Abstract
Background: Extracellular vesicles are particles ranged from 30 nm to 5μm and subcategorized into three groups; exosomes, microvesicles and apoptotic bodies, each of which have different biological impact. Lack of a standard method for the detection and isolation of MVs has led to a challenging issue that is a worth considering. In this study, we isolated MVs from the conditioned medium of UC-MSCs by four different schemes of ultracentrifugation.
Methods: We examined the efficacy of differential centrifugation ranging from 10,000×g to 60,000×g on UCMSCs- derived microvesicles yield and purity. The fractions were evaluated by Dynamic Light Scattering (DLS) method, total protein quantification and flow cytometry.
Results: UC-MSCs were spindle cells that adhered to plastic culture flasks. These cells expressed MSC markers such as CD44 and CD73, whereas were negative for hematopoietic markers CD45 and CD34. UC-MSCparticles were successfully isolated. Particles were heterogeneous vesicles of approximately 50 to 1250 nm in diameter that bear the surface-expressed molecules UC-MSCs such as; CD90, CD106, CD166 and CD44, and negative for CD34, CD63, and CD9. According to the results of DLS method, centrifugation at 10,000, 20,000, 40,000 and 60,000 ×g, all gave MVs of less than 1000 nm. It is of notion that only at the centrifugation rates of 40,000 and 60,000×g, particles of less than 100 nm in diameter were also obtained.
Conclusion: The choice of exact speed greatly influences the purity of MVs and their yield. Our findings indicate that centrifugation at 20,000×g is appropriate for the purification of UC-MSC-MVs.
Keywords: extracellular vesicles, exosomes, microvesicles, DLS method differential centrifugation
Introduction
Cell-derived vesicles were first discovered in 1940 when elementary studies were carried out to show the “biological importance of the thromboplastic protein of blood” (1). Many years later, in 1967, electron microscopy was used to recognize these subcellular particles (1). Almost all cells release several types of vesicles, which are generally denominated extracellular vesicles (EVs) (1-4).
EVs are spherical particles encircled by a phospholipid bilayer. The diameter of EV mostly ranges from 30nm to 5µm, the smallest being some 100-fold smaller than the smallest cells. In general, EVs have been subcategorized into three groups, exosomes (30-100nm), microvesicles (100-1000 nm) and apoptotic blebs or bodies (50–5,000nm); each of which have different biological impact (1,2,5-7). Determination of Size, density, morphology, lipid composition, protein composition, and subcellular origin are the evaluation criteria for assortment about the type of cell-derived vesicles. In the early future, it is expected that also the refractive index, ζ potential, and chemical composition will be accessible from individual vesicles to become relevant novel characteristics (1,7,8).
Apoptotic bodies are typically larger than 1000 nm in size and have commonly been isolated at 2000×g (1,4,5,9). Exosomes are small particles of 30–100 nm in diameter with intracellular origin. The multivesicular bodies are originated from endosome compartments and are released after fusing with the plasma membrane as exosomes. This release is dependent on the cytoskeletal activation (1,2,9). MVs (also called shedding vesicles, shedding microvesicles, or microparticles) is a main class of EVs (1). This type of EVs has a typical diameter of 100 nm to 1000 nm and bigger than exosomes. In addition, they are particles shed from the plasma membrane of viable cells (1,9,10). Calcium influx and cytoskeleton reorganization play more important role in the formation of shedding microvesicles (MVs) (5). Membrane budding and shedding of MVs are resulted from the inactivation of some enzymes including flippase and floppase and activation of the enzyme, scramblase (11,12).
It is known that MVs includes surface receptors and a number of biologically active molecules such as microRNA, mRNA, lipids and proteins. These molecules can be delivered between the cells by MVs (1,2). Consequently, MVs may exchange the behavior of recipient cells by this method. There is a growing interest in the utilization of vesicles. It has been revealed that MVs released from Umbilical Cord mesenchymal stem cells (UC-MSCs) may imitate the beneficial effect of UC-MSCs (13,14).
Thus, UC-MSCs may be a suitable source for therapeutic MVs. UC-MSCs can be easily isolated from umbilical cord blood through a non-invasive and painless method. Various studies have demonstrated that UC-MSC exhibit a higher proliferation capacity than bone marrow (BM-MSC) (15-18).
Nevertheless, due to the small size of MVs, their detection is unreliable by conventional detection methods. Isolation of MVs is a challenging procedure. Lack of a standard method for the detection and isolation of MVs lead to the differences in classification criteria and simply reveals one of the principal issues to be solved. For this, we isolated MVs the conditioned medium of UC-MSCs by four different schemes of ultracentrifugation. We examined the effect of using different ultracentrifugation rates on the vesicle size by flow cytometry, dynamic light scattering (DLS) and total protein quantification. The prospect of our study was to choose the most convenient speed of ultracentrifugation for the isolation from the conditioned medium MVs of UC-MSCs.
Methods
Isolation and culture of UC- MSCs
Fresh human umbilical cords that are generally discarded after delivery were obtained with the written informed consent of the parents. The research ethics committee at Iranian Blood Transfusion Organization approved this project.
After washing human umbilical cords with PBS, the umbilical cord vessels (one vein and two arteries) were removed. Residual umbilical cords were cut into pieces of 1 mm size and were transported into culture plates containing low-glucose Dulbecco, Modified Eagle’s Medium (DMEM, Bioiea Company) with 10% fetal bovine serum and 1% penicillin and streptomycin (FBS, Gibco BRL Co., USA). Plates were kept at 37°C in a humidified atmosphere with 5% CO2. The culture medium was exchanged after three days and large pieces were removed. The adherent cells were separated by 0.1% trypsin (Gibco BRL Co., USA) and sub cultured. Only cells from three to seven passages were used for further experiments. Characterization of UC-MSCs was examined by flow cytometry technique.
Isolation of MVs
When the mesenchymal stem cells reached to 80% confluence, the DMEM medium was replaced with fresh RPMI deprived of FBS and added 0.5% bovine serum album (BSA) (Sigma-Aldrich) incubated at 37°C in a humidified atmosphere with 5% CO2 for 72h. The viability of the cell cultured after 72h was >92% as detected by trypan blue. The conditioned medium was individually transferred into four tubes for a series of sequential centrifugation steps. A multi-step different centrifugation and ultracentrifugation are the most common methods used for the isolation of MVs (12,13). The first steps are to eliminate large dead cells and large cell debris by consecutive centrifugations at increasing speeds. The samples were centrifuged at 400× g for 10 min and at 2000×g for 20 min. At each of these steps, the supernatant was collected and transferred to new tubes for the next step and the pellet was thrown away. The final supernatant of each tube was collected and ultracentrifuged at each of stated below conditions, 10,000×g, 20,000×g, 40,000×g and 60,000×g in a 12110 angle rotor (Sigma, 3K30, Germany) for 70min at 4°C. The pellet was washed in the plenty of PBS to remove proteins and with centrifugation at the same high speed. The result of this step was microvesicles pellet. MVs were stored at -80°C for the subsequent experiments. Protein content was quantified by Bradford assay kit, (BIO-RAD PROTEIN ASSAY, Cat.No.500-0006).
MVs surface marker analysis
Flow cytometry was used to characterize the isolated MVs. A sample of isolated MV (50μL) was incubated with 4μL of different antibodies CD90, CD106, CD166, CD44, CD63, CD9 and CD34 with appropriate isotype control IgG for 30 min at 4°C. Then 200 μL latex beads less than 1µm (FluoSpheres polystyrene, yellow- green) (molecular probes, USA) were added and samples were immediately analyzed using a flow cytometry.
Dynamic Light Scattering (DLS)
Dynamic Light scattering is one of the most commonly used techniques that frequently utilizes to estimate the size of small particles and the molecular weights of large protein complexes. We applied DLS (Malvern Instruments) to assess the size distributions of UC-MVs. MVs were obtained from the supernatants of UC-MSCs, as previously described. Then MVs size was measured using DLS method. The analyzer presented the size distribution of MVs graphically as intensity plots.
Results
Isolation and expansion of UC-MSCs
UC-MSCs were isolated and identified as previously explained (23). Briefly, the cells had a spindle-shaped morphology and adhered to plastic culture wells or flasks (Fig. 1).
Fig. 1 .
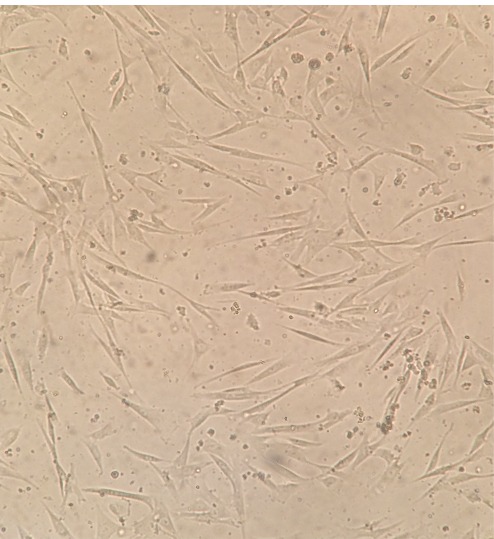
The morphology of mesenchymal stem cells (MSCs) derived from Umbilical cord (UC-MSCs) were isolated and cultured in a T25 flask. The cells displayed a fibroblast-like phenotype. The image shows cells in passage 3, x10
With flow cytometry analysis, we realized that the expression of MSC markers CD44 and CD73 was positive, whereas the expression of hematopoietic markers CD45, CD34 was negative (Fig. 2).
Fig. 2 .
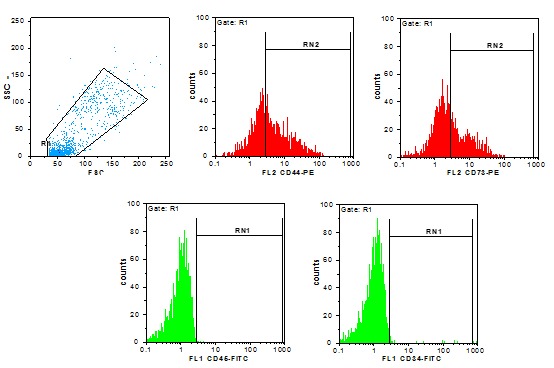
Flow cytometric analysis of Umbilical cord mesenchymal stem cell (UC-MSC) makers. Cells at passage 3-7 were applied. Conjugated fluorescent antibodies were: CD44-PE, CD73-PE, CD34-FITC and CD45 FITC.
UC-MSC-MVs were successfully isolated and determined as previously described (24). Particles were heterogeneous lipid bilayer vesicles of approximately 50 to 1250 nm in diameter (Fig. 3a, b). Flow cytometric analysis showed UC-MSC-MVs bear the surface-expressed molecules typically expressed by UC-MSCs, such as, CD90, CD106, CD166 and CD44, and negative for CD34, CD63 and CD9. Microvesicles were pelleted after 70min centrifugation at 10,000× g, 20,000× g, 40,000× g and 60,000× g. It was revealed that the rate of 20,000× g was more efficient for the isolation of MVs. (Fig. 3)
Fig. 3.
Fig. 3a .
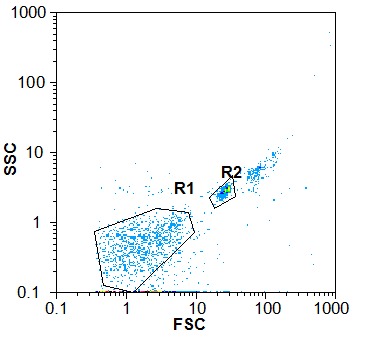
A. Representative dot plots from the flow cytometer. R1 is the gate for MVs and R2 is the gate for beads. The size of MVs in R1 was less than 1μm.
Fig. 3b .
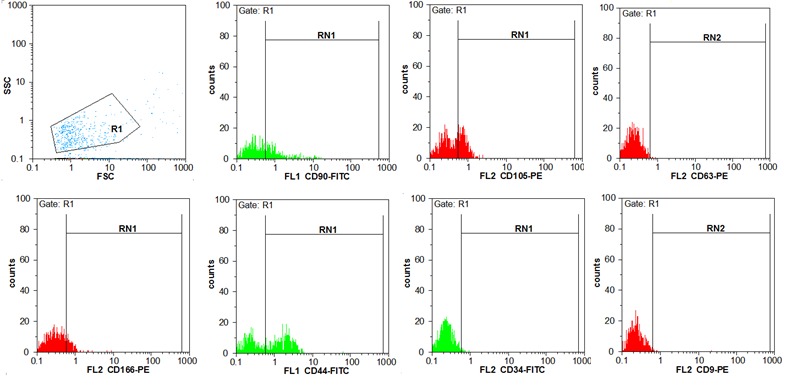
The isolated microvesicles were stained with fluorochrome–conjugated antibodies; CD90-FITC, CD105-PE, CD166-PE, CD44-FITC, CD63-PE, CD9PE, and CD34-FITC plus reagent. They were positive for CD90 (36.96%), CD105 (39.47%), CD166 (17.41%), CD44 (55.33%) and negative for CD34 (1.48%), CD63 (0.4) and CD9 (0.45).
DLS analysis results for particle size distribution
Supernatants of UC-MSCs contained MVs. The size of MVs was assessed using Malvern Instruments. The result of DLS method was shown in Figure 4. The first setting (10,000×g) gave two distinct peaks at 971 and 132nm with Z-Average (d.nm) of 879 (Fig. 4a). The second protocol (20,000 × g) gave a single peak at 542nm with Z-Average of 845 (Fig. 4b). The third and fourth protocol (40,000 × g and 60,000 × g) gave two separate peaks at 450 and 81.2 nm with Z-Average (d.nm) of 898 and 400nm, 102nm with Z-Average of 527, respectively. The size of particles that were obtained by centrifugation at 40,000×g and 60,000 × g was different between less than 100nm and between 100-1000 nm (Fig. 4c, d). This may contribute to the observed difference in sedimentation characteristics by different speeds.
Fig. 4.
Fig. 4a .
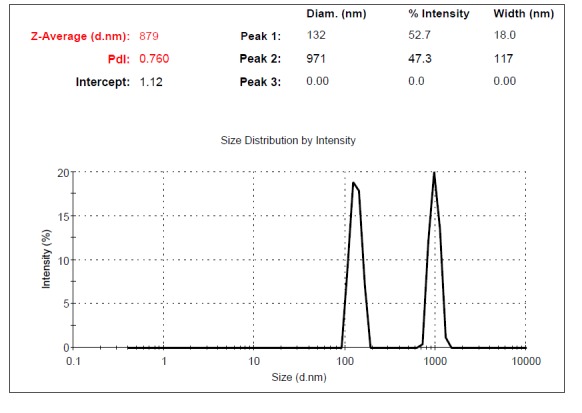
Dynamic Light Scattering (DLS) results for the size distribution of MVs obtained at the centrifugation rate of 10,000× g. From the relative intensities of the size distribution peaks, appears that there is likely some apoptotic debris within the sample.
Fig. 4b .
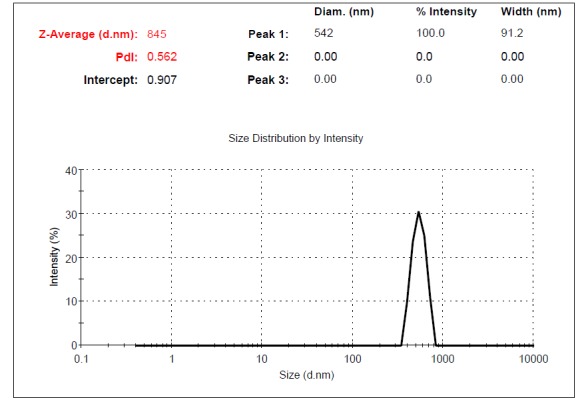
Dynamic Light Scattering (DLS) results for the size distribution of MVs obtained at the centrifugation rate of 20,000× g.
Fig. 4c .
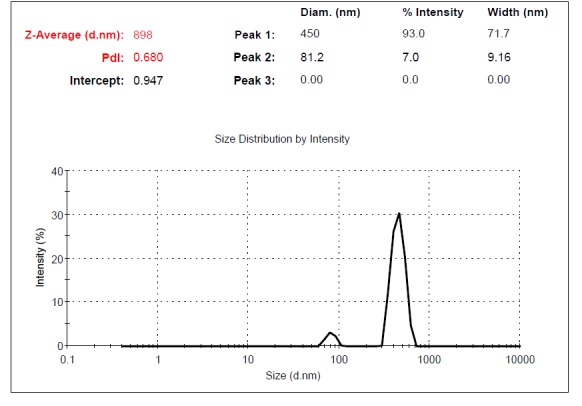
Dynamic Light Scattering (DLS) results for the size distribution of MVs obtained at the centrifugation rate of 40,000× g. The contamination possibility with exosomes at 40,000× g is less than at 60,000× g.
Fig. 4d .
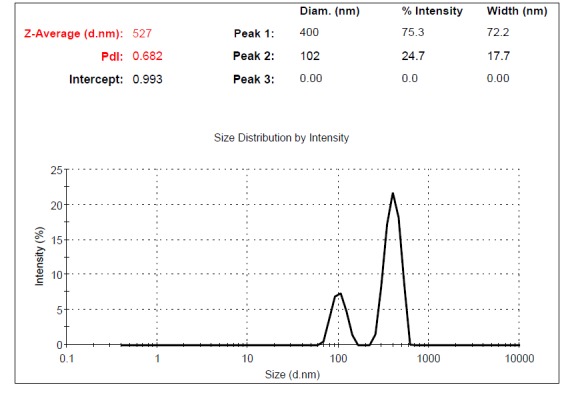
Dynamic Light Scattering (DLS) results for the size distribution of MVs obtained at the centrifugation rate of 60,000× g.
Discussion
Different types of cells release extracellular vesicles. Various mechanisms such as oncogenic transformation, hypoxia, cellular activation, oxidative stress or death can boost the Production of EVs (1).
One of the significant constraints in this evolving field is the lack of a standard method for isolation of EVs. As with other methods, there are variations depending on the laboratory setting to isolate EVs. The efficiency for the isolation of vesicles depends on size, density of vesicles, temperature of the fluid in which the vesicles existed, the fraction volume of the vesicles and most importantly the centrifugation speed (6). For this reason, it is essential to adopt the easy and cost-effective protocol enabling the isolation of EVs with a size between 100-1000nm.
The methods used for the purification and isolation of released EVs including:
a) Absorption to magnetic/non-magnetic microbeads
b) Size exclusion chromatography
c) Differential centrifugation/ ultra-centrifugation with/ without a sucrose gradient cushion. Although this method is time-consuming (4–5h) and requires an ultracentrifuge but due to its facility and high capacity, it is still the most common method (6).
The possibility of contamination with apoptotic bodies is one of a major issue in the isolation of microvesicles from cell culture medium that results from cells undergoing apoptosis. To inhibit the contamination of samples with apoptotic debris, we used only cultures of cells with a high viability (>95%) and differential centrifugation/ultracentrifugation. In this study it was found that contamination with apoptotic bodies was decreased at speeds above 10, 000×g (20, 000×g, 40, 000×g, 60, 000×g) although a small degree of dead or dying cells remained and cannot be removed entirely (Fig. 4).
Contamination with exosomes is another potential issue in the isolation of microvesicles. When reviewing the field of microvesicles, a basic protocol with a 70 minutes centrifugation at approximately 100,000×g is most often applied (19,20). In a review by Xiaomin Zhang et al. in 2014, exosomes are precipitated by centrifugation at ≥100,000× g (21). Based on the graphs derived from DLS method (Fig. 4), the chance of contamination with exosomes increased when the speed above 20,000×g was used. The density of exosome in the pelleted MVs was higher at the centrifugation rates of 40,000×g and 60,000×g. This indicated that contamination with exosomes could be reduced at lower speeds.
The choice of exact speed greatly influences the purity and yield of isolated of MVs. Our findings indicated that the centrifugation rate of 20,000×g is optimal speed for UC-MVs purification.
Based on our results, it is possible to eliminate most of the contamination when we use appropriate and optimal speeds. However, increasing the purity of samples in this manner will also reduce the yield.
Conclusion
It was concluded that there was no need to use the speed higher than 20,000×g for the isolation of microvesicles. The centrifugation rate of 20,000×g is enough to obtain MVs with optimal purity and yield. We observed differences in the fractions obtained by differential centrifugation in terms of vesicle size. The choice of exact speed greatly influences MVs purity and yield. Our findings indicate that 20,000×g is optimal speed for microvesicles purification.
Acknowledgments
This study was the result of a thesis financially supported by Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Iranian Blood Transfusion Organization, Tehran. The authors wish to thank all the staff of IBTO Research Center for the cooperation. None of the authors have any conflicts of interest to declare.
Cite this article as: Rad F, Pourfathollah AA, Yari F, Mohammadi S, Kheirandish M. Microvesicles preparation from mesenchymal stem cells. Med J Islam Repub Iran 2016 (16 July). Vol. 30:398.
References
- 1.van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacological reviews. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 2.Momen-Heravi F, Balaj L, Alian S, Mantel P-Y, Halleck AE, Trachtenberg AJ. et al. Current methods for the isolation of extracellular vesicles. Biological chemistry. 2013;394(10):1253–62. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L. et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PloS one. 2010;5(7):e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. Journal of neuro-oncology. 2013;113(1):1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleissner F, Goerzig Y, Haverich A, Thum T. Microvesicles as novel biomarkers and therapeutic targets in transplantation medicine. American Journal of Transplantation. 2012;12(2):289–97. doi: 10.1111/j.1600-6143.2011.03790.x. [DOI] [PubMed] [Google Scholar]
- 6.Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M. Isolation of extracellular vesicles: Determining the correct approach (Review) International journal of molecular medicine. 2015;36(1):11–7. doi: 10.3892/ijmm.2015.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrology Dialysis Transplantation. 2012;27(8):3037–42. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 8.Huang YC, Parolini O, Deng L. The potential role of microvesicles in mesenchymal stem cell-based therapy. Stem cells and development. 2012;22(6):841–4. doi: 10.1089/scd.2012.0631. [DOI] [PubMed] [Google Scholar]
- 9.Barteneva NS, Fasler-Kan E, Bernimoulin M, Stern JN, Ponomarev ED, Duckett L. et al. Circulating microparticles: square the circle. BMC cell biology. 2013;14(1):23. doi: 10.1186/1471-2121-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200(4):373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international. 2010;78(9):838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 12.Camussi G, C Deregibus M, Tetta C. Tumor-derived microvesicles and the cancer microenvironment. Current molecular medicine. 2013;13(1):58–67. [PubMed] [Google Scholar]
- 13.Zou X, Zhang G, Cheng Z, Yin D, Du T JG, Miao S. et al. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5(2):40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fibbe W. Mesenchymal stem cells A potential source for skeletal repair. Annals of the rheumatic diseases. 2002;61(suppl 2):ii29–ii31. doi: 10.1136/ard.61.suppl_2.ii29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baksh D, Song L, Tuan R. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. Journal of cellular and molecular medicine. 2004;8(3):301–16. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9(1):12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W. et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. haematologica. 2006;91(8):1017–26. [PubMed] [Google Scholar]
- 18.Bakhshi T, Zabriskie RC, Bodie S, Kidd S, Ramin S, Paganessi LA. et al. Mesenchymal stem cells from the Wharton's jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long‐term ex vivo culture. Transfusion. 2008;48(12):2638–44. doi: 10.1111/j.1537-2995.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. International journal of oncology. 2012;40(1):130–8. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 20.Zhang HC, Liu XB, Huang S, Bi XY, Wang HX, Xie LX. et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem cells and development. 2012;21(18):3289–97. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. International journal of molecular sciences. 2014;15(3):4142–57. doi: 10.3390/ijms15034142. [DOI] [PMC free article] [PubMed] [Google Scholar]


