Abstract
Background:
Hydrogen peroxide (HP) at lower concentration can provide less alteration on enamel surface and when combined with laser therapy, could decrease tooth sensitivity. This in situ study evaluated the influence of 15% and 35% HP gel activated by lighting-emitting diode (LED)/laser light for in-office tooth bleaching.
Materials and Methods:
Forty-four bovine enamel slabs were polished and subjected to surface microhardness (load of 25 g for 5 s). The specimens were placed in intraoral palatal devices of 11 volunteers (n = 11). Sample was randomly distributed into four groups according to the bleaching protocol: 15% HP, 15% HP activated by LED/laser, 35% HP, and 35% HP activated by LED/laser. The experimental phase comprised 15 days and bleaching protocols were performed on the 2nd and 9th days. Surface microhardness (KHN) and color changes were measured and data were analyzed by ANOVA (α = 0.05).
Results:
There were no significant differences in microhardness values neither in color alteration of enamel treated with 15% HP and 35% HP activated or not by LED/laser system (P > 0.05).
Conclusions:
Both concentrations of HP (15 or 35%), regardless of activated by an LED/laser light, did not affect the surface microhardness and had the same effectiveness in enamel bleaching.
Key words: Hydrogen peroxide, laser, microhardness, tooth bleaching
Introduction
Tooth bleaching is one of the most requested esthetic treatments by patients, and its effectiveness is directly related to the concentration of the bleaching agent and the exposure time of this gel on tooth surface. With increased exposure time and concentration of the bleaching agent, the oxidation process becomes more intense and can cause changes in the morphology of tooth surface.[1,2,3]
The bleaching technique is based on the application of hydrogen peroxide (HP) on the tooth surface. The peroxide breaks down and releases highly reactive molecules called “free radicals” that penetrate the tooth structure through redox reaction with pigments of dental hard tissue. Since the by-products that result are colorless, the tooth receives a lightening effect.[4,5] Some in vitro studies using 35% HP found that the bleaching process can modify surface roughness,[6,7] decrease microhardness,[8,9,10,11,12] and change the calcium/phosphorus rates.[13,14] However, in the presence of saliva, fluorides, and other remineralizing solutions, such changes may not occur.[10]
To reduce the adverse effects of bleaching using 35% HP and to consider that the effectiveness of bleaching might not change with decreasing concentration of gel, HP at low concentration has been proposed for in-office dental bleaching.[15,16]
HP has been used alone or activated by a light source.[17,18] The combination of lighting-emitting diode (LED) matrix with infrared diode lasers can accelerate the action of the bleaching gel.[5,12,17,19] The advantage of a light source is its ability to heat the HP, thereby increasing the kinetic energy of the molecules and the rate of oxygen decomposition to form oxygen-free radicals and enhance the bleaching process. The use of a light source makes the bleaching process faster,[17] probably without reducing the mineral content and microhardness of enamel.[12]
Based on the lack of information on the effect of 15% HP activated by LED/laser system on dental enamel and considering that HP at small concentration can produce good results without damaging tooth surface, it is important to conduct in situ studies on the action of HP on the tooth surface.
The aim of this in situ study was to evaluate the influence of 15% HP activated or not by LED/laser on enamel and compare it with 35% HP on the microhardness and color alteration. The null hypothesis tested was that (1) 15% HP causes less change on enamel microhardness and (2) 15% HP has the same effectiveness in tooth bleaching when compared to 35% HP.
Materials and Methods
Ethical aspects
This study was approved by the Local Ethics Research Committee (#38824713.6.0000.5419). The volunteers were informed about the methodology of the experiment, its risks and benefits, and signed a “Statement of Consent,” agreeing to participate in the experiment.
Twelve volunteers of both genders who fulfilled inclusion criteria (good general and oral health with no active caries lesions or periodontal treatment needs, good oral hygiene, nonsmokers, ability to comply with the experimental protocol, not using fixed or removable orthodontic devices or had undergone previous bleaching treatment) were selected to participate in the study.[18,19] The sample size was determined based on the previous studies;[20,21] however, during the research, there was the withdrawal of one volunteer, totalizing 11 volunteers.
Sample preparation
Freshly extracted bovine incisors were prepared. The teeth were cleaned and stored in 0.1% thymol solution at 9°C. The absence of cracks, hypoplasia, and hypomineralization was confirmed by inspection under stereomicroscope with increase of 20× (Leica, S6 D StereoZoom, Leica Microsystems AG, Switzerland). After that, teeth were sectioned, obtaining enamel slabs (5 mm × 5 mm × 3 mm). Then, they were fixed in Teflon matrices using casting wax and were flattened and polished 1200-grit silicon carbide papers (Hermes Abrasives Ltd., VA, EUA) in a polish machine (DP-9U2; Struers A/S, Copenhagen, Denmark) and 0.3 μm alumina paste (Arotec S/A Ind. Com., Sao Paulo, Brazil). After polished, specimens were cleaned for 1 min in an ultrasonic cleaner to remove debris from the surface.
Initial analysis
To standardize the sample, slabs were subjected to initial reading of microhardness and received three indentations in the central region of each fragment with a Knoop-type indenter (HMV-2000, Shimadzu Corporation, Kyoto, Japan) with a static load of 25 g for 5 s.[20] The general average was calculated and slabs with average 20% above or below were excluded[21] and 44 enamel blocks were selected.
After that, the color analysis was realized using a portable colorimeter (SP62S; X-Rite, Grand Rapid, Michigan, EUA), with 4 mm focal opening and diffuse geometry (D/8°). The color change (ΔE*) was calculated L*, a*, and b* using the formula (ΔE* = (ΔL*)2 + (Δa*)2 + (Δb*)2 /2).[5]
The slabs were sterilized in a microwave oven (CMW30, Consul, Sao Paulo, SP, Brazil) as described by Viana et al.[22]
Staining procedures
Enamel slabs were submitted to staining with coffee (Melitta Extra Forte; Melitta do Brasil Ind. e Com. Ltda, Sao Paulo, Brazil), which was prepared in the ratio of 300 ml water to 6 g of powder in a coffee maker. The specimens were immersed individually in 20 mL of this solution at 37°C. The solution was changed daily until complete 72 h.[23]
Intraoral phase
For the intraoral phase, palatal intraoral appliances were made with acrylic resin using models that were produced from alginate impressions of the maxillary and mandibular dental arches of all volunteers.
During 7 days of the preexperimental phase and in the experimental period, volunteers brush their natural teeth with toothbrush (Oral-B Indicator 35; Gillette do Brasil Ltda., Manaus, Amazonas, Brazil) and toothpaste (Colgate-Palmolive, Osasco, Sao Paulo, Brazil) provided by the researcher.
In the experimental phase, volunteers were instructed to brush the specimens three times a day, with standardized toothbrush and slurry (Colgate-Palmolive, Osasco, Sao Paulo, Brazil) (3:1) to mimic oral health conditions.[21]
On the 1st day, volunteers use the palatal device to form acquired pellicle, simulating the clinical situation.[7] Dental bleaching was realized on the 2nd and 9th days in the laboratory under standardized conditions of illumination by a previously trained researcher.
On the 2nd day, it was realized the first session of bleaching according to the four different protocols: 15% HP (Lase Peroxide 15% Lite; DMC Equipamentos, Sao Carlos, SP, Brazil); 15% HP activated by LED/laser (Whitening lase light plus; DMC Equipamentos); 35% HP (Lase Peroxide 35% Sensy; DMC Equipamentos); and 35% HP activated by LED/laser (Whitening lase light plus; DMC Equipamentos).
The LED/laser system is formed by LEDs matrix, which generates blue light with 470 nm wavelength and three infrared diode lasers with a wavelength of 808 nm and 0.2 watt power each one. The researcher conducted the bleaching treatments following the required security standards.
The bleaching gel was applied with a spatula covering the whole enamel surface with approximately 2 mm thick and the bleaching protocols were realized according to the manufacturer's instruction [Table 1].
Table 1.
Materials used in this study
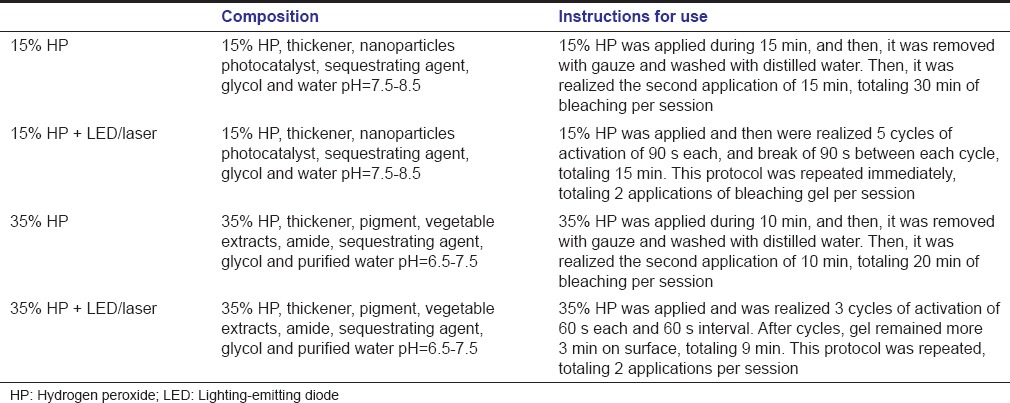
After the first session of bleaching, the volunteers use the palatal device for 7 days continuously, removing only to eat and to realize oral hygiene.
On the 9th day, the second session of bleaching was realized following the same protocol. Each specimen received the same treatment realized in the first session defined by random drawing.
After this, the volunteers used the palatal device for more 7 days,[7] and on the 15th day, the slabs were removed and submitted to color assessment and microhardness test.
Final analysis
After intraoral phase, the slabs were removed from the palatal appliances and were ultrasonically cleaned for 10 min. Next, the color analysis and the microhardness test were performed on enamel slabs as described previously. Three readings of microhardness were averaged and used as the outcome value for each slab.
Statistical analysis
Data were analyzed using Statgraphics Centurion XV statistical software. Homogeneity of variance and normality were tested by Barlett and Kolmonov-Smirnov tests, and then, data were submitted to two-way ANOVA. Significance level was set at 5%.
Results
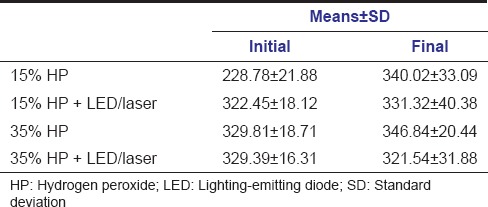
Mean values and standard deviation of microhardness are shown in Table 2. Two-way ANOVA showed no significant difference between groups bleached with 15% HP and 35% HP, activated or not by LED/laser, in both tested moments (initial and after bleaching).
Table 2.
Means and standard deviation of microhardness (KHN)
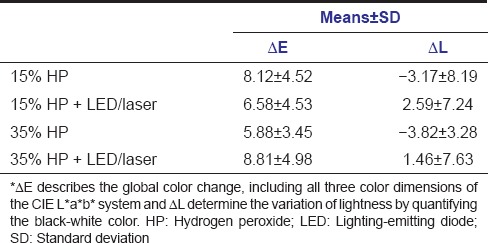
For the color data, the two-way ANOVA showed no significant differences among groups bleached with 15% HP and 35% HP, activated or not by LED/laser for the values of ΔE and ΔL. Mean values and standard deviations are shown in Table 3.
Table 3.
Means and standard deviations of color analysis
Discussion
In the present study, the first null hypothesis was rejected since there was no difference between microhardness for teeth treated with 15% HP and 35% HP activated or not by LED/laser. These results can be explained by the fact that, in the oral cavity, saliva is supersaturated with minerals, such as calcium and phosphate, which act in the remineralization process.[24] Furthermore, the saliva prevents the reduction of dental hardness by eliminating direct contact of teeth with acidic substances, thus acting as a diffusion barrier for calcium and phosphate.[25]
The effect of human saliva on bleached enamel is an important consideration when evaluating the real potential of these remineralizing agents. Lia Mondelli et al. evaluated the effect of bleaching treatments using different HP concentrations with or without light activation and found that all bleaching procedures lead to a decrease in surface microhardness when compared with control group after 24 h. However, after 7 days of remineralization, the surface microhardness returned to normal levels for all bleached specimens.[3] Zeczkowski et al. verified the effect of different storage conditions on bleached enamel microhardness and change in hardness was observed only in enamel storage in purified water.[26] Araujo et al. observed in vitro the reduction on enamel microhardness after bleaching; however, this effect was not found in in situ design, suggesting that human saliva improved remineralizing capability.[27]
A reduction in hardness after bleaching has been reported in some studies[9,28,29] and has often been associated with demineralization of the surface by the action of bleaching agents.[7] Some studies assigned these structural modifications mostly to the gel pH.[7,30,31] Neutral gels are recommended for tooth bleaching with the purpose of reducing deleterious effects on tooth enamel. In this study, the pH of the gels ranged from 6.5 to 8.5 and this may explain the results since the neutral pH caused no changes on enamel and did not alter microhardness.
Bistey et al. reported that structural changes of the enamel surface are time dependent, with considerable changes after 60 min of exposure to peroxide.[32] The time of exposure used in this study was 30 min for 35% HP and 45 min for 15% HP, as recommended by manufacturers, which might be insufficient to promote enamel demineralization.
In bleaching protocols, a light source is widely used to reduce treatment time by increasing the release of oxygen, the ion responsible for the bleaching effect.[27] Kossatz et al. found faster bleaching for the LED/laser group than those without light activation after the first session of bleaching.[17] In fact, the light sources are not responsible for tooth bleaching. The rationale behind the benefits of light activation is that a small fraction of light is absorbed by the bleaching product and its energy is converted to heat. Most likely, this is the main mechanism of action of all light-activated bleaching procedures, and it leads to increased release of hydroxyl radicals through a rise in temperature (thermocatalysis).[33]
The light source used in this study is made of a matrix of LEDs and three diode lasers. This association, besides accelerate the bleaching process, has analgesic effect due to the presence of diode laser, which may reduce the sensitivity.[34] Gurgan et al. found similar bleaching esthetic results between groups with and without diode laser; however, laser-treated teeth had lower sensitivity.[35] The analgesic effect provided by diode laser may sometimes be neutralized by the heat generated by the LED system.[19,36]
Regarding the color changes, when analyzing the three color dimensions of the CIE L* a* b* system separately, the L* values determine the lightness by quantifying the black-white color, a* and b* values describe chrome and are less used. ΔE describes the global color change, including all three color dimensions of the CIE L* a* b* system, and can be used to compare the efficacy of different bleaching agents.[37]
In the present study, ΔE and ΔL were evaluated and the second null hypothesis was accepted since HP in different concentrations activated or not by LED/laser did not influence the effectiveness of dental bleaching. Our results are in agreement with de Almeida et al., which evaluated the color alteration and diffusion of 20% HP and 35% HP in teeth and found that despite greater peroxide diffusion in teeth bleached with 35% HP, both protocols showed the same effectiveness.[16]
Although some studies found that the effectiveness of dental bleaching is directly proportional to HP concentration,[1,2] Matis et al. suggested that the time that tooth in contact with the product has a greater influence on the bleaching effect than the concentration of the bleaching agent.[38] This statement can explain the results of this study since contact time of 15% HP was longer than 35% HP; hence, although the difference of concentration, the bleaching agents tested had the same effectiveness.
Considering the above-mentioned facts, it can be concluded that HP in oral environment did not affect the surface microhardness and when used at low concentration, has the same effectiveness on dental bleaching, regardless of using LED/laser light to accelerate the process. Further studies should be conducted to verify the performance of bleaching agents on the oral cavity, as well as to evaluate the HP at lower concentrations, to reduce postoperative sensitivity.
Financial support and sponsorship
This study was supported by CNPq (National Counsel of Technological and Scientific Development) process n° 140208/2014-3.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gopinath S, James V, Vidhya S, Karthikeyan K, Kavitha S, Mahalaxmi S. Effect of bleaching with two different concentrations of hydrogen peroxide containing sweet potato extract as an additive on human enamel: An in vitro spectrophotometric and scanning electron microscopy analysis. J Conserv Dent. 2013;16:45–9. doi: 10.4103/0972-0707.105298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borges AB, Zanatta RF, Barros AC, Silva LC, Pucci CR, Torres CR. Effect of hydrogen peroxide concentration on enamel color and microhardness. Oper Dent. 2015;40:96–101. doi: 10.2341/13-371-L. [DOI] [PubMed] [Google Scholar]
- 3.Lia Mondelli RF, Garrido Gabriel TR, Piola Rizzante FA, Magalhães AC, Soares Bombonatti JF, Ishikiriama SK. Do different bleaching protocols affect the enamel microhardness? Eur J Dent. 2015;9:25–30. doi: 10.4103/1305-7456.149634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamoto K, Tsujimoto Y. Effects of the hydroxyl radical and hydrogen peroxide on tooth bleaching. J Endod. 2004;30:45–50. doi: 10.1097/00004770-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Mondelli RF, Azevedo JF, Francisconi AC, Almeida CM, Ishikiriama SK. Comparative clinical study of the effectiveness of different dental bleaching methods - Two year follow-up. J Appl Oral Sci. 2012;20:435–43. doi: 10.1590/S1678-77572012000400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez JA, Bittencourt B, Michel M, Sabino N, Gomes JC, Gomes OM. Ultrastructural evaluation of enamel after dental bleaching associated with fluoride. Microsc Res Tech. 2012;75:1093–8. doi: 10.1002/jemt.22035. [DOI] [PubMed] [Google Scholar]
- 7.Sa Y, Sun L, Wang Z, Ma X, Liang S, Xing W, et al. Effects of two in-office bleaching agents with different pH on the structure of human enamel: An in situ and in vitro study. Oper Dent. 2013;38:100–10. doi: 10.2341/11-173-L. [DOI] [PubMed] [Google Scholar]
- 8.Barros-Matoso F, de Souza-Gabriel AE, Furtado-Messias DC, de Sousa-Neto MD, Alfredo E. Microhardness of intracoronal dentin exposed to bleaching and fluoride treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e1–5. doi: 10.1016/j.tripleo.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Borges AB, Samezima LY, Fonseca LP, Yui KC, Borges AL, Torres CR. Influence of potentially remineralizing agents on bleached enamel microhardness. Oper Dent. 2009;34:593–7. doi: 10.2341/08-081-L. [DOI] [PubMed] [Google Scholar]
- 10.De Abreu DR, Sasaki RT, Amaral FL, Flório FM, Basting RT. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness. J Esthet Restor Dent. 2011;23:158–68. doi: 10.1111/j.1708-8240.2010.00394.x. [DOI] [PubMed] [Google Scholar]
- 11.Zanet CG, Fava M, Alves LA. In vitro evaluation of the microhardness of bovine enamel exposed to acid solutions after bleaching. Braz Oral Res. 2011;25:562–7. doi: 10.1590/s1806-83242011000600015. [DOI] [PubMed] [Google Scholar]
- 12.Parreiras SO, Vianna P, Kossatz S, Loguercio AD, Reis A. Effects of light activated in-office bleaching on permeability, microhardness, and mineral content of enamel. Oper Dent. 2014;39:E225–30. doi: 10.2341/13-031-L. [DOI] [PubMed] [Google Scholar]
- 13.Poorni S, Kumar RA, Shankar P, Indira R, Ramachandran S. Effect of 10% sodium ascorbate on the calcium: Phosphorus ratio of enamel bleached with 35% hydrogen peroxide: An in vitro quantitative energy-dispersive X-ray analysis. Contemp Clin Dent. 2010;1:223–6. doi: 10.4103/0976-237X.76388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesan SM, Narayan GS, Ramachandran AK, Indira R. The effect of two bleaching agents on the phosphate concentration of the enamel evaluated by Raman spectroscopy: An ex vivo study. Contemp Clin Dent. 2012;3(Suppl 2):S172–6. doi: 10.4103/0976-237X.101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basting RT, Amaral FL, França FM, Flório FM. Clinical comparative study of the effectiveness of and tooth sensitivity to 10% and 20% carbamide peroxide home-use and 35% and 38% hydrogen peroxide in-office bleaching materials containing desensitizing agents. Oper Dent. 2012;37:464–73. doi: 10.2341/11-337-C. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida LC, Soares DG, Gallinari MO, de Souza Costa CA, Dos Santos PH, Briso AL. Color alteration, hydrogen peroxide diffusion, and cytotoxicity caused by in-office bleaching protocols. Clin Oral Investig. 2015;19:673–80. doi: 10.1007/s00784-014-1285-3. [DOI] [PubMed] [Google Scholar]
- 17.Kossatz S, Dalanhol AP, Cunha T, Loguercio A, Reis A. Effect of light activation on tooth sensitivity after in-office bleaching. Oper Dent. 2011;36:251–7. doi: 10.2341/10-289-C. [DOI] [PubMed] [Google Scholar]
- 18.de Almeida LC, Costa CA, Riehl H, dos Santos PH, Sundfeld RH, Briso AL. Occurrence of sensitivity during at-home and in-office tooth bleaching therapies with or without use of light sources. Acta Odontol Latinoam. 2012;25:3–8. [PubMed] [Google Scholar]
- 19.Moncada G, Sepúlveda D, Elphick K, Contente M, Estay J, Bahamondes V, et al. Effects of light activation, agent concentration, and tooth thickness on dental sensitivity after bleaching. Oper Dent. 2013;38:467–76. doi: 10.2341/12-335-C. [DOI] [PubMed] [Google Scholar]
- 20.de Arruda AM, dos Santos PH, Sundfeld RH, Berger SB, Briso AL. Effect of hydrogen peroxide at 35% on the morphology of enamel and interference in the de-remineralization process: An in situ study. Oper Dent. 2012;37:518–25. doi: 10.2341/11-112-L. [DOI] [PubMed] [Google Scholar]
- 21.Hara AT, Turssi CP, Ando M, González-Cabezas C, Zero DT, Rodrigues AL, Jr, et al. Influence of fluoride-releasing restorative material on root dentine secondary caries in situ. Caries Res. 2006;40:435–9. doi: 10.1159/000094290. [DOI] [PubMed] [Google Scholar]
- 22.Viana PS, Machado AL, Giampaolo ET, Pavarina AC, Vergani CE. Disinfection of bovine enamel by microwave irradiation: Effect on the surface microhardness and demineralization/remineralization processes. Caries Res. 2010;44:349–57. doi: 10.1159/000318528. [DOI] [PubMed] [Google Scholar]
- 23.Bazzi JZ, Bindo MJ, Rached RN, Mazur RF, Vieira S, de Souza EM. The effect of at-home bleaching and toothbrushing on removal of coffee and cigarette smoke stains and color stability of enamel. J Am Dent Assoc. 2012;143:e1–7. doi: 10.14219/jada.archive.2012.0188. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter GH. The secretion, components, and properties of saliva. Annu Rev Food Sci Technol. 2013;4:267–76. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 25.Vukosavljevic D, Custodio W, Buzalaf MA, Hara AT, Siqueira WL. Acquired pellicle as a modulator for dental erosion. Arch Oral Biol. 2014;59:631–8. doi: 10.1016/j.archoralbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Zeczkowski M, Tenuta LM, Ambrosano GM, Aguiar FH, Lima DA. Effect of different storage conditions on the physical properties of bleached enamel: An in vitro vs. in situ study. J Dent. 2015;43:1154–61. doi: 10.1016/j.jdent.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Araujo Fde O, Baratieri LN, Araújo E. In situ study of in-office bleaching procedures using light sources on human enamel microhardness. Oper Dent. 2010;35:139–46. doi: 10.2341/08-033-C. [DOI] [PubMed] [Google Scholar]
- 28.da Costa Soares MU, Araújo NC, Borges BC, Sales Wda S, Sobral AP. Impact of remineralizing agents on enamel microhardness recovery after in-office tooth bleaching therapies. Acta Odontol Scand. 2013;71:343–8. doi: 10.3109/00016357.2012.681119. [DOI] [PubMed] [Google Scholar]
- 29.Borges AB, Yui KC, D’Avila TC, Takahashi CL, Torres CR, Borges AL. Influence of remineralizing gels on bleached enamel microhardness in different time intervals. Oper Dent. 2010;35:180–6. doi: 10.2341/09-117-L. [DOI] [PubMed] [Google Scholar]
- 30.Magalhães JG, Marimoto AR, Torres CR, Pagani C, Teixeira SC, Barcellos DC. Microhardness change of enamel due to bleaching with in-office bleaching gels of different acidity. Acta Odontol Scand. 2012;70:122–6. doi: 10.3109/00016357.2011.600704. [DOI] [PubMed] [Google Scholar]
- 31.Salomão D, Santos D, Nogueira R, Palma-Dibb R, Geraldo-Martins V. Acid demineralization susceptibility of dental enamel submitted to different bleaching techniques and fluoridation regimens. Oper Dent. 2014;39:E178–85. doi: 10.2341/13-140. [DOI] [PubMed] [Google Scholar]
- 32.Bistey T, Nagy IP, Simó A, Hegedus C. In vitro FT-IR study of the effects of hydrogen peroxide on superficial tooth enamel. J Dent. 2007;35:325–30. doi: 10.1016/j.jdent.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Joiner A. The bleaching of teeth: A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Torres CR, Batista GR, César PD, Barcellos DC, Pucci CR, Borges AB. Influence of the quantity of coloring agent in bleaching gels activated with LED/laser appliances on bleaching efficiency. Eur J Esthet Dent. 2009;4:178–86. [PubMed] [Google Scholar]
- 35.Gurgan S, Cakir FY, Yazici E. Different light-activated in-office bleaching systems: A clinical evaluation. Lasers Med Sci. 2010;25:817–22. doi: 10.1007/s10103-009-0688-x. [DOI] [PubMed] [Google Scholar]
- 36.Polydorou O, Wirsching M, Wokewitz M, Hahn P. Three-month evaluation of vital tooth bleaching using light units-a randomized clinical study. Oper Dent. 2013;38:21–32. doi: 10.2341/12-041-C. [DOI] [PubMed] [Google Scholar]
- 37.Dietschi D, Rossier S, Krejci I. In vitro colorimetric evaluation of the efficacy of various bleaching methods and products. Quintessence Int. 2006;37:515–26. [PubMed] [Google Scholar]
- 38.Matis BA, Cochran MA, Franco M, Al-Ammar W, Eckert GJ, Stropes M. Eight in-office tooth whitening systems evaluated in vivo: A pilot study. Oper Dent. 2007;32:322–7. doi: 10.2341/06-135. [DOI] [PubMed] [Google Scholar]


