Abstract
Background:
Chronic periodontitis, an inflammatory disease, is closely related to certain systemic conditions such as cardiovascular diseases, obesity, and Type 2 diabetes mellitus. These conditions, occurring as comorbidities, synergically affect periodontal tissues.
Aim:
This study aims to examine whether chronic gingivitis and chronic generalized severe periodontitis in patients with Type 2 diabetes mellitus are associated with increased left ventricular mass (LVM).
Materials and Methods:
A total of 45 patients affected with Type 2 diabetes mellitus were recruited and divided into three groups with 15 patients each according to their periodontal status: Group I consisting of healthy individuals, Group II consisting of chronic gingivitis, and Group III consisting of chronic generalized severe periodontitis. They were assessed clinically, biochemically, and echocardiographically. LVM was calculated according to Devereux formula and was indexed to height.
Results:
The differences in the means for LVM and LVM index (LVMI) were statistically significant in three groups with a P = 0.006 and 0.014, respectively. After adjusting for the confounders, the mean values of LVM in Group I, II, and III were 149.35 ± 35.51 g, 147.95 ± 31.59 g, and 156.36 ± 36.57 g, respectively and for LVMI, the mean values were 43.61 ± 12.16 g/m2.7 (Group I), 47.12 ± 10.84 g/m2.7 (Group II), and 46.34 ± 12.55 g/m2.7 (Group III).
Conclusions:
A positive association between chronic generalized severe periodontitis and increased LVM in Type 2 DM patients was observed, suggesting the role of periodontal disease in the left ventricular hypertrophy.
Key words: Cardiovascular disease, diabetes mellitus, left ventricular hypertrophy, periodontitis
Introduction
Periodontal diseases are among the most common, chronic polymicrobial diseases affecting the human race. Anaerobic Gram-negative microorganisms along with a susceptible host are primarily considered to be important etiologic determinants in chronic periodontal disease.[1] It is believed that periodontal diseases do not merely occur as a consequence of plaque accretion, but are also coupled with various host factors, which could alter the consequence of the plaque on a particular individual. A number of diverse studies have indicated that periodontal diseases may also be associated with a wide array of systemic diseases and conditions.[2,3]
Diabetes mellitus has been identified as one of the critical risk factors in chronic periodontal disease and especially so when the glycemic control is inadequate.[4] The immune-inflammatory response is affected in diabetic patients due to alteration in the function of inflammatory cells involved such as macrophages, neutrophils, and monocytes. Periodontal disease, once established; the chronicity of the lesion contributes toward a worsening diabetic status, adding up to more severe diabetes-related complications.[5,6] It has been established that treatment of periodontal disease results in improvement of glycemic control and a decrease in diabetes treatment requirements.[7]
The negative consequences of the periodontal disease in patients with diabetes mellitus, however, are not restricted to the oral cavity and worsening glycemic control, but may also be identified in distant organ systems.[8] Preliminary evidence suggests that periodontal disease infection is a contributing risk factor for cardiovascular disease.[9,10] It has been hypothesized that the periodontal pathogens such as Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Streptococcus sanguis and Tannerella forsythia because of their presence in atherosclerotic plaques, play an important role in the development and progression of atherosclerosis.[11,12]
Another possible explanation for the increased risk of cardiovascular events and an ominous prognostic sign is left ventricular hypertrophy (LVH) and is often present in Type 2 diabetes mellitus patients. LVH caused by an increase in mass of the left ventricle of heart can be secondary to an increase in wall thickness, an increase in cavity size, or both. It is also known that periodontal disease results in elevated systemic levels of C-reactive protein, interleukin-6, and neutrophils which cause increased arterial stiffness, atherosclerosis, and blood pressure, which is thought to contribute to an increased left ventricular mass (LVM).[13] Thus, periodontal diseases may have a direct or indirect association with an increase in LVM. Since there is a persistent ambiguity between this association, further evidence is required to clarify this relationship. Hence, this study was planned to examine whether chronic gingivitis and chronic generalized severe periodontitis in patients with Type 2 diabetes mellitus is associated with increased LVM.
Materials and Methods
A total of 45 patients, ≥30 years of age and affected with Type 2 diabetes mellitus were selected from those visiting the Department of Periodontics and Implantology of Vidya Shikshan Prasarak Mandal's Dental College and Research Centre, Nagpur, India. These patients were assessed clinically, biochemically, and echocardiographically. The study was initiated after the clearance from Institutional Ethics Committee of our institute and adhered to the provisions of Declaration of Helsinki of 1973 (as revised in 2000). The study has been registered with Clinical Trial Registry of India with registration number CTRI/2016/01/006532. A special pro forma was designed, so as to have a systematic recording of information and observations which included a detailed case history, clinical examination, periodontal indices, biochemical, and echocardiographic parameters. All the patients were apprised of the purpose and study design, and a written informed consent was obtained.
The study comprised three groups, each having 15 patients. Group I - Periodontally healthy patients with Type 2 diabetes mellitus. Group II - Chronic gingivitis patients with Type 2 diabetes mellitus and Group III - Chronic generalized severe periodontitis patients with Type 2 diabetes mellitus. Chronic periodontitis was diagnosed based on the 1999 consensus classification of periodontal disease characterized by at least 8 sites with probing pocket depth (PPD) of ≥5 mm and clinical attachment loss (CAL) of ≥5 mm.[14]
Inclusion criteria
Subjects ≥30 years of age
Subjects diagnosed with controlled Type II diabetes mellitus
Periodontally healthy subjects in Group I
Subjects with chronic gingivitis in Group II
Subjects with chronic generalized severe periodontitis in Group III
All subjects having seven or more remaining teeth.
Exclusion criteria
Patients exhibiting the following conditions were excluded from the study:
Patients with a history of any periodontal treatment or systemic antibiotic therapy in the past 6 months
Patients undertaking drugs which have an influence on the periodontium
Patients who were smokers or had a history of smoking
Pregnant women or lactating mothers
Patients with gestational diabetes and any other systemic disease other than Type 2 diabetes mellitus
Hypertensive patients.
Clinical parameters
PPD and CAL were measured using Hu Friedy Williams Graduated Periodontal Probe (Chicago, IL, USA) on four sites of each tooth. Periodontal indices such as plaque index (PI), gingival index (GI), and papillary bleeding index (PBI) were measured to assess periodontal status.[15,16,17] To exclude hypertensive patients, medical history, and office blood pressure was recorded in the sitting position after a rest of 15 min and an average of 3 readings was calculated. The patients’ height, weight, and waist circumference were recorded. Parameters used to measure body mass index (BMI) were height in meters and weight in kilograms and calculated using formula:
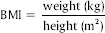
BMI categorization of the study population was done according to the World Health Organization criteria.[18]
Biochemical parameters
The area over the antecubital fossa was disinfected, and 2 ml of blood was withdrawn. The samples were stored until analysis at −80°C for biochemical tests. The parameters such as glycated hemoglobin (HbA1c) and serum lipid profile were evaluated using standard laboratory methods.
Electrocardiographic parameters
Dimensions of the left ventricle of heart were measured using two-dimensional Echocardiography. Echocardiography was performed (Toshiba Nemio XG SSA-580A Machine, Tokyo, Japan) with a 3 MHz cardiac probe by one trained sonographist (AM) [Figure 1]. To exclude endocardial vegetation, B-mode presentation was applied, and M-mode presentation was used. The LVM was calculated as an average value of three readings obtained for every patient according to the Devereux formula.[19] Results were corrected according to the formula:
Figure 1.
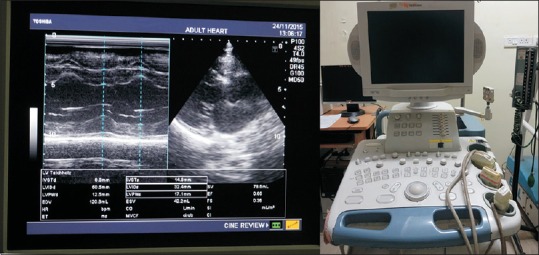
Quantification of left ventricular mass by two-dimensional M-mode echocardiography using Toshiba Nemio XG SSA-580A machine
LVMcorrected = 0.8 × LVM + 0.6 (expressed in grams) and LVM index (LVMI) was obtained by dividing the LVMcorrected by height2.7 and expressed as g/m2.7.
The clinical, biochemical, and electrocardiographic parameters were recorded by the single trained examiner (AK), (GB), and (AM) respectively who were calibrated before the study and were not aware of the other parametric measurements.
The data on demographic parameters like age, gender were obtained on 15 individuals from each of the three study groups. Frequency distribution and descriptive statistics like mean and standard deviation were obtained for demographic parameters. Furthermore, descriptive statistics were obtained for periodontal parameters and compared across three groups. Anthropometric parameters such as BMI, waist circumference, and biochemical parameters like serum lipid profile and glycemic control parameter (HbA1c) were also compared across groups using one-way analysis of variance (ANOVA). LVM and LVMI were compared across study groups using one-way ANOVA. In addition, these two parameters were adjusted to confounders like BMI, age, and HbA1c using analysis of covariance (ANCOVA) and compared across groups using one-way ANOVA. ANCOVA was used to evaluate the statistical significance of difference in the adjusted means of dependent variable across levels of factor. This combines both regression and ANOVA to control the effects of covariates in the analysis. All the analyses were performed using R-3.0.0 programming language, and statistical significance was evaluated at 5% level.
Results
This cross-sectional study compared LVM and LVMI in clinically healthy, gingivitis, and chronic generalized severe periodontitis patients with Type 2 diabetes mellitus. After periodontal examination, a total of 45 Type 2 diabetes mellitus patients (24 males, 21 females, mean age of 49.22 ± 7.6) were divided into three groups: Periodontally healthy (Group I, n = 15), chronic gingivitis (Group II, n = 15), and chronic generalized severe periodontitis (Group III, n = 15). The basal parameters of the patients from all groups are shown in Table 1.
Table 1.
Characteristics of healthy controls and patients with gingivitis and chronic periodontitis and potential determinants of periodontitis severity
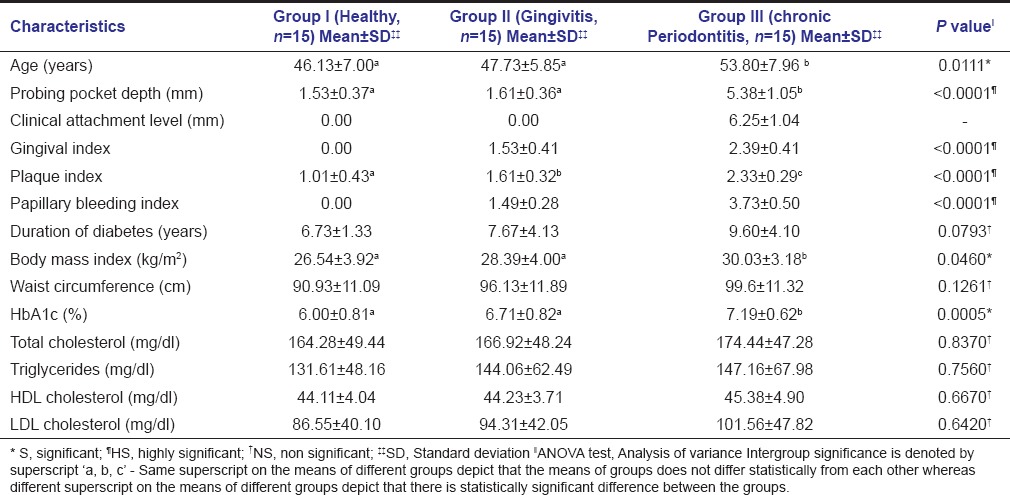
Periodontally healthy patients did not differ significantly from those with chronic gingivitis and chronic generalized severe periodontitis with regards to gender, duration of diabetes, antidiabetic medication, peripheral blood pressure, waist circumference, and serum lipid profile. However, there was a significant difference in age of Group I, II, and III. When long-term glucose control was evaluated using HbA1c assay and an intergroup comparison done, statistically significant difference between Group I (6.00 ± 0.81%) and Group III (7.19 ± 0.62%) with P = 0.0003, as well as Group II (6.71 ± 0.82%) and Group III (7.19 ± 0.62%) with P < 0.05 was seen, while insignificant difference between Group I and II was observed. Another finding observed was that majority of the subjects had a moderate diabetic control as can be concluded from their mean HbA1c values.
Pairwise analysis using Tukey's post hoc test revealed statistically insignificant difference in the mean values of BMI between Group I and Group II, but significant difference between Group I and Group III with P = 0.0357 (P < 0.05). Mean LVM, when estimated by Devereux formula, was found to be 136.31 ± 35.79 g, 149.60 ± 34.14 g, and 167.86 ± 47.34 g in Group I, II, and III, respectively. On indexing LVM to height, mean LVMI in Group I, II, and III was 39.36 ± 11.70 g/m2.7, 47.79 ± 15.59 g/m2.7, and 49.81 ± 15.49 g/m2.7, respectively [Table 2]. On comparison across the three study groups with respect to LVM and LVMI using one-way ANOVA, a statistically insignificant difference was found with a P = 0.102 and 0.120, respectively.
Table 2.
Comparison of mean and SD of LVM and LVMI amongst the 3 study groups before and after adjusting for confounders (age, BMI, HbA1c)
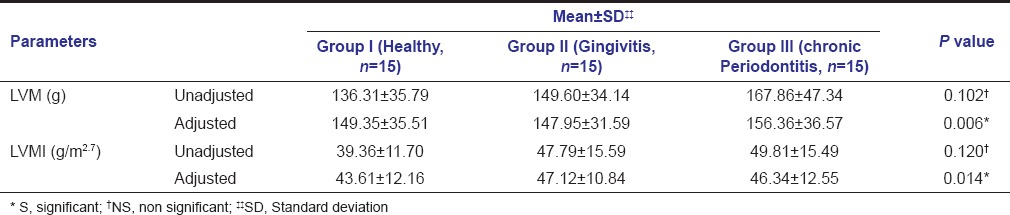
Our results showed that there was statistically significant difference for age, HbA1c, and BMI, among the three study groups. This indicates that these above-mentioned parameters may have had confounding effect on our primary outcome variable. Hence, to better understand the effect of severity of periodontal disease on LVM and LVMI, they were adjusted to age, HbA1c and BMI using ANCOVA and compared across groups using one-way ANOVA. The adjusted model shows that the differences in the means for LVM and LVMI were statistically significant, in the three groups with a P = 0.006 and 0.014, respectively. After adjusting for the confounders, the mean values of LVM in Group I, II, and III were 149.35 ± 35.51 g, 147.95 ± 31.59 g, and 156.36 ± 36.57 g, respectively and for LVMI, the mean values were 43.61 ± 12.16 g/m2.7 (Group I), 47.12 ± 10.84 g/m2.7 (Group II), and 46.34 ± 12.55 g/m2.7 (Group III) [Table 2].
Discussion
The patients included in the study were ≥30 years of age with a mean age of 49.22 ± 7.6 years. We specifically recruited patients with ≥30 years of age because the long-standing chronicity of the periodontal infection would have the time to affect the process of increased LVM leading to a ventricular hypertrophy if at all it occurs.
Diabetes mellitus is considered to be one of the most common chronic human endocrine disorder manifesting with hyperglycemia, whereas chronic periodontitis is one of the most prevalent periodontal infections within the oral cavity, manifesting in periodontal tissue destruction. Both the diseases express themselves in terms of inflammation within the tissues, which also appears to be a critical factor in their pathogenesis. When diabetic control was monitored using HbA1c assay, the difference between mean levels was statistically significant in Group III as compared to the other two groups suggesting a direct or indirect link between diabetic control and periodontal tissue breakdown. The explanation to this could be the possible mechanism by which chronic inflammation (periodontitis) results in an increase in serum inflammatory mediators, thereby causing insulin resistance and further worsening glycemic control. Our results are in agreement with the previous study, in which prevalence and severity of periodontal disease was reported to increase with increased HbA1c levels in Type 2 diabetes mellitus patients.[20] In the present trial serum, lipid profile parameters demonstrated a marginal increase from Group I to Group II with the maximum levels being found with Group III patients, but these differences did not yield any statistical significance. It was revealed that patients with chronic generalized severe periodontitis have higher values for LVM and LVMI compared to chronic gingivitis and patients with healthy periodontium, and the difference reached the level of significance. The findings of the previous studies, in renal patients, in patients with essential hypertension, in diabetic patients and sample from general population reported a positive association between increased LVM, LVMI and chronic periodontitis and our findings are consistent with them.[8,21,22,23,24,25]
The excogitative design of our study is the fact that we recruited all diabetic patients without hypertension, which is in discordance to the inclusion criteria of the above-mentioned studies. In additon, in the present trial, we quantified periodontal inflammatory status based on an overall intraoral assessment of clinical parameters such as PPD, CAL, bleeding on probing, PI, GI, and PBI, which seem to be better as compared to only community periodontal index of treatment, needs score used in the previous trials. The mean LVM in chronic periodontitis patients found in a study by Franek et al. was 238.6 g, whereas in this study it was 156.36 ± 36.57 g.[8] The deviations in results found in our study can strongly be attributed to the ethinicity as our study was conducted on Indian population, the difference in the way the periodontal conditions were evaluated and so, altogether not allowing for the direct comparison of the results.
In this study, BMI was used as an assessor of obesity. Interestingly, the mean BMI in all the three groups was found to be above the upper limit of normal values (BMI >24.9 kg/m2). Increased BMI among the study population is a peculiar trait identified with the presence of diabetes. Mean BMI was found to be highest in Group III (30.03 ± 3.18 kg/m2), showing a positive association between obesity and periodontal disease. Similarly, a significant relationship between obesity and periodontal disease was found in the Nutrition Health and Nutrition Examination Survey III, which concluded that obesity is a significant predictor of periodontal disease, and insulin resistance appears to mediate this relationship.[26] Moreover, in obesity, there is excess fat mass which is known to increase metabolic demand, and thus both cardiac output and total blood volume are elevated which can contribute to volume overload LVH.[27]
Confounding effect of age, BMI, and HbA1c may have attenuated the strength of association between chronic generalized severe periodontitis and LVM. Hence, these parameters were adjusted, and the adjusted model depicts the role of chronic severe periodontitis on LVH by showing significant difference among the three groups.
From the correlations drawn in our study and from the accumulated epidemiologic and laboratory evidence, we can comment that age, obesity, and Type 2 diabetes mellitus are well-known risk factors, playing an important role in the development of LVH. Furthermore the possibility that periodontitis may stimulate inflammatory change in adipose tissue creating a triangular self-generating cycle of morbidity linking obesity, diabetes mellitus, and periodontal disease cannot be underestimated.
In obesity, adipocytes produce pro-inflammatory cytokines such as tumor necrosis factor-α, matrix metalloproteinases, which enhance periodontal tissue destruction. In obese people, there is also a possibility of increased LVM due to volume overload. When hyperinsulinemia was studied in relation to LVH, it was seen that insulin exerts a growth stimulating effect causing the proliferation of myocytes along with stimulation of collagen synthesis in vascular smooth muscle cells.[28] This shows a possible link connecting diabetes mellitus, periodontitis, and LVH. Thus, periodontal infection may play an indirect role in pathophysiology of increased LVM increasing the risk of cardiac events.
The cross-sectional design of the study does not allow us to assess the cause-effect relationship between the parameters examined, and further prospective studies would be required to prove or refute the above-mentioned results. Periodontal interventional studies are desired to assess the effect of periodontal therapy on LVM and LVMI.
Conclusions
The observations of the present study confirm a positive association between chronic severe periodontitis and increased LVM in Type 2 diabetes mellitus, patients suggesting the role of periodontal disease in LVH. Regardless of the underlying mechanisms, it may, however, be said that LVH caused by increased LVM is an independent cardiovascular risk factor and may result in cardiovascular morbidity and mortality. Periodontal disease could provide an easily accessible biological assay for a more accurate definition of cardiovascular risk profile in individual subjects, and thus periodontal evaluation might contribute to refine cardiovascular risk assessment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bodet C, Chandad F, Grenier D. Porphyromonas gingivalis-induced inflammatory mediator profile in an ex vivo human whole blood model. Clin Exp Immunol. 2006;143:50–7. doi: 10.1111/j.1365-2249.2005.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morita T, Yamazaki Y, Mita A, Takada K, Seto M, Nishinoue N, et al. A cohort study on the association between periodontal disease and the development of metabolic syndrome. J Periodontol. 2010;81:512–9. doi: 10.1902/jop.2010.090594. [DOI] [PubMed] [Google Scholar]
- 3.Southerland JH, Moss K, Taylor GW, Beck JD, Pankow J, Gangula PR, et al. Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis. 2012;222:196–201. doi: 10.1016/j.atherosclerosis.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Ryan ME, Carnu O, Kamer A. The influence of diabetes on the periodontal tissues. J Am Dent Assoc. 2003;134:34S–40S. doi: 10.14219/jada.archive.2003.0370. [DOI] [PubMed] [Google Scholar]
- 5.Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: A two-way relationship. Ann Periodontol. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- 6.Karjalainen KM, Knuuttila ML, von Dickhoff KJ. Association of the severity of periodontal disease with organ complications in type 1 diabetic patients. J Periodontol. 1994;65:1067–72. doi: 10.1902/jop.1994.65.11.1067. [DOI] [PubMed] [Google Scholar]
- 7.Grossi SG, Skrepcinski FB, DeCaro T, Robertson DC, Ho AW, Dunford RG, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–9. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 8.Franek E, Napora M, Blach A, Budlewski T, Gozdowski D, Jedynasty K, et al. Blood pressure and left ventricular mass in subjects with type 2 diabetes and gingivitis or chronic periodontitis. J Clin Periodontol. 2010;37:875–80. doi: 10.1111/j.1600-051X.2010.01613.x. [DOI] [PubMed] [Google Scholar]
- 9.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(10 Suppl):1123–37. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 10.López R, Oyarzún M, Naranjo C, Cumsille F, Ortiz M, Baelum V. Coronary heart disease and periodontitis - A case control study in Chilean adults. J Clin Periodontol. 2002;29:468–73. doi: 10.1034/j.1600-051x.2002.290513.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiu B. Multiple infections in carotid atherosclerotic plaques. Am Heart J. 1999;138(5 Pt 2):S534–6. doi: 10.1016/s0002-8703(99)70294-2. [DOI] [PubMed] [Google Scholar]
- 12.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–60. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 13.Danesh J. Coronary heart disease, Helicobacter pylori, dental disease, Chlamydia pneumoniae, and cytomegalovirus: Meta-analyses of prospective studies. Am Heart J. 1999;138(5 Pt 2):S434–7. doi: 10.1016/s0002-8703(99)70270-x. [DOI] [PubMed] [Google Scholar]
- 14.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 16.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 17.Mühlemann HR. Psychological and chemical mediators of gingival health. J Prev Dent. 1977;4:6–17. [PubMed] [Google Scholar]
- 18.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i. [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Apoorva SM, Sridhar N, Suchetha A. Prevalence and severity of periodontal disease in type 2 diabetes mellitus (non-insulin-dependent diabetes mellitus) patients in Bangalore city: An epidemiological study. J Indian Soc Periodontol. 2013;17:25–9. doi: 10.4103/0972-124X.107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franek E, Blach A, Witula A, Kolonko A, Chudek J, Drugacz J, et al. Association between chronic periodontal disease and left ventricular hypertrophy in kidney transplant recipients. Transplantation. 2005;80:3–5. doi: 10.1097/01.tp.0000158716.12065.24. [DOI] [PubMed] [Google Scholar]
- 22.Franek E, Blaschyk R, Kolonko A, Mazur-Psonka L, Langowska-Adamczyk H, Kokot F, et al. Chronic periodontitis in hemodialysis patients with chronic kidney disease is associated with elevated serum C-reactive protein concentration and greater intima-media thickness of the carotid artery. J Nephrol. 2006;19:346–51. [PubMed] [Google Scholar]
- 23.Angeli F, Verdecchia P, Pellegrino C, Pellegrino RG, Pellegrino G, Prosciutti L, et al. Association between periodontal disease and left ventricle mass in essential hypertension. Hypertension. 2003;41:488–92. doi: 10.1161/01.HYP.0000056525.17476.D7. [DOI] [PubMed] [Google Scholar]
- 24.Franek E, Klamczynska E, Ganowicz E, Blach A, Budlewski T, Gorska R. Association of chronic periodontitis with left ventricular mass and central blood pressure in treated patients with essential hypertension. Am J Hypertens. 2009;22:203–7. doi: 10.1038/ajh.2008.330. [DOI] [PubMed] [Google Scholar]
- 25.Shimazaki Y, Saito T, Kiyohara Y, Kato I, Kubo M, Iida M, et al. Relationship between electrocardiographic abnormalities and periodontal disease: The Hisayama Study. J Periodontol. 2004;75:791–7. doi: 10.1902/jop.2004.75.6.791. [DOI] [PubMed] [Google Scholar]
- 26.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11 Suppl):2075–84. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 27.Rider OJ, Francis JM, Ali MK, Byrne J, Clarke K, Neubauer S, et al. Determinants of left ventricular mass in obesity; a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2009;11:9. doi: 10.1186/1532-429X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill DJ, Milner RD. Insulin as a growth factor. Pediatr Res. 1985;19:879–86. doi: 10.1203/00006450-198509000-00001. [DOI] [PubMed] [Google Scholar]


