Abstract
Background:
Curcuma xanthorrhiza is a native Indonesian plant and traditionally utilized for a range of illness including liver damage, hypertension, diabetes, and cancer.
Objective:
The study determined the effects of C. xanthorrhiza extracts (ethanol and aqueous) and their constituents (curcumene and xanthorrhizol) on UDP-glucuronosyltransferase (UGT) and glutathione transferase (GST) activities.
Materials and Methods:
The inhibition studies were evaluated both in rat liver microsomes and in human recombinant UGT1A1 and UGT2B7 enzymes. p-nitrophenol and beetle luciferin were used as the probe substrates for UGT assay while 1-chloro-2,4-dinitrobenzene as the probe for GST assay. The concentrations of extracts studied ranged from 0.1 to 1000 μg/mL while for constituents ranged from 0.01 to 500 μM.
Results:
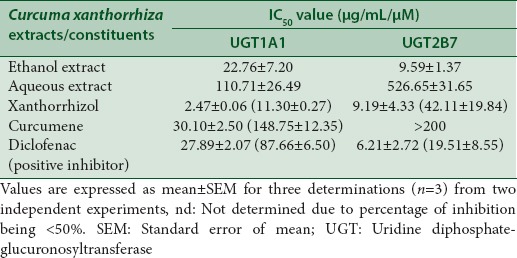
In rat liver microsomes, UGT activity was inhibited by the ethanol extract (IC50 =279.74 ± 16.33 μg/mL). Both UGT1A1 and UGT2B7 were inhibited by the ethanol and aqueous extracts with IC50 values ranging between 9.59–22.76 μg/mL and 110.71–526.65 μg/Ml, respectively. Rat liver GST and human GST Pi-1 were inhibited by ethanol and aqueous extracts, respectively (IC50 =255.00 ± 13.06 μg/mL and 580.80 ± 18.56 μg/mL). Xanthorrhizol was the better inhibitor of UGT1A1 (IC50 11.30 ± 0.27 μM) as compared to UGT2B7 while curcumene did not show any inhibition. For GST, both constituents did not show any inhibition.
Conclusion:
These findings suggest that C. xanthorrhiza have the potential to cause herb-drug interaction with drugs that are primarily metabolized by UGT and GST enzymes.
SUMMARY
Findings from this study would suggest which of Curcuma xanthorrhiza extracts and constituents that would have potential interactions with drugs which are highly metabolized by UGT and GST enzymes. Further clinical studies can then be designed if needed to evaluate the in vivo pharmacokinetic relevance of these interactions
Abbreviations Used: BSA: Bovine serum albumin, CAM: Complementary and alternative medicine, cDNA: Complementary deoxyribonucleic acid, CDNB: 1-Chloro-2,4-dinitrobenzene, CuSO4.5H2O: Copper(II) sulfate pentahydrate, CXEE: Curcuma xanthorrhiza ethanol extract, CXAE: Curcuma xanthorrhiza aqueous extract, GC-MS: Gas chromatography-mass spectroscopy, GSH: Glutathione, GST: Glutathione S-transferase, KCl: Potassium chloride, min: Minutes, MgCl2: Magnesium chloride, mg/mL: Concentration (weight of test substance in milligrams per volume of test concentration), mM: Milimolar, Na2CO3: Sodium carbonate, NaOH: Sodium hydroxide, nmol: nanomol, NSAIDs: Non-steroidal antiinflammatory drug, p-NP: para-nitrophenol, RLU: Relative light unit, SEM: Standard error of mean, UDPGA: UDP-glucuronic acid, UGT: UDP-glucuronosyltransferase.
Key words: Curcuma xanthorrhiza, glutathione transferase, UDP-glucuronosyltransferase, xanthorrhizol
INTRODUCTION
Curcuma xanthorrhiza or commonly known as “temulawak” or Javanese turmeric is a member of the ginger family (Zingeberaceae), which is a native Indonesian plant. It is planted in Thailand, Philippines, Sri Lanka, and Malaysia. C. xanthorrhiza is a low-growing plant with a root (rhizome) that looks like ginger.[1] Traditionally, this plant is used as an ingredient in health supplements known as “jamu” or to cure certain health problems including hepatitis, liver complaints, diabetes, rheumatism, anticancer, hypertension, and heart disorders.[1,2]
C. xanthorrhiza has also shown antidiuretic, anti-inflammatory, antioxidant, antihypertensive, antihepatotoxic, antibacterial, and antifungal effects.[1] Different phytochemicals found in the herbs have the potential to modulate drug-metabolizing enzymes activities, thus resulting in herb-drug interaction.[3] The traditional benefits of C. xanthorrhiza were further confirmed by the isolation and identification of several constituents, including xanthorrhizol and curcumene, and a few volatile substances. Xanthorrhizol is the major component of the essential oil of C. xanthorrhiza, which is a bisabolene-type sesquiterpenoid obtained through hydrodistillation.[4,5,6] The presence of xanthorrhizol in C. xanthorrhiza differentiates this plant from other Curcuma species. Xanthorrhizol has been reported to encompass a wide range of biological activities such as antibacterial, antiseptic, and antibiotic.[7] Curcumene, which is also a bisabolene-type sesquiterpenoid, is one of the constituents found in the essential oil of C. xanthorrhiza with content ranging from 2.6% to 13.6%.[5,6]
Phase II enzymes are becoming increasingly crucial in drug discovery and drug development. UDP-glucuronosyltransferase (UGT) and glutathione transferase (GST) are the major Phase II enzymes catalyzing the glucuronidation and glutathione (GSH) conjugation, respectively. Glucuronidation of xenobiotics is an important pathway for many diverse organisms to protect themselves against toxins, and the enzymes that detoxify xenobiotics by glucuronidation are the UGTs.[8] GSH conjugation, typically regarded as a detoxification reaction which is catalyzed by GST, is an important host defense mechanism, serving as a scavenger of electrophilic xenobiotics and their re-metabolites.[9]
Herb-drug interaction is becoming an important area of research due to increased concomitant use of herb and conventional drugs. The interaction may involve having a herb component causing an increase or decrease in the amount of drug in the blood through induction or inhibition of drug-metabolizing enzymes.[10] Alteration of drug metabolism by interference with herbal medicines is significantly important since most of the reported clinical adverse drug interactions were due to the modulation of drug-metabolizing enzymes activity. Furthermore, the rapid increase in the consumption of herbal products among herbal practitioners, and although health benefits may be derived from the use of C. xanthorrhiza, this herbal plant extracts and its constituents may have the potential to interact with coadministered drugs. Most of the studies of C. xanthorrhiza focused on its pharmacological activity, and up to date, no report of herb-drug interactions involving C. xanthorrhiza and Phase II drug-metabolizing enzymes has been published. However, previous studies have reported that this herbal plant did show a very low inhibition toward cytochrome P450 (CYP450) enzymes.[11,12] This situation has triggered the investigation on the effects of C. xanthorrhiza extracts and its constituents on Phase II drug-metabolizing enzymes activity in vitro.
MATERIALS AND METHODS
Chemical and reagents
Bovine serum albumin, potassium sodium tartrate tetrahydrate (KNaC4H4O6.4H2O), copper (II) sulfate pentahydrate (CuSO4.5H2O), magnesium chloride (MgCl2), Triton X-100, p-nitrophenol (p NP), tris (hydroxymethyl) aminomethane hydrochloride (Tris-HCl), UDP-glucuronic acid (UDPGA) (trisodium salt), and diclofenac sodium salt were purchased from Sigma-Aldrich Co.(St. Louis, MO, USA). Ethanol (96%) was purchased from Merck (Darmstadt, Germany). Glycerol was purchased from BDH Chemicals LTD (Poole, UK). Potassium dihydrogen orthophosphate (KH2 PO4) was purchased from AJAX Chemicals (New South Wales, Australia). Sodium nitrate (NaNO3) and potassium chloride (KCl) were purchased from Bendosen Laboratory Chemicals (Selangor, Malaysia). Sodium carbonate (Na2 CO3) and sodium hydroxide (NaOH) were purchased from Systerm® (Shah Alam, Malaysia). Folin and Ciocalteu's phenol reagent were purchased from R and M Chemicals (Canada). Dipotassium hydrogen phosphate (KH2 PO4) was purchased from Riedel-de Häen, Seelze (Germany). Tannic acid was purchased from HmbG Chemicals (Germany). UGT-Glo™ Screening System was purchased from Promega, USA. Purified recombinant human GSTP1 was a gift from Associate Professor Dr. Mohd Nizam Mordi and Mr. Mohammed Nooraldeen Mahmod (Centre for Drug Research, Universiti Sains Malaysia).
Plant collection
Fresh rhizomes of C. xanthorrhiza were purchased from Temerloh, Pahang, Malaysia, in February 2010. The plant was authenticated by Mr. Shanmugam and deposited at the Herbarium Unit of the School of Biological Sciences, USM. The voucher specimen number of the plant is USM11022. The rhizomes were air-dried, ground into fine powder, and stored prior usage.
Standardization of extracts
C. xanthorrhiza extracts were standardized in reference to the content of its constituents, xanthorrhizol, and curcumene. Both xanthorrhizol and curcumene were measured using gas chromatography mass spectrometry as reported by Ab-Halim et al.[5] Compared to curcumene, xanthorrhizol was found to be higher in both ethanol and aqueous extracts (ethanol: 22.89%, aqueous: 3.41%), followed by curcumene (ethanol extract: 13.61%, aqueous extract: 2.11%).[5]
Experimental animals
Male Sprague Dawley rats (150–200 g) were obtained from the Animal House of USM. The rats were maintained under controlled temperature (25°C ± 2°C), 12 h light/12 h dark conditions for 1 week before the start of the experiments. They were provided with water and food ad libitium. Animals were maintained and handled according to the recommendations of the USM Ethical Committee which approved the design of the animal experiments with the reference number USM/Animal Ethics Approval/2011/(72) (340).
Preparation of rat liver cytosolic fraction and microsome
Rat livers were removed immediately after sacrifice and were rinsed with ice-cooled distilled water followed by ice-cooled 67 mM potassium phosphate buffer (pH 7.4), blotted dry, and weighed. Isolated rats liver samples were homogenized in 67 mM potassium phosphate buffer (pH 7.4) containing 1.15% (w/v) KCl where the volume of buffer is 3 times the weight of the liver samples using a Potter-Elvehjem homogenizer. After centrifugation of the homogenate fraction at 12,500 ×g for 20 min at 4°C, the resultant supernatant was decanted to ultracentrifuge tubes (Optiseal™) and centrifuged at 100,000 ×g for 60 min in Optima™ TLX refrigerated ultracentrifuge (Beckman Coulter, Inc., USA). The supernatant obtained was the cytosolic fraction. The microsomal pellet obtained was scrapped and each was resuspended in 300 µL 67 mM potassium phosphate buffer with 1.15% KCl and 20% (v/v) glycerol. The pooled microsomes were homogenated again to mix the solution properly. The cytosolic fractions and microsomes were aliquoted into several microfuge tubes and were stored at −80°C until used. Protein content of microsomes and cytosolic fractions were determined by a method by Lowry et al.[13]
Inhibition of rat liver UDP-glucuronosyltransferase enzyme by Curcuma xanthorrhiza extracts and constituents
The inhibition studies with rat liver microsome as the UGT source was carried out according to a previous method by Embola et al.,[14] with slight modification.[15] The typical 200 µL incubation mixture contained 0.01% (v/v) Triton X-100, 0.5 mg/mL of UGT enzymes (rat liver microsomes), different concentrations of C. xanthorrhiza, 0.1 M Tris HCl buffer, 0.005 MgCl2, and 0.5 mM p NP in polypropylene microfuge tubes. Microsomes were preincubated with Triton X-100 on ice for 5 min to activate UGT enzymes. The range of C. xanthorrhiza extracts determined was 0.1–1000 µg/mL while the concentration range for the positive inhibitor, diclofenac ranged from 0.1 to 1000 µM. The concentration range of C. xanthorrhiza constituents determined was from 0.02 to 200 µM. The final concentration of ethanol and dimethyl sulfoxide used in this study was <2% (v/v). Controls were prepared as described above except that no extract or constituent was added. Blank for control, xanthorrizol, and ar-curcumene contained the same as described above except without addition of Triton X-100, microsomes, constituents, and UDPGA. However, blank for each extract differ from the others since each concentration determined have their own blank. This is due to the presence of color in the extracts that may interfere with the absorbance reading. The reaction was started by addition of 30 mM UDPGA which was dissolved in distilled water. All tubes were vortexed and incubated in a shaking water bath at 37°C for 30 min. At the end of each incubation time, the reaction was stopped by adding 200 µL of 20% (w/v) ice-cold trichloroacetic acid. All tubes were vortexed for at least 2 min and incubated in ice for at least 10 min. Then, all tubes were centrifuged at 2000 rpm for 10 min. After centrifugation, 200 µL of supernatant from each tube was transferred into new polypropylene microfuge tubes and 800 µL of 0.5 M NaOH was added. Tubes were vortexed and incubated at room temperature for 10 min. After 10 min incubation, 200 µL of mixture was transferred to a 96 microtiter well plate. The absorbance at 405 nm was measured using a Plate CHAMELEON™ multitechnology plate reader, 425-106 (Hidex Oy, Finland).
Inhibition of human UDP-glucuronosyltransferase recombinant expressed enzyme by Curcuma xanthorrhiza extracts and constituents
The luminescent assay was conducted as described in the UGT-Glo™ Screening System protocol. For each inhibition study, the concentrations of extracts and positive control ranged from 0.1–1000 µg/mL to 0.1–1000 µM whereas the concentrations for constituents ranged from 0.02 to 200 µM. The highest concentration determined for constituents was only at 200 µM since maximum inhibition was observed at this concentration. Reactions were conducted in white 96-well luminometer plate using a 37°C incubator. The final reaction volume (40 µL per 96-well) consisted of UGT reaction buffer, 4 mM UDPGA or an equivalent volume of water, and 20 µM UGT multienzyme substrate with 0.1 mg/mL UGT1A1 or UGT2B7 supersomes. Negative control reactions contained the same components as UGT reactions, except the UGT supersomes were replaced with an equivalent amount of control microsomes, devoid of UGT activity. The amount of incubation time for the UGT1A1 reaction was 90 min while it was 60 min for the UGT2B7 reaction. The reaction was stopped by addition of 40 µL reconstituted luciferin detection reagent (LDR) and D-cysteine to all determined wells and further incubated at room temperature for 20 min to stabilize the luminescence signal. UGT activity was detected by the addition of LDR and luminescence signal was recorded as relative light units.
Inhibition of glutathione transferase enzyme by Curcuma xanthorrhiza extracts and constituents
The inhibition studies using rat liver GST and human GST Pi-1 (hGSTP1) were carried out according to a previous method[16] with slight modifications[17,18] using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. Incubation mixtures (300 μL for rat liver GST, 200 μL for hGSTP1) contained 0.1 M potassium phosphate buffer (pH 6.5) and 0.001 M of GSH. This was followed by addition of GST enzyme (0.125 mg/mL of rat liver GST, 0.003 mg/mL of hGSTP1) and different concentrations of extracts and compounds. The control sample contained the same as mentioned except that no extracts or compounds were added. The mixture for blank sample for each concentration of extracts and compounds also contained the same as mentioned except that the rat liver cytosolic fraction was denatured at 60°C for 20 min while blank sample for hGSTP1 was replaced with 0.1 M of potassium phosphate buffer (pH 6.5). The concentrations of plant extracts and tannic acid used ranged from 0.01 to 1000 µg/mL. The concentrations of pure compounds used in this study ranged from 0.01 to 500 µM. The reason for not determining the activity above 1000 µg/mL and 500 µM was because maximum absorbance was reached at the highest concentration determined. GST-mediated conjugation of CDNB to GSH was measured using a Plate CHAMELEON™ multitechnology plate reader, 425-106 (Hidex Oy, Finland) at wavelength of 340 nm for 5 min for rat liver GST while 20 min for hGSTP1. All experiments were carried out in 5 replicates.
Statistical analysis
All results are presented as mean ± standard error of mean of five determinations except for UGT luminescence assay (three determinations from two independent experiments). The IC50 values, concentration of inhibitor causing 50% inhibition of enzyme activity, were calculated by the nonlinear regression analysis of percentage of remaining enzyme activity (expressed as percent of control) versus the logarithm of inhibitor concentration. ANOVA followed by Dunnett test was used to evaluate the significant differences of the results obtained. P < 0.05 was considered statistically significant. All computations were performed using GraphPad Prism® 5 for Windows software (Version 5.01, GraphPad Software, Inc., USA).
RESULTS
Inhibition of rat liver UDP-glucuronosyltransferase enzyme by Curcuma xanthorrhiza extracts and constituents
Among the two extracts studied, ethanol extract gave an IC50 value of 279.74 ± 16.33 µg/mL, whereas the IC50 value for aqueous extract could not be determined since percentage of inhibition was <50% at the highest concentration determined [Figure 1]. However, the ethanol extract of C. xanthorrhiza showed weak inhibition since the activity of UGT enzyme was inhibited higher than 50% only at concentration of 1000 µg/mL. None of the C. xanthorrhiza constituents inhibited rat liver UGT enzyme activity; therefore, an accurate IC50 could not be determined [Figure 2]. Diclofenac which acted as the positive control for UGT enzymes showed an IC50 value of 402.04 ± 25.84 µM.
Figure 1.
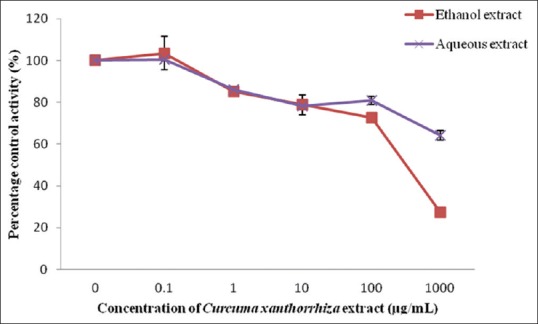
Effect of Curcuma xanthorrhiza extracts on UDP-glucuronosyltransferase activity in rat liver microsomes. Values are expressed as mean of relative UDP-glucuronosyltransferase-specific activity over control ± standard error of mean for five determinations (n = 5)
Figure 2.
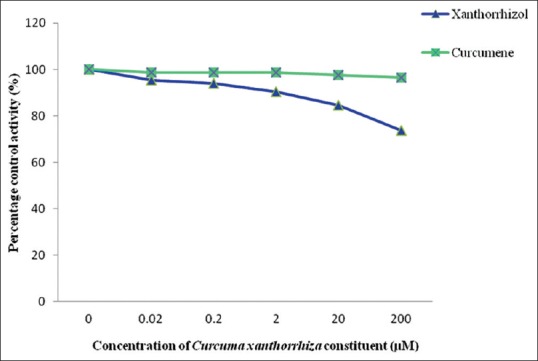
Effect of Curcuma xanthorrhiza constituents on UDP-glucuronosyltransferase activity in rat liver microsomes. Values are expressed as mean of relative UDP-glucuronosyltransferase-specific activity over control ĕ standard error of mean for five determinations (n = 5)
Inhibition of human UDP-glucuronosyltransferase recombinant expressed enzyme by Curcuma xanthorrhiza extracts and constituents
Ethanol extract exhibited stronger inhibition toward UGT2B7 with an IC50 value of 9.59 ± 1.37 µg/mL whereas a lower inhibition on UGT1A1 with an IC50 value of 22.76 ± 7.20 µg/mL was observed [Figure 3]. The IC50 values for the inhibition of UGT1A1 and UGT2B7 by the aqueous extract of C. xanthorrhiza were lower compared with the values of 110.71 ± 26.49 and 526.65 ± 31.65 µg/mL, respectively [Figure 4]. As for the two constituents of C. xanthorrhiza, xanthorrhizol exhibited higher inhibition toward UGT1A1 with an IC50 value of 11.30 ± 0.27 µM. With UGT2B7, xanthorrhizol exhibited moderate inhibition with an IC50 value of 42.11 ± 19.84 µM [Figure 5]. However, curcumene was found to have weaker inhibition toward UGT1A1 and UGT2B7 [Figure 6]. Diclofenac acted as the positive inhibitor for UGT1A1 and UGT2B7. Diclofenac was found to be a stronger inhibitor toward UGT2B7 rather than UGT1A1 with an IC50 value of 19.51 ± 8.55 µM compared to 87.66 ± 6.50 µM for UGT1A1. The IC50 value for UGT1A1 was 148.75 ± 12.35 µM while the IC50 value for UGT2B7 was higher than 200 µM. The IC50 values for extracts and constituents are summarized in Table 1.
Figure 3.
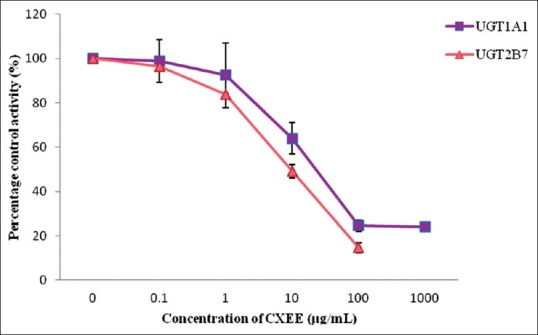
Effect of Curcuma xanthorrhiza ethanol extract on human recombinant UDP-glucuronosyltransferase activities. Values are expressed as mean of relative UDP-glucuronosyltransferase-specific activity over control ± standard error of mean for three determinations (n = 3) from two independent experiments
Figure 4.
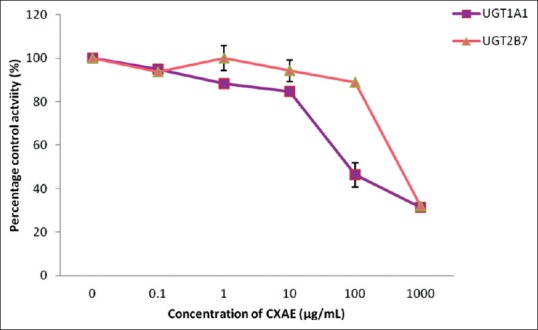
Effect of Curcuma xanthorrhiza aqueous extract on human recombinant UDP-glucuronosyltransferase activities. Values are expressed as mean of relative UDP-glucuronosyltransferase-specific activity over control ± standard error of mean for three determinations (n = 3) from two independent experiments
Figure 5.
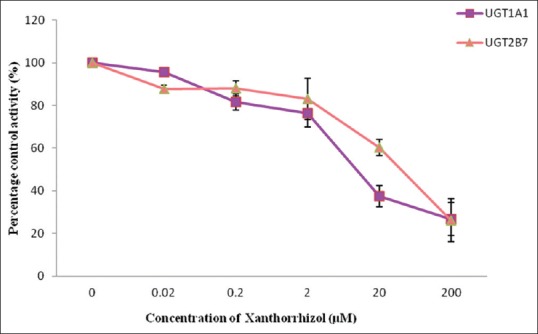
Effect of xanthorrhizol on human recombinant UDP-glucuronosyltransferases activities. Values are expressed as mean of relative UDP-glucuronosyltransferase-specific activity over control ± standard error of mean for three determinations (n = 3) from two independent experiments
Figure 6.
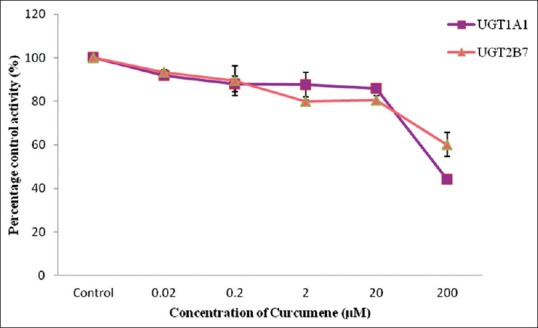
Effect of curcumene on human recombinant UDP-glucuronosyltransferases activities. Values are expressed as mean of relative UDP-glucuronosyltransferase-specific activity over control ± standard error of mean for three determinations (n = 3) from two independent experiments
Table 1.
IC50 values of Curcuma xanthorrhiza extracts and constituents on human UGT1A1 and UGT2B7 activities
Inhibition of rat liver glutathione transferase enzyme by Curcuma xanthorrhiza extracts and constituents
In this study, tannic acid which is the known inhibitor for GST enzyme showed an IC50 value of 4.83 ± 2.29 µg/mL. Among the two extracts determined, only the ethanol extract gave an IC50 value of 292.38 ± 20.16 µg/mL, whereas the IC50 value for aqueous extract could not be determined due to percentage of inhibition being <50% at the highest concentration determined none of the C. xanthorrhiza constituents inhibited GST enzyme activity in this study; therefore, an IC50 could not be determined since none of the constituents studied exhibited more than 50% of inhibition [Figures 7 and 8].
Figure 7.
Effect of Curcuma xanthorrhiza extracts on rat liver glutathione transferase enzyme activity. Values are expressed as mean of relative glutathione transferase-specific activity over control ± standard error of mean for five determinations (n = 5)
Figure 8.
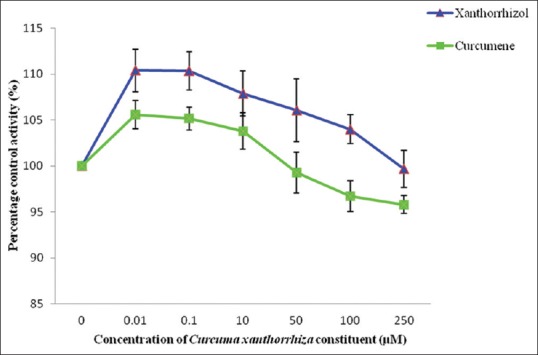
Effect of Curcuma xanthorrhiza constituents on glutathione transferase enzyme activity. Values are expressed as mean of relative glutathione transferase-specific activity over control ± standard error of mean for five determinations (n = 5)
Inhibition of human glutathione transferase Pi-1 by Curcuma xanthorrhiza extracts and constituents
Tannic acid which acted as the positive control for hGSTP1 showed an IC50 value of 4.39 ± 1.37 µM. Among the two extracts studied, ethanol extract showed an IC50 value of 128.88 ± 8.61 µg/mL while the IC50 value for aqueous extract was 726.04 ± 57.35 µg/mL. Ethanol extract was only determined until 250 µg/mL because maximum absorbance was reached at concentrations higher than that. As for the constituents, curcumene and xanthorrhizol did not exhibit 50% of inhibition toward hGSTP1 activity even at the highest concentration determined [Figures 9 and 10].
Figure 9.
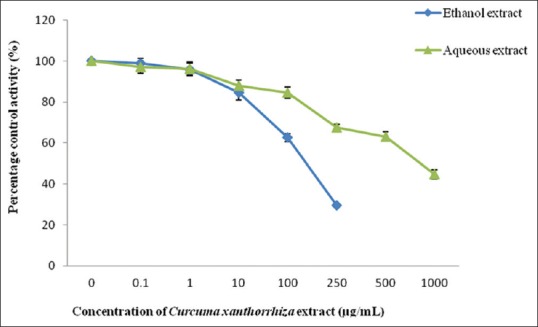
Effect of Curcuma xanthorrhiza extracts on human glutathione transferase Pi-1 activity. Values are expressed as mean of relative glutathione transferase-specific activity over control ± standard error of mean for five determinations (n = 5)
Figure 10.
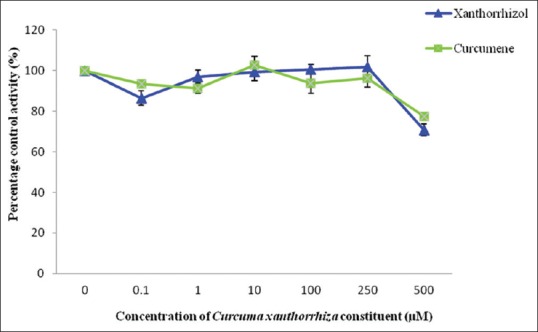
Effect of Curcuma xanthorrhiza constituents on human glutathione transferase Pi-1 activity. Values are expressed as mean of relative glutathione transferase-specific activity over control ± standard error of mean for five determinations (n = 5)
DISCUSSION
In this study, two different extracts of C. xanthorrhiza were used which were ethanol and aqueous extracts. The effects of the C. xanthorrhiza, as extracts in different solvents, were investigated since there might be chemical constituents with different solubility present which can modulate drug-metabolizing enzymes activity.[19] A total of two isolated constituents (curcumene and xanthorrhizol) of C. xanthorrhiza have been chosen in this study as these compounds have been reported as the major compounds, to which the therapeutic activities of this herb are mostly attributed.[5,6]
Taking into consideration of its ease of solubility and color changing property, pNP was chosen in this study to measure the UGTs activity in general, using rat liver microsomes as the enzyme source. pNP is primarily metabolized by the UGT1A6 isoform in the rat and human liver.[14] The unreacted amount of pNP can be measured by the spectrophotometric procedure.[20] UGT activity in rat liver microsomes was only inhibited by C. xanthorrhiza ethanol extract (CXEE) with an IC50 value of 279.74 ± 16.33 µg/mL. The aqueous extract weakly inhibited the UGT activity with 36% inhibition at 1000 µg/mL. None of C. xanthorrhiza constituents studied inhibited pNP-UGT enzyme activity in this study even at the highest concentration investigated; therefore, IC50 values could not be determined. Although this shows that metabolic interaction between C. xanthorrhiza and coadministered drugs metabolized by UGT enzymes are unlikely to occur, the possibility that the effect of C. xanthorrhiza on glucuronidation would be isoform specific cannot be ruled out. Hence, to justify the potential of C. xanthorrhiza extracts and constituents to modulate specific UGT isoforms activities, a UGT luminescence assay was conducted on human recombinant expressed UGT1A1 and UGT2B7 isoforms.
UGT1A1 and UGT2B7 are the most important UGT enzymes since both of them contribute to the glucuronidation of top 200 drugs prescribed in the United States in 2002.[21] Recombinant expressed enzymes are commonly used to predict drug-drug interactions risk which relates to the inhibition of enzyme.[22] The simplicity of this model allows the determination of the activity of specific isoforms involved in drug metabolism.[23] The evaluation of UGT1A1 inhibition demonstrated higher inhibition by ethanol extract of C. xanthorrhiza with an IC50 value of 22.76 ± 7.20 µg/mL versus an IC50 value of 110.71 ± 26.49 µg/mL for the aqueous extract. Similarly for UGT2B7, the ethanol extract of C. xanthorrhiza demonstrated a much higher inhibition than the aqueous extract. The IC50 values for the inhibition of UGT2B7 by ethanol extract were 9.59 ± 1.37 µg/mL compared to the aqueous extract of C. xanthorrhiza with an IC50 value of 526.65 ± 31.65 µg/mL. These findings may be due to the presence of a higher content of constituents such as phenols, flavonoids, coumarins, terpenoids, saponins, and cardiac glycosides in the ethanol extract as compared to their content in the aqueous extract which may serve to modulate UGT activity.[5]
The results as presented in Table 1 show that the ethanol extract is a better inhibitor for UGT2B7 than for UGT1A1. Interestingly, it can be seen that the inhibitory effect of the ethanol extract on UGT2B7 was not due to xanthorrizol alone since the content of xanthorrhizol was the highest in the ethanol extract (since xanthorrhizol individually inhibit UGT1A1 better than UGT2B7). This suggests that the presence of other constituents together with xanthorrhizol in the ethanol extract may contribute to the higher inhibition on UGT2B7. This implies that any uptake of products with high percentage of CXEE concurrently with therapeutic drugs primarily metabolized by UGT1A1 and UGT2B7 may cause herb-drug interaction. To exemplify, it can be speculated that when lovastatin, a drug to lower cholesterol level is metabolized by UGT1A1 or lorazepam, a drug for the treatment of anxiety disorders is metabolized by UGT2B7.[24] Thus, when these drugs were to be coadministered with products that contain high percentage of CXEE, inhibition of the metabolism of both drugs may occur.
A closer look at the UGT inhibitory capacities of the constituents showed xanthorrhizol having 3 times higher inhibitory effect on UGT1A1 compared to UGT2B7 (IC50 UGT1A1 = 11.30 ± 0.27 µM, UGT2B7 = 42.11 ± 19.84 µM). These findings indicated that products with high content of xanthorrhizol may cause metabolic interaction when metabolized with drugs that are highly metabolized by UGT1A1 and UGT2B7 isoforms. Another constituent found in C. xanthorrhiza, curcumene, did not show any inhibition toward UGT activity in both animal and human models used in this study. This is probably due to the structure of curcumene which does not possess any functional group that can be involved in glucuronidation (hydroxyl, phenols, amines, tertiary and heterocyclic amines, amides thiols, and acidic carbon atoms).[25]
The inhibition of GST by C. xanthorrhiza extracts and constituents was studied in rat liver cytosol and human recombinant GST Pi-1 enzyme. The reaction catalyzed by GST between GSH and an electrophilic substrate results in the formation of a thioether. CDNB can react enzymatically or nonenzymatically with nucleophiles.[17] The assessment of rat liver GST inhibition by C. xanthorrhiza extracts demonstrated that the higher inhibition was by CXEE with an IC50 value of 292.38 ± 20.16 µg/mL. This value was 60-fold higher than the IC50 value for tannic acid. However, an accurate IC50 value for aqueous extract could not be determined because percentage of inhibition was <50% at the highest concentration investigated. Previous study by Tan et al.[26] had also reported that the aqueous extract of C. xanthorrhiza did not show any significant inhibition toward rat liver GST activity. All two constituents of C. xanthorrhiza, xanthorrhizol, and curcumene demonstrated very low inhibition or no inhibition toward rat liver GST. In hGSTP1, the ethanol extract demonstrated higher inhibition (IC50 =128.88 ± 8.61 µg/mL) compared to inhibition observed for the aqueous extract, with an IC50 value of 726.04 ± 57.35 µg/mL, respectively. Using similar estimation made for UGT enzymes, the ethanol extract of C. xanthorrhiza exhibited higher inhibition than the aqueous extract toward rat liver GST and hGSTP1 activities. These findings indicated that inhibition of both GST enzymes by the ethanol extract may be caused by other constituents present in the ethanol extract since both xanthorrhizol and curcumene did not show inhibition when studied individually. Hence, inhibition demonstrated by the ethanol extract on hGSTP1 suggested that precautions should be taken when co-administering product with high CXEE with drugs that are highly metabolized by hGSTP1.
Since GSTs are a major group of Phase II detoxification enzymes, strong inhibition by CXEE could contribute to toxicity in humans, since in normal cells, GST inhibition results in increased toxicity due to a reduced protection against electrophilic chemicals or metabolites.[27] Herb-drug interaction involving GST isoforms remains unclear since there are no reports regarding drugs that are metabolized by specific GST isoform. However, the strong inhibitory activities exhibited by the ethanol extract of C. xanthorrhiza could have useful application in chemotherapy. The overexpression of GSTs in tumor cells after administration of anticancer drugs including chlorambucil, melphalan, and nitrosourea has stimulated the search for nontoxic GST inhibitors that could be useful therapeutic agents to modulate anticancer drug resistance.[28] Drug resistance in cancer cells can be overcome by the administration of chemomodulators that react as nontoxic reversing agents together with the anticancer agents.[29]
CONCLUSION
Based on our findings, inhibition of UGT activity shown by CXEE and xanthorrhizol indicates that product with high content of CXEE or xanthorrhizol may inhibit glucuronidation of substrates or drugs that are primarily metabolized by UGT1A1 and UGT2B7. Although C. xanthorrhiza extracts have lower inhibitory potential for herb-drug interactions toward rat liver GST and hGSTP1 activity, inhibition of GST by the ethanol extract suggests that this extract may act as an anticancer agent as well as chemopreventive agent. Nevertheless, the focus of this study was on UGT and GST enzymes; the modulatory effects of C. xanthorrhiza extracts and its constituents on other drug-metabolizing enzymes were not examined including CYP450, flavin-containing monooxygenases, sulfotransferase, and drug transporters such as P-glycoproteins. Hence, the mechanisms by which C. xanthorrhiza may interfere with these proteins are currently unknown. Further studies are needed to examine the clinical relevance of these observations in vivo.
Financial support and sponsorship
NKEA Research Grant Scheme (NRGS) from the Ministry of Agriculture and Agro-based Industry (MOA), Malaysia (Grant no: 304/CDADAH/650584).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Sabariah Ismail
Sabariah Ismail, is a Professor at the Centre for Drug Research, Universiti Sains Malaysia, Penang, Malaysia. She is currently appointed as the Director of Centre for Herbal Standardization, Universiti Sains Malaysia, Penang, Malaysia. Her research interest is in metabolic drug-drug interactions focusing on herb-drug interactions which involved phase II drug metabolizing enzymes including uridine diphospho glucuronosyltransferase (UGT) and glutathione S-transferase (GST).
REFERENCES
- 1.Sears B. Anti-Inflammation Zone – Reversing the Silent Epidemic That's Destroying Our Health. New York, USA: HarperCollins Publishers; 2005. pp. 75–87. [Google Scholar]
- 2.Ruslay S, Abas F, Shaari K, Zainal Z, Maulidiani, Sirat H, et al. Characterization of the components in the active fractions of health gingers (Curcuma xanthorrhiza and Zingiber zerumbet) by HPLC-DAD-ESIMS. Food Chem. 2007;104:1183–91. [Google Scholar]
- 3.Pan Y, Tiong KH, Abd-Rashid BA, Ismail Z, Ismail R, Mak JW, et al. Effect of eurycomanone on cytochrome P450 isoforms CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2E1 and CYP3A4 in vitro. J Nat Med. 2014;68:402–6. doi: 10.1007/s11418-013-0794-8. [DOI] [PubMed] [Google Scholar]
- 4.Cheah YH, Nordin FJ, Sarip R, Tee TT, Azimahtol HL, Sirat HM, et al. Combined xanthorrhizol-curcumin exhibits synergistic growth inhibitory activity via apoptosis induction in human breast cancer cells MDA-MB-231. Cancer Cell Int. 2009;9:1. doi: 10.1186/1475-2867-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ab-Halim MR, Tan MS, Ismail S, Mahmud R. Standardization and phytochemical studies of Curcuma xanthorrhiza Roxb. Int J Pharm Pharm Sci. 2012;4:606–10. [Google Scholar]
- 6.Jantan I, Saputri FC, Qaisar MN, Buang F. Correlation between chemical composition of curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evid Based Complement Alternat Med. 2012;2012:438356. doi: 10.1155/2012/438356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nur SW. Comparison of extraction system and validation technique to determine xanthorrhizol from Temulawak (Curcuma xanthorrhiza) using High Performance Liquid Chromatography (HPLC) Bogor: Faculty of Mathematics and Natural Science, Agricultural Institute of Bogor; 2006. [Google Scholar]
- 8.Kenneth B. Drug metabolism. In: Hacker P, Messer WS, Bachmann KA, editors. Pharmacology: Principles and Practice. United States of America: Academic Press; 2009. pp. 131–72. [Google Scholar]
- 9.Kumar GN, Surapaneni S. Role of drug metabolism in drug discovery and development. Med Res Rev. 2001;21:397–411. doi: 10.1002/med.1016. [DOI] [PubMed] [Google Scholar]
- 10.Fugh-Berman A, Ernst E. Herb-drug interactions: Review and assessment of report reliability. Br J Clin Pharmacol. 2001;52:587–95. doi: 10.1046/j.0306-5251.2001.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usia T, Iwata H, Hiratsuka A, Watabe T, Kadota S, Tezuka Y. CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine. 2006;13:67–73. doi: 10.1016/j.phymed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Hanapi NA, Azizi J, Ismail S, Manso SM. Evaluation of selected Malaysian medicinal plants on phase I drug metabolizing enzymes, CYP2C9, CYP2D6, and CYP3A4 activities in vitro. Int J Pharm. 2010;6:494–9. [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Embola CW, Sohn OS, Fiala ES, Weisburger JH. Induction of UDP-glucuronosyltransferase 1 (UDP-GT1) gene complex by green tea in male F344 rats. Food Chem Toxicol. 2002;40:841–4. doi: 10.1016/s0278-6915(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 15.Azizi J, Ismail S, Mansor SM. Mitragyna speciosa Korth leaves extracts induced the CYP450 catalyzed aminopyrine-N-demethylase (APND) and UDP-glucuronosyl transferase (UGT) activities in male Sprague-Dawley rat livers. Drug Metabol Drug Interact. 2013;28:95–105. doi: 10.1515/dmdi-2012-0039. [DOI] [PubMed] [Google Scholar]
- 16.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 17.Azizi J, Ismail S, Mordi MN, Ramanathan S, Said MI, Mansor SM. In vitro and in vivo effects of three different Mitragyna speciosa korth leaf extracts on phase II drug metabolizing enzymes – glutathione transferases (GSTs) Molecules. 2010;15:432–41. doi: 10.3390/molecules15010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musdal Y, Ertan-Bolelli T, Bolelli K, Yilmaz S, Ceyhan D, Hegazy U, et al. Inhibition of human glutathione transferase P1-1 by novel benzole derivatives. Turk J Biochem. 2012;37:431–6. [Google Scholar]
- 19.Pan Y, Abd-Rashid BA, Ismail Z, Ismail R, Mak JW, Pook PC, et al. In vitro effects of active constituents and extracts of Orthosiphon stamineus on the activities of three major human cDNA-expressed cytochrome P450 enzymes. Chem Biol Interact. 2011;190:1–8. doi: 10.1016/j.cbi.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Bock KW, Forster A, Gschaidmeier H, Brück M, Münzel P, Schareck W, et al. Paracetamol glucuronidation by recombinant rat and human phenol UDP-glucuronosyltransferases. Biochem Pharmacol. 1993;45:1809–14. doi: 10.1016/0006-2952(93)90437-2. [DOI] [PubMed] [Google Scholar]
- 21.Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, et al. Drug-drug interactions for UDP-glucuronosyltransferase substrates: A pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–8. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- 22.Bjornsson TD, Callaghan JT, Einolf HJ, Fischer V, Gan L, Grimm S, et al. The conduct of in vitro and in vivo drug-drug interaction studies: A pharmaceutical research and manufacturers of america (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–32. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- 23.Baranczewski P, Stanczak A, Sundberg K, Svensson R, Wallin A, Jansson J, et al. Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep. 2006;58:453–72. [PubMed] [Google Scholar]
- 24.Pharmacology Weekly. 2014. [Last accessed on 2014 Jul 10]. Available from: http://www.pharmacologyweekly.com/content/pages/ugt-enzymes-medications-herbs-substrate-inhibitor-inducer .
- 25.Lu H, Fang ZZ, Cao YF, Hu CM, Hong M, Sun XY, et al. Isoliquiritigenin showed strong inhibitory effects towards multiple UDP-glucuronosyltransferase (UGT) isoform-catalyzed 4-methylumbelliferone (4-MU) glucuronidation. Fitoterapia. 2013;84:208–12. doi: 10.1016/j.fitote.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Tan MS, Ab-Halim MR, Mustaffa F, Mohd-Ali NI, Ismail S, Mahmud R. Inhibitory effect of selected Malaysian herbal plants on glutathione S-transferase activity. Int J Pharmacol. 2011;7:349–55. [Google Scholar]
- 27.Appiah-Opong R, Commandeur JN, Axson C, Vermeulen NP. Interactions between cytochromes P450, glutathione S-transferases and Ghanaian medicinal plants. Food Chem Toxicol. 2008;46:3598–603. doi: 10.1016/j.fct.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Gyamfi MA, Ohtani II, Shinno E, Aniya Y. Inhibition of glutathione S-transferases by thonningianin A, isolated from the African medicinal herb, Thonningia sanguinea, in vitro. Food Chem Toxicol. 2004;42:1401–8. doi: 10.1016/j.fct.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Hayeshi R, Mukanganyama S, Hazra B, Abegaz B, Hasler J. The interaction of selected natural products with human recombinant glutathione transferases. Phytother Res. 2004;18:877–83. doi: 10.1002/ptr.1481. [DOI] [PubMed] [Google Scholar]


