones and dihydroflavonols, Band A is frequently observed with little reduction more than a shoulder at 300–330 nm and Band B with range of the 277–295 nm range, is the characteristic peak.[7]
It is reported that plants containing polyhydroxy substituted flavonoids have highest antioxidant activity. O-di-OH substitution of the B-ring has appeared to be the most favorable structural characteristic in these compounds. Inactive quinines are formed due to free radicals abstraction of the two hydroxyl hydrogens of B-ring. C-ring plays an important role in antioxidant potential. Entirely substituted C-ring (catecholic flavonols) represent greater antioxidant than corresponding members of the group.[8,9] UV-Vis spectra provide informative as well as interpretative tool for the characterization of C-ring, but MS could provide much accurate information ion the molecular mass (parent ion) as well as and on structural analogues.[10]
Flavonols act as scavengers of nitric oxide synthesis, inhibitors of xanthine oxidase, growth regulators, photosensitizors and chemotaxonomic markers.[3,11,12] They inhibit cyclooxygenase as well as lipoxygenase enzymes resulting in reduced platelet activation and aggregation, offers protection against cardiovascular diseases and inflammation, anticancer, anti-viral, anti-microbial, anti-hepatotoxic, anti-osteoporotic, anti-allergic, anti-spasmodic, and antiulcer agents.[2,10,13,14,15,16,17,18]
Researchers have interpreted the results based on mass elucidation with structural identification of different classes of flavonoids. However, fragmentation data was not satisfactory enough to analyze subgroups of these class of compounds. There are losses of smaller molecules or radicals from the (M + H) + ion e. g. 18 a.m.u. (H2O), 28 a.m.u. (CO), and 42 a.m.u. (CH2 CO). The depiction of C-ring structures is commonly based on the UV-Vis spectra or spectral information libraries which are not the trusted sources of information.[10]
Flavonols have 3-hydroxy substitution and are bounded with sugar moiety attached to carbon-carbon bond which makes them unique since they are resistant to acid hyrdrolysis.[19] Though there are hundreds of flavonal aglycones known, kaempferol, quercetin, and myricetin are commonly elucidated by MS. In quercetin, different O-substituted compounds are observed. In addition, more than 200 quercetin glycosides have been noticed of which quercetin-3-rutinoside is locally known as rutin. In current research studies, a logical positive ionization mode LC-MS analysis of flavonols was attempted. The research paper illustrates fragmentation patterns of rutin and quercetin from Carissa congesta (CC), Benincasa hispida (BH), and Polyalthia longifolia (PL) plant extracts as depicted in graphical abstract. The studies provide researchers with a view that the selected plant extracts elucidated good amount of flavonoids.
MATERIALS AND METHODS
Fresh selected parts of CC, PL and BH were collected, dried, authenticated, and extracted and preliminary phytochemical constituents were identified as reported by us. In addition, we have identified rutin as a constituent from PL leaves extract by high-performance thin layer chromatography and high-performance LC.[20,21,22]
The instrument used in the experimentation was as follows:
LC-MS instrument: Varian Inc, USA
Model: 410 Prostar Binary LC with 500 MS IT PDA Detectors
Column: ZORBAX ECLIPSE XDB-C18 with Marrow Bore 2.1 × 150 mm 5 micron
-
Mobile phase: The plant extracts were dissolved in mobile phase as mentioned below:
- CC and PL − 0.1% formic acid + H2O (60: 40)
- BH – Acetonitrile.
RESULTS
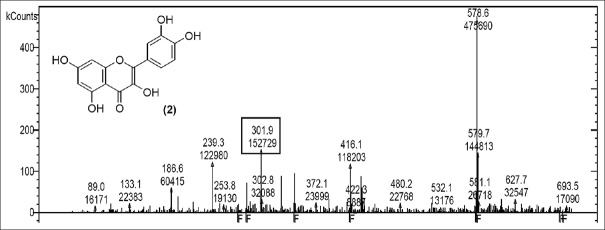
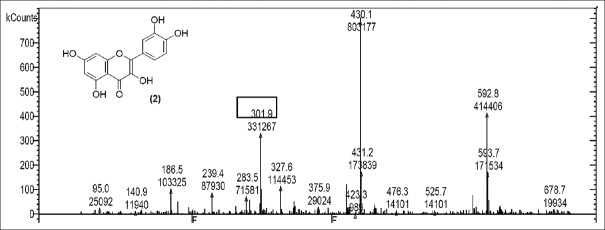
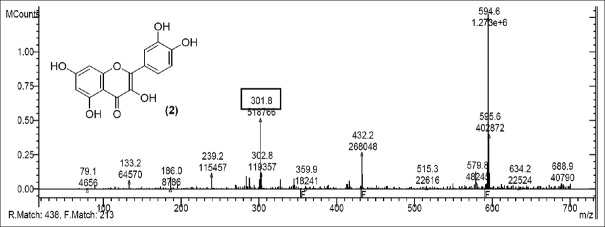
LC-MS studies revealed the presence of the components from the CC, PL, and BH extracts as mentioned in Tables 1–3. Quercetin [Figure 1] was found in roots of CC (Apocyanaceae) having a characteristic peak at m/z 301.9 (M) [Figure 1] confirmed with available references in the literature.[5]
Table 1.
Various components and their fragments in Carissa congesta extract
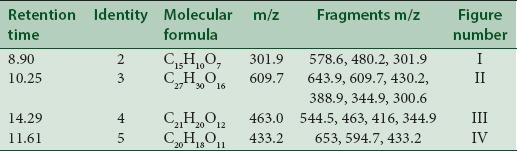
Table 3.
Various components and their fragments in Benincasa hispida extract
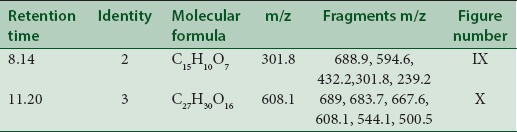
Figure 1.
Mass spectrum showing presence of quercetin (2) in Carissa congesta extract
Table 2.
Various components and their fragments in Polyalthia longifolia extract
MS spectrum showing m/z 480.2 (M + glucoside) consisted of glucoside m/z 178 suggested the addition of glucoside molecule and m/z at 578.6 (M + glucosyl glucoside) consisted of glucosyl glucoside m/z 276 suggesting addition of glucosyl gluconide.
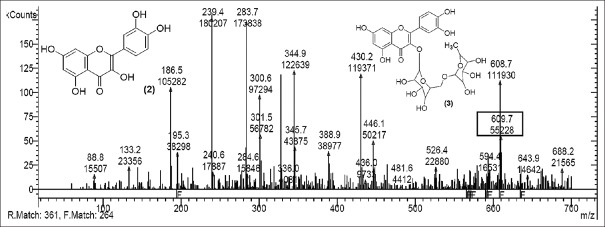
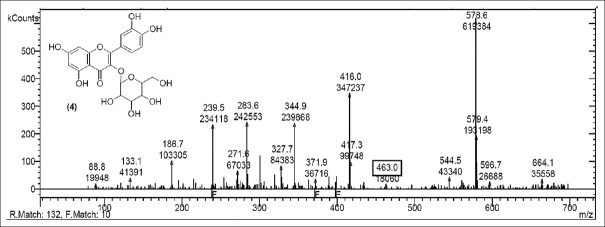
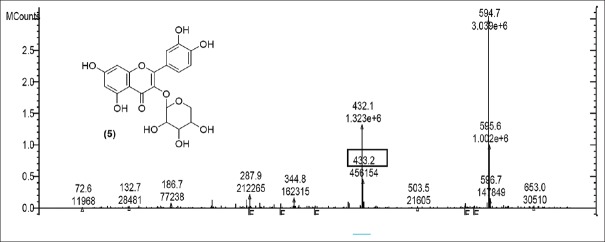
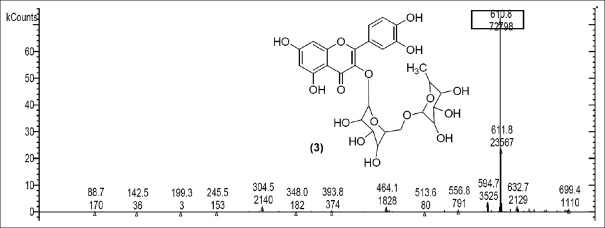
Rutin [Figure 2] was present in CC extract showed molecular ion at m/z 609.7 (M). Further peaks obtained at m/z 643.9 (M + 2H2O) refers to addition of formate ion. m/z 430.2 (M – O – glucosyl) refers to loss of O-glucosyl as a fragment. (M – O-glucosyl – CO2) refers to loss of O-glucosyl with CO2 while (M – O-glucosyl – HCOO−) having m/z 344.9 refers to loss of O-glucosyl with three molecules of CO2. Other expected compounds detected from the CC extract were quercertin-O-hexoside [Figure 3] having m/z 463.0 and its fragment ion observed at m/z 544.5, indicating loss of formic acid which was seen at m/z 416.0 and m/z 344.9 (M – HCOOH). Isomers quercetin-3-O-xyloside/arabinoside [Figure 4] has shown a characteristic peak at m/z 433.2. Fragments at m/z 594.7 (M + glucosyl) referred addition of glucose and m/z 653.0 (M + glucosylacetate) showed addition of glucosylacetate.
Figure 2.
Mass spectrum showing presence of quercetin (2) and Rutin (3) in Carissa congesta extract
Figure 3.
Mass spectrum showing presence of quercetin-O-hexoside (4) in Carissa congesta extract
Figure 4.
Mass spectrum showing presence of quercetin-3-O-xyloside/arabinoside (5) in Carissa congesta extract
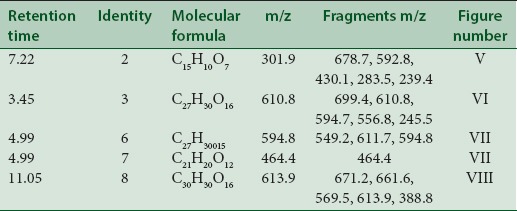
Quercetin [Figure 5] was found in the leaves of PL at m/z 301.9 (M). Fragments were observed at m/z 678.7 which were assumed to be diacyl prenyl quercetin-3-O-glycoside. Fragments ions observed at m/z 592.8 were expected to be derivative of prenyl-3-O-glycoside. Another fragment was observed at m/z 430.1 shows [prenyl quercetin + CO2].
Figure 5.
Mass spectrum showing presence of quercetin (2) in Polyalthia longifolia extract
Ions at m/z 283.5 shows (M-H2O) removal of water molecule while ions at m/z 239.4 indicate elimination of formic acid with water molecule (M – HCOOH – H2O) or elimination of water molecule with CO2 (M-H2O-CO2).
Rutin [Figure 6] was spotted at m/z 610.8 (M). Fragment ions at m/z 699.4 (M + 2CO2) while 594.7 (M – O+) consisting oxyen ion, m/z 556.8 [M– 3H2O] indicates elimination of three water molecules and m/z 245.5 (M – CH3 COOH) indicates elimination of acetic acid.
Figure 6.
Mass spectrum showing presence of rutin (3) in Polyalthia longifolia extract
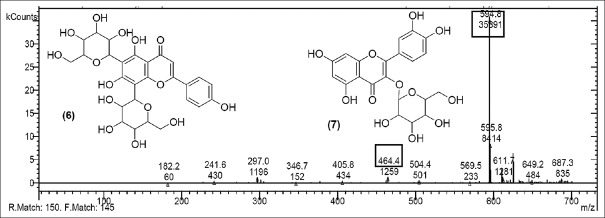
Quercetin derivative vicenin 2 [Figure 7] was seen at m/z 594.8 (M) with molecular peaks at m/z 611.7 (M + O+) which shows addition of oxygen ion and m/z 649.2 (M + 3H2O) shows addition of three water molecules.
Figure 7.
Mass spectrum showing presence of Vicenin 2 (6) and quercetin-3-O-glucoside (7) in Polyalthia longifolia extract
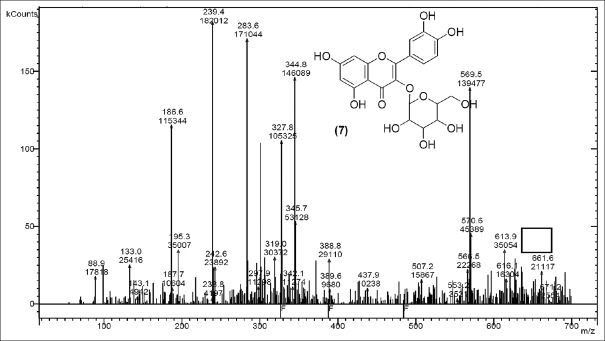
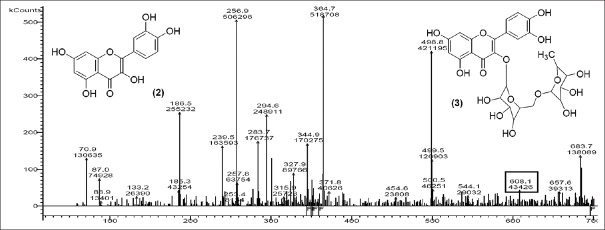
Quercetin-3-O-glucoside [Figure 7] with m/z at 464.4 (M) and quercetin-O-(O-galloyl) hexoside [Figure 8] m/z 613.9 (M) were identified. The other fragments obtained were m/z 661.6 (M + HCOOH) referred as addition of formic acid, m/z 569.5 [M – CO2] referred as elimination of CO2, m/z 388.8 (M – CO2– hexoside) refers to elimination of hexoside with CO2 and m/z 671.2 (M + CH3 COO−) shows addition of acetate ion.
Figure 8.
Mass spectrum showing presence of quercetin-O-(O-galloyl)-hexoside (8) in Polyalthia longifolia extract
Quercetin [Figure 9] was probably identified in the seeds of BH which showed molecular ion peak at m/z 301.8. The fragment ion obtained at m/z 432.2 (M + 3-O-arabinoside) or (M + 3-O-xyloside) showed addition of O-arabinoside or O-xyloside, respectively. Another fragment having m/z 594.6 (M + 3-O-glucosylarabinoside/xyloside) suggests addition of O-glucosylarabinoside/xyloside while m/z 634.2 (M + 3-O-glucosyl arabinoside/xyloside + 2H2O) suggests addition of O-glucosylarabinoside/xyloside with two water molecules. Two more fragments with m/z 688.9 (M + 3-O-glucosylarabinoside/xyloside) and 239.2 (M + CH3 COOH) indicates addition of O-glucosylarabinoside/xyloside with five molecules of water and two molecules of acetic acid.
Figure 9.
Mass spectrum showing presence of quercetin (2) in Benincasa hispida extract
Rutin [Figure 10] was characterised at m/z 608.1 (M) along with fragments at m/z 667.6 (M + CH3 COOH) showing addition of acetic acid. Other peaks at m/z 683.7 (M + CH3 CH2 COOH) showing addition of propionic acid and m/z 544.1 (M – CH3 COOH-4) suggesting the elimination of acetic acid with four hydrogen ions. Another fragment having m/z 500.5 (M – CH3 COOH-48) showed elimination of acetic acid, CO2 and four hydrogen ions. The m/z at 689 indicates presence of rutin sulfate.
Figure 10.
Mass spectrum showing presence of quercetin (2) and rutin (3) in Benincasa hispida extract
DISCUSSION
In the current study, flavonols (rutin and quercetin) and their analogues were characterized from plant. LC-MS analysis revealed that both compounds and their analogues possess fragmentation behavior with protonation which was concerned with dehydration, loss of CO and C-ring fission. Any unknown flavanol aglycone can be directly interpreted according to C-ring identification, dehydration pattern and CO losses of protonated molecule as well as produced ions. Fragementation pattern was associated with the applied collision energy. If the collision energy is less than main fragment in the MS, the spectra produced was (M + H)+. However, by enhancing collision energy, a complete fragmentation of the protonated molecule can be obtained.[23,24,25,26,27]
CONCLUSIONS
Thus, CC, BH and PL extracts revealed the presence of flavanols belonging such as rutin and quercetin as parent ions and close analogues such as quercetin-O-hexoside, Vicenin 2, quercetin-3-O-xyloside/arabinoside, and quercetin-3-O-glucoside were identified as fragments.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Gaurav M. Doshi
Mr. Gaurav M. Doshi, Presently working as Assistant Professor, Department of Pharmacology, Vivekanand Education Society's College of Pharmacy, Chembur (East), Mumbai- 400074. He has 33 journal papers; 82 citations to his credit. He is Ph.D Research Scholar of Department of Pharmaceutical Sciences, Pacific Academy of Higher Education and Research University, Udaipur, Rajasthan, India.
Acknowledgment
We acknowledge the management of Vivekanand Education Society's College of Pharmacy, Mumbai for providing all facilities to carry out this work. We would also like to acknowledge Indian Institute of Technology, Mumbai, for their help in LC-MS analysis.
REFERENCES
- 1.Shropshire W., JR . Action spectroscopy. In: Mitrakos K, Shropshire W Jr, editors. Phytochrome. London: Academic Press; 1972. pp. 161–81. [Google Scholar]
- 2.Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–53. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 3.Iwashina T. The structure and distribution of the flavonoids in plants. J Plant Res. 2000;113:287–99. [Google Scholar]
- 4.Shahidi F. Chemistry, Health Effects and Practical Applications. Champaign, Illinois: AOCS Press; 1997. Flavonoids as antioxidants. Natural Antioxidants; p. 174. [Google Scholar]
- 5.Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V. Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules. 2007;12:593–606. doi: 10.3390/12030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dangles O, Fargeix G, Dufour C. One-electron oxidation of quercetin and quercetin derivatives in protic and non protic media. J Chem Soc. 1999;2:1387–95. [Google Scholar]
- 7.Tsimogiannis DI, Oreopoulou V. The contribution of flavonoid C-ring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3',4'-hydroxy substituted members. Innov Food Sci Emerg Technol. 2006;7:140–6. [Google Scholar]
- 8.Boué SM, Carter-Wientjes CH, Shih BY, Cleveland TE. Identification of flavone aglycones and glycosides in soybean pods by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003;991:61–8. doi: 10.1016/s0021-9673(03)00209-7. [DOI] [PubMed] [Google Scholar]
- 9.Bohm B. Introduction to Flavonoids. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998. Extraction, purification and identification of flavonoids; p. 200. [Google Scholar]
- 10.Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 11.van Acker SA, Tromp MN, Haenen GR, van der Vijgh WJ, Bast A. Flavonoids as scavengers of nitric oxide radical. Biochem Biophys Res Commun. 1995;214:755–9. doi: 10.1006/bbrc.1995.2350. [DOI] [PubMed] [Google Scholar]
- 12.Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, et al. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–6. doi: 10.1021/np970237h. [DOI] [PubMed] [Google Scholar]
- 13.Cos P, Calomme M, Sindambiwe JB, De Bruyne T, Cimanga K, Pieters L, et al. Cytotoxicity and lipid peroxidation-inhibiting activity of flavonoids. Planta Med. 2001;67:515–9. doi: 10.1055/s-2001-16472. [DOI] [PubMed] [Google Scholar]
- 14.Le Marchand L. Cancer preventive effects of flavonoids – A review. Biomed Pharmacother. 2002;56:296–301. doi: 10.1016/s0753-3322(02)00186-5. [DOI] [PubMed] [Google Scholar]
- 15.Pelzer LE, Guardia T, Osvaldo Juarez A, Guerreiro E. Acute and chronic antiinflammatory effects of plant flavonoids. Farmaco. 1998;53:421–4. doi: 10.1016/s0014-827x(98)00046-9. [DOI] [PubMed] [Google Scholar]
- 16.Hollman PC, Katan MB. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed Pharmacother. 1997;51:305–10. doi: 10.1016/s0753-3322(97)88045-6. [DOI] [PubMed] [Google Scholar]
- 17.Hollman PC. Evidence for health benefits of plant phenols: Local or systemic effects. J Sci Food Agric. 2001;81:842–52. [Google Scholar]
- 18.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 19.Harborne JB. First Indian Reprint. 3rd ed. New Delhi, India: Springer (India) Private Limited Publication House; 2005. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; pp. 1–295. [Google Scholar]
- 20.Doshi GM, Chaskar PK, Zine SP, Une HD. Cold extraction of Carissa congesta wight monitored by a comparative revision of HPLC and HPTLC. Pharmacogn Commun. 2014;4:29–33. [Google Scholar]
- 21.Doshi GM, Une HD. Chromatographic studies on Benincasa hispida (thunb.) Cogn Seed extract scrutinized by HPLC and HPTLC. Pharmacogn J. 2014;6:42–8. [Google Scholar]
- 22.Doshi GM, Zine SP, Chaskar PK, Une HD. Solicitation of HPLC and HPTLC techniques for determination of rutin from Polyalthia longifolia Thwaites. Pharmacognosy Res. 2014;6:234–9. doi: 10.4103/0974-8490.132601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabre N, Rustan I, de Hoffmann E, Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J Am Soc Mass Spectrom. 2001;12:707–15. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 24.March ER, Miao XS. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int J Mass Spectrom. 2004;231:157–67. [Google Scholar]
- 25.Hughes JR, Croley RT, Metcalfe DC, March ER. A tandem mass spectrometric study of selected characteristic flavonoids. Int J Mass Spectrom. 2001;210-211:371–85. [Google Scholar]
- 26.Wu W, Yan C, Li L, Liu Z, Liu S. Studies on the flavones using liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr A. 2004;1047:213–20. doi: 10.1016/j.chroma.2004.06.128. [DOI] [PubMed] [Google Scholar]
- 27.Wu W, Liu Z, Song F, Liu S. Structural analysis of selected characteristic flavones by electrospray tandem mass spectrometry. Anal Sci. 2004;20:1103–5. doi: 10.2116/analsci.20.1103. [DOI] [PubMed] [Google Scholar]