Abstract
Context:
This study evaluated the efficacy of denture adhesive, cleanser, chlorhexidine, and brushing against Candida albicans biofilm developed on an acrylic surface and predicted the most effective, simple, and inexpensive way to maintain denture health, thereby preventing denture stomatitis.
Aims:
To find the best possible method for maintaining denture hygiene.
Settings and Design:
This retrospective analysis was conducted in the Guru Nanak Institute of Dental Sciences and Research, Kolkata, and this in vitro study was designed to minimize denture stomatitis among denture wearing population.
Subjects and Methods:
Sixty acrylic discs of equal dimensions after exposure to C. albicans were treated for a duration of 24 h with denture adhesive, cleanser, 0.2% chlorhexidine individually, or in combinations simulating clinical conditions dividing in six groups, ten samples each (n = 10).
Statistical Analysis Used:
After treatment, colony count was evaluated and statistically analyzed by post hoc Tukey's test and Dunnett's test to determine the most effective way of prevention.
Results:
The statistical post hoc analysis (Tukey's test and Dunnett's test) showed high significance (P < 0.0001). The group treated with adhesive showed high fungal growth compared to the control group, whereas chlorhexidine showed high potency to prevent C. albicans, whereas adhesive increased the adhesion of C. albicans to acrylic surface.
Conclusions:
Denture adhesive increases the adherence of C. albicans to denture surface. Other cleaning chemicals such as cleanser and chlorhexidine decrease the adherence. Moreover, among the all denture cleaning protocol, chlorhexidine drastically inhibit the adherence, as well as growth of C. albicans over denture surface.
Key words: Candida albicans, chlorhexidine, denture adhesive, denture cleanser, denture stomatitis
Introduction
Increasing life expectancy has led to a rising number of elderly people worldwide, resulting in a high prevalence of edentulism and complete denture wearing.[1] It has been reported that the fitting surface of maxillary and mandibular dentures is more susceptible for microbial contamination and contains significantly high plaque due to stagnation, pooling of saliva, and the absence of contact with the tongue[2] along with its inherent unpolished nature which contains microscopic pores and irregularities. This often leads to bacterial and fungal colonization causing denture stomatitis.[3] Denture stomatitis is one of the most common inflammatory conditions that affects denture wearers.[4] Although different microbes are responsible for denture stomatitis, most of the cases are caused by colonization of Candida species on denture materials among which Candida albicans are the most commonly concerned yeast.[5]
Different products are commonly used to clean and maintain dentures with their specific efficacy. Studies have revealed that chemical cleansers contain a variety of active agents. Effective disinfection can be attained by enzymes, hypochlorite solutions, acids, mouthwashes, and peroxide solutions.[6] The sodium hypochlorite-based denture cleansers are fungicidal and are known to be effective by dissolving mucin and other organic substances.[7] Alkaline peroxides are the most commonly used denture cleaners due to its good antimicrobial activity against denture biofilms in the absence of odor and after taste.[8] Chlorhexidine is one of the most widely used agents in dentistry and has been used as an adjunct in the treatment of oral candidiasis since the 1970s. Being an antiseptic agent with a broad spectrum of antimicrobial activity including C. albicans and other common nonalbican yeast species, chlorhexidine-based treatments have the ability to remove denture biofilm.[9] Many denture wearers use denture adhesives for better retention and stability of denture. Various studies have shown conflicting results. While some in vitro studies showed that some denture adhesives supported C. albicans growth, inducing hyphal formation,[10] others have suggested that denture adhesives possessed antifungal activity.[11] In light of these observations, this study was undertaken to evaluate the efficacy of denture adhesive, cleanser, chlorhexidine, and combination of these agents against C. albicans biofilm developed on the acrylic surface to find the most effective, simple, and inexpensive way to maintain denture health, thereby preventing denture stomatitis.
Subjects and Methods
Instruments and materials
The instruments and materials used in this study are given in Tables 1a and b, respectively. To avoid cross-contamination, strict principle of sterilization was followed as shown in Table 1c.
Table 1a.
List of instruments
Table 1b.
List of materials
Table 1c.
Methods of sterilization
Methods
In this study, sixty acrylic samples were divided into six groups of ten each. Each acrylic sample was 10 mm in diameter and 1.5 mm in thickness. Polymerization was done in an acrylizer at 74°C for 90 min and 100°C for 30 min following which it was allowed to bench cool for 2 h. All acrylic specimens were removed from the flask and immersed in distilled water at 37°C for 12 h for residual monomer release followed by an ultrasonic cleaning in an ultrasonic cleaner for 20 min in distilled water. Finishing and polishing were not done as the samples were meant to simulate the rough intaglio surface of the denture.
Instead of direct spore application, a suspension preparation of C. albicans in artificial saliva was used in this study to enable a uniform adherence on all samples. The fungal agent (C. albicans MTCC-227) was cultured on Sabouraud dextrose agar (SDA) at 37°C for 48 h aerobically, and the inoculum of this fungal agent was prepared in 50 ml Sabouraud dextrose broth (SDB) by incubating at 37°C for 48 h with vigorous shaking and adjusted to 1 × 107 cells/ml according to 0.5 McFarland test standard turbidimetrically.[12] After affluent fungal growth, cells were harvested by centrifugation at 4000 ×g for 10 min and transferred to a sterile conical flask containing 60 ml of artificial saliva and mixed homogeneously to prepare a cell suspension. This was divided into six test tubes each containing 10 ml of cell suspension. Ten sterilized acrylic discs were added to each test tube which were kept still for 6 h and then shaken well at every 1 h interval to achieve adherence of C. albicans.
Following exposure to C. albicans, each group as described in Table 2 was treated by a particular cleaning protocol over a period of 24 h with adhesive (Group I) or cleanser (Group II) or chlorhexidine mouthwash (Group III) or adhesive + chlorhexidine (Group IV) or adhesive + chlorhexidine (Group V). Cleaning under regular tap water mechanically without any other chemical was considered as the control group (Group VI). The duration of the cleaning protocol with each agent was chosen such that it simulated clinical conditions as depicted in Table 2. The summarized flowchart depicting the methodology of the study is shown in Figure 1.
Table 2.
Study design of different treatment group

Figure 1.
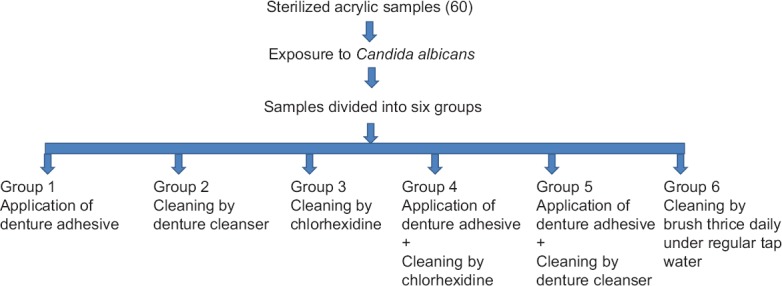
The summarized flowchart depicting the methodology of the study
Cleaning protocol
For Group I
All the acrylic discs (n = 10) were incubated with C. albicans cells in artificial saliva for 12 h initially and then discs were aseptically immersed in a thin suspension of adhesive paste and mixed homogeneously for 10 min to coat adhesive on the whole surface of acrylic discs. Coated acrylic discs were then retransferred to the test tube containing C. albicans suspension and further reincubated for more 12 h duration. After incubation of overall 24 h, the acrylic discs were then recoated once with adhesive.
For Group II
All acrylic discs (n = 10) were incubated with C. albicans suspension in artificial saliva for 24 h. A freshly prepared cleanser solution was made by dropping one-half of a tablet in distilled water to give instant effervescence. At the 24th h, samples were kept in solution for 3 min as instructed by the manufacturer.
For Group III
Treatment was performed as in Group II. After exposure of acrylic discs with fungal suspension and incubation, samples were kept in 0.2% chlorhexidine for 5 min at the 24th h.
For Group IV
All the acrylic discs (n = 10) were incubated with C. albicans cells in artificial saliva for 12 h initially and then discs were aseptically immersed in a thin suspension of adhesive and mixed homogeneously for 10 min to coat adhesive on the whole surface of acrylic discs. Coated acrylic discs were then retransferred to the test tube containing C. albicans suspension and further reincubated for more 12 h duration. After incubation of overall 24 h, the acrylic discs were then recoated once with adhesive and each acrylic disc was gently collected from the tubes and immersed in 0.2% chlorhexidine for 5 min with gentle shaking.
For Group V
Acrylic discs (n = 10) were incubated with C. albicans suspension in artificial saliva for 12 h initially and then discs were aseptically immersed in a thin suspension of adhesive and mixed homogeneously for 10 min to coat adhesive on the whole surface of acrylic discs. Coated acrylic discs were then retransferred to the test tube containing C. albicans suspension and again reincubated further 12 h duration. After incubation of overall 24 h, the acrylic discs were then recoated with adhesive one more time. Samples were then treated with freshly prepared cleanser solution; one-half of a tablet (clanden) was dropped in distilled water which gave instant effervescence and readily made a greenish color cleanser solution. Samples were kept in solution for 3 min at the 24th h as instructed by the manufacturer.
For Group VI
In the case of the control group (Group VI), acrylic discs (n = 10) were incubated with C. albicans suspension in artificial saliva for overall 24 h with intermittent washing under regular water flow for a duration of 2 min at every 8 h interval.
After treatment, each acrylic disc across all groups was then transferred to corresponding test tubes containing 10 ml sterilized SDB and incubated for 24 h, shaken 8 hourly, at 37°C. After incubation, each acrylic disc was gently discarded from the tubes, and all the tubes containing fungal growth were diluted to 10−4 and 100 μl amount of specimen was seeded onto SDA medium and incubated for 48 h at 37°C. After incubation, the colonies were counted and expressed as colony-forming units per milliliter to evaluate the effectiveness of different cleaning protocol.
Statistical analysis of data
Statistical analysis was performed by one-way analysis of variance (ANOVA) and post hoc analysis was done to compare in between two groups by Tukey's multiple comparison test and also for each group to the control by Dunnett's multiple comparison test using GraphPad Prism 5.03 (GraphPad Software, Inc. San Diego, CA, USA). The significance level was set at P ≤ 0.05.
Results
The residual colony-forming unit of C. albicans after treatment with different protocol is given Table 3a. Comparative examination showed that chlorhexidine (Group III) was the most effective agent in reducing the growth of C. albicans followed by the treatment with a combination of adhesive and chlorhexidine (Group IV) and treatment with cleanser (Group II).
Table 3a.
Residual colony-forming units of Candida albicans after treatment with different methods
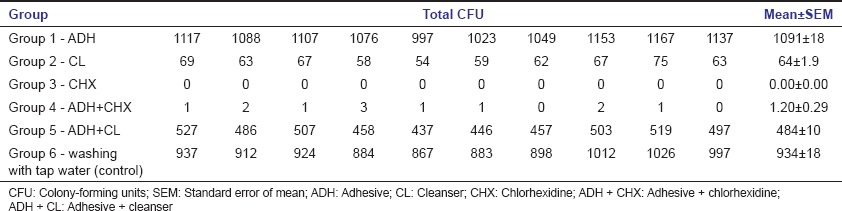
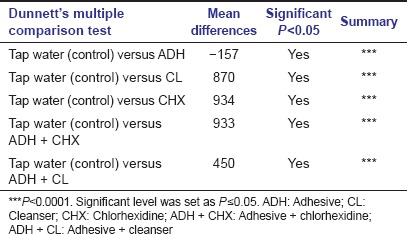
Treatment with combination product of adhesive and cleanser (Group V) also showed reduced fungal growth with respect to control group (Group VI), while denture material treated with adhesive (Group I) alone showed markedly high fungal growth not only with respect to control group but also with respect to all other groups of treatment [Figure 2]. Mean of fungal colony-forming unit after treatment with different groups was statistically compared by one-way ANOVA which indicated significant differences among these groups. Post hoc Tukey's multiple comparison analysis between groups [Table 3b] indicated that mean of most of the treatment groups was highly significant (P < 0.0001). Besides, post hoc Dunnett's multiple comparison test between the control group (Group VI) and treatment groups (Group I, II, III, IV, and V) showed high significance (P < 0.0001). Though, Group I which was treated with adhesive alone showed noticeably high fungal growth compared to control group (Group VI) and was statistically highly significant (P < 0.0001) according to post hoc Dunnett's multiple comparison test [Table 3c]. Whereas, other groups of treatment also showed statistically high significance (P < 0.0001) compare to control group, but it was on the basis of suppressing the fungal growth.
Figure 2.
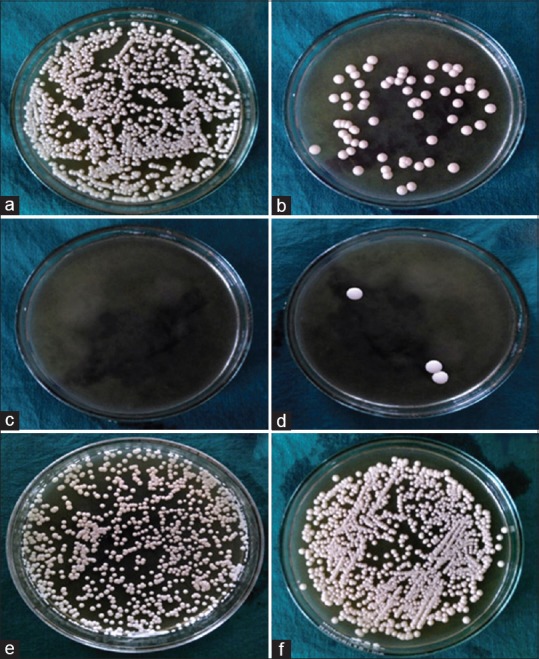
Residual candidal growth after treatment with different methods: (a) adhesive, (b) cleanser, (c) chlorhexidine, (d) adhesive + chlorhexidine, (e) adhesive + cleanser, (f) control
Table 3b.
Post hoc Tukey’s multiple comparison test between treatment groups. Significant level was set as P≤0.05
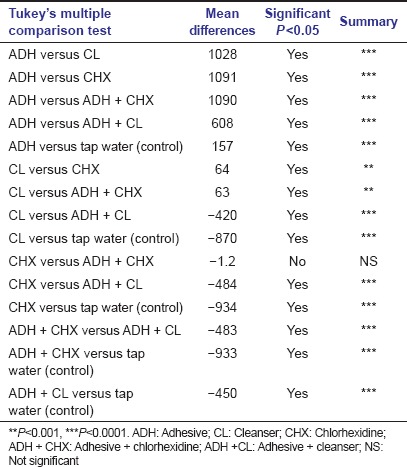
Table 3c.
Post hoc Dunnett's multiple comparison test between treated group with control group
Discussion
The guidelines outlined by the American College of Prosthodontics recommend that dentures should be cleaned daily by soaking and brushing with an effective, nonabrasive denture cleanser.[13] However, for denture wearers with limited motor function, use of denture adhesives are a commonly suggested method to maintain dentures. The effect of different denture adhesives on controlling the candidal growth on denture materials is quite controversial. Several past studies have suggested that denture adhesives suppress fungal growth.[6,11] In the present study, it has been seen that denture adhesive promotes the growth of C. albicans on denture material. This finding supports the study of Stafford and Russell[10,11] who found that most denture adhesives promoted the growth of C. albicans by inducing hyphal formation. This study also supported the findings of Sampaio-Maia et al.[14] where they found that among ten studied adhesives, four adhesives had an inducing effect on fungal growth while others had an inhibitory effect. In an in vivo study conducted by Oliveira et al.,[15] it was found that denture adhesives did not significantly alter the oral microbiota over a 14-day trial period, the results of which may have been influenced by the level of oral hygiene maintenance across different subjects. The present in vitro study was an attempt to keep most of the variables same for all the samples. The discrepancies in the previous studies may be attributed to the fact that each study evaluated different denture adhesives and employed different techniques to assay microbial growth. The present study showed that acrylic treated with adhesive had the highest contamination by C. albicans which may have been caused due to adhesive increasing the surface area of acrylic sample. In the present study, Group II treated with cleanser (clanden, GLOBAL DENT AIDS PVT LTD NOIDA, New Delhi, India), contains sodium perborate, showed a significant inhibitory effect on fungal growth, and exhibited almost 94% fungicidal activity with respect to control. Many authors have proved the efficacy of alkaline perborate denture cleanser like sodium perborate (Na2 H4 B2 O8) tablets to be efficient in removing biofilm.[16,17] The fungicidal mechanism of cleanser might be explained by the fact that it contains sodium perborate (Na2 H4 B2 O8). In aqueous solution, it produces hydrogen peroxide (H2 O2) which chemically possesses both the oxidizing and reducing properties and easily decomposes to form water and nascent oxygen. H2 O2 is a strong oxidizer, containing oxygen-oxygen single bond (O-O) which makes it thermodynamically unstable. This nascent oxygen or singlet oxygen creates oxidative stress on the fungal cells and arrests their metabolic machinery and the fungal cells eventually die. Again in basic solution, H2 O2 produces hydroxyl radicals (· OH) and reduces many inorganic and organic elements. These hydroxyl radicals readily react with and damage vital cellular components. Alkaline peroxides when dissolved in water forms solution of hydrogen peroxide and liberate nascent oxygen or hydroxyl radicals depending on the pH of the solution and damage vital cellular components.[18] The oxygen bubbles also exert a mechanical cleansing effect.[19] Thus, alkaline peroxide cleansers are able to remove Candida from the acrylic surface either by oxidation or reduction or both.
Chlorhexidine showed a remarkable fungicidal activity which was most potent among all the studied groups in the present study. Chlorhexidine is a cationic polybiguanide (bisbiguanide) that shows a broad spectrum antimicrobial activity against many Gram-positive and Gram-negative bacteria and fungi. It has both bacteriostatic and bactericidal mechanisms of action, depending on its concentration. Chlorhexidine acts against fungi, as well as bacteria, by disrupting the cell membrane and inducing cytoplasmic precipitation. Chlorhexidine is a positively-charged molecule that binds to the negatively charged sites on the cell wall which destabilizes the cell wall and interferes with osmosis.[20,21] McDonnell and Russell[21] proposed that the bacterial uptake of chlorhexidine was very rapid, typically working within 20 s. In low concentrations, it affects the integrity of the cell wall. Once the cell wall is damaged, chlorhexidine then crosses into the cell itself and attacks the cytoplasmic membrane (inner membrane). Damage to the cytoplasm's delicate semipermeable membrane allows for leakage of components leading to lysis and cell death. In high concentrations, chlorhexidine causes the cytoplasm to congeal or solidify. The present study proved the efficacy of chlorhexidine to be 100% against C. albicans in a concentration of 0.2% for 5 min. This could be correlated with a study by Vianna et al. in 2004 on disinfection of infected root canals where 2.0% chlorhexidine showed its efficacy against C. albicans >99.99%. Pusateri et al.[22] tested the sensitivity of chlorhexidine on C. albicans grown on denture acrylic. This study also suggested chlorhexidine to be significantly effective against C. albicans. de Andrade et al.[23] proposed that 0.12% for 20 min and 2.0% for 5 min worked with equal efficacy to remove denture biofilm and suggested any of these methods as an auxiliary for cleaning denture.
The present study also investigated two groups which contained a combination of two denture products, namely “adhesive + chlorhexidine” (Group IV) and “adhesive + cleanser” (Group V). No previous study using this combination was found although these situations are very common clinically where a patient uses both these products. It has been seen that Group IV was more efficient than Group V with respect to fungicidal activity (99% and 52%, respectively). This may be explained by the fact that although adhesive promoted C. albicans to colonize efficiently, the strong fungicidal activity of chlorhexidine was still potent against C. albicans. In Group V, i.e., in the case of “adhesive + cleanser” group, the fungal count was higher when compared to “adhesive + chlorhexidine” group because the main fungicidal agent of cleanser (H2 O2) might not have efficiently penetrated the adhesive-coated acrylic samples due to its low half-life. Thus, C. albicans in the outer surface of the adhesive layer was affected by H2 O2, but fungal cells protected by the adhesive layer were unaffected as they are beyond the reach of H2 O2. Moreover, in this study, contamination of the acrylic samples was avoided by directly inoculating the acrylic discs in SDB containing fungal growth which might have influenced the results.
Limitation
The limitations of the study could be the use of acrylic discs instead of dentures where the surface roughness of acrylic surface did not mimic the roughness of intaglio surface of the denture. Moreover, there was no simulation of the oral environment. Various alternative cleaning methods such as ultraviolet radiation and microwaves were not included as a parameter in this study.
Conclusion
Within limitations of the study, the following conclusions can be drawn: Denture adhesive increases the adherence of C. albicans to denture surface to a large extent. When denture adhesives are to be used special care to clean the denture with chlorhexidine or a cleanser must be taken to remove the Candida biofilm. Chlorhexidine should be the first choice for cleaning rather than a cleanser used alone or in combination with denture adhesives.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Orsi IA, Junior AG, Villabona CA, Fernandes FH, Ito IY. Evaluation of the efficacy of chemical disinfectants for disinfection of heat-polymerised acrylic resin. Gerodontology. 2011;28:253–7. doi: 10.1111/j.1741-2358.2010.00400.x. [DOI] [PubMed] [Google Scholar]
- 2.Keng SB, Lim M. Denture plaque distribution and the effectiveness of a perborate-containing denture cleanser. Quintessence Int. 1996;27:341–5. [PubMed] [Google Scholar]
- 3.Jagger DC, Al-Akhazam L, Harrison A, Rees JS. The effectiveness of seven denture cleansers on tea stain removal from PMMA acrylic resin. Int J Prosthodont. 2002;15:549–52. [PubMed] [Google Scholar]
- 4.Webb BC, Thomas CJ, Willcox MD, Harty DW, Knox KW. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust Dent J. 1998;43:45–50. doi: 10.1111/j.1834-7819.1998.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 5.Budtz-Jörgensen E. The significance of Candida albicans in denture stomatitis. Scand J Dent Res. 1974;82:151–90. doi: 10.1111/j.1600-0722.1974.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 6.Jagger DC, Harrison A. Denture cleansing - the best approach. Br Dent J. 1995;178:413–7. doi: 10.1038/sj.bdj.4808788. [DOI] [PubMed] [Google Scholar]
- 7.Harrison Z, Johnson A, Douglas CW. An in vitro study into the effect of a limited range of denture cleaners on surface roughness and removal of Candida albicans from conventional heat-cured acrylic resin denture base material. J Oral Rehabil. 2004;31:460–7. doi: 10.1111/j.1365-2842.2004.01250.x. [DOI] [PubMed] [Google Scholar]
- 8.Paranhos HF, Silva-Lovato CH, de Souza RF, Cruz PC, de Freitas-Pontes KM, Watanabe E, et al. Effect of three methods for cleaning dentures on biofilms formed in vitro on acrylic resin. J Prosthodont. 2009;18:427–31. doi: 10.1111/j.1532-849X.2009.00450.x. [DOI] [PubMed] [Google Scholar]
- 9.Ellepola AN, Samaranayake LP. Adjunctive use of chlorhexidine in oral candidoses: A review. Oral Dis. 2001;7:11–7. [PubMed] [Google Scholar]
- 10.Stafford GD, Russell C. Efficiency of denture adhesives and their possible influence on oral microorganisms. J Dent Res. 1971;50:832–6. doi: 10.1177/00220345710500040701. [DOI] [PubMed] [Google Scholar]
- 11.Makihira S, Nikawa H, Satonobu SV, Jin C, Hamada T. Growth of Candida species on commercial denture adhesives in vitro. Int J Prosthodont. 2001;14:48–52. [PubMed] [Google Scholar]
- 12.Mcfarland J. The nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA. 1907;49:1176–8. [Google Scholar]
- 13.Felton D, Cooper L, Duqum I, Minsley G, Guckes A, Haug S, et al. Evidence-based guidelines for the care and maintenance of complete dentures: A publication of the American College of Prosthodontists. J Prosthodont. 2011;20(Suppl 1):S1–12. doi: 10.1111/j.1532-849X.2010.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Sampaio-Maia B, Figueiral MH, Sousa-Rodrigues P, Fernandes MH, Scully C. The effect of denture adhesives on Candida albicans growth in vitro. Gerodontology. 2012;29:e348–56. doi: 10.1111/j.1741-2358.2011.00478.x. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira MC, Oliveira VM, Vieira AC, Rambob I. In vivo assessment of the effect of an adhesive for complete dentures on colonisation of Candida species. Gerodontology. 2010;27:303–7. doi: 10.1111/j.1741-2358.2009.00345.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar MN, Thippeswamy HM, Raghavendra Swamy KN, Gujjari AK. Efficacy of commercial and household denture cleansers against Candida albicans adherent to acrylic denture base resin: An in vitro study. Indian J Dent Res. 2012;23:39–42. doi: 10.4103/0970-9290.99036. [DOI] [PubMed] [Google Scholar]
- 17.Dhamande MM, Pakhan AJ, Thombare RU, Ghodpage SL. Evaluation of efficacy of commercial denture cleansing agents to reduce the fungal biofilm activity from heat polymerized denture acrylic resin: An in vitro study. Contemp Clin Dent. 2012;3:168–72. doi: 10.4103/0976-237X.96820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prousek J. Fenton chemistry in biology and medicine. Pure Appl Chem. 2007;79:2325–38. [Google Scholar]
- 19.Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 20.Gomes BP, Vianna ME, Matsumoto CU, Rossi Vde P, Zaia AA, Ferraz CC, et al. Disinfection of gutta-percha cones with chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:512–7. doi: 10.1016/j.tripleo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pusateri CR, Monaco EA, Edgerton M. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch Oral Biol. 2009;54:588–94. doi: 10.1016/j.archoralbio.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Andrade IM, Cruz PC, Silva-Lovato CH, de Souza RF, Souza-Gugelmin MC, Paranhos Hde F. Effect of chlorhexidine on denture biofilm accumulation. J Prosthodont. 2012;21:2–6. doi: 10.1111/j.1532-849X.2011.00774.x. [DOI] [PubMed] [Google Scholar]


